Ultrasound diagnosis of malaria: examination of the spleen, liver, and optic nerve sheath diameter
Yuanting Zha, Michelle Zhou, Anjali Hari, Bradley Jacobsen, Neha Mitragotri, Bianca Rivas, Olga Gabriela Ventura, Janice Boughton, John Christian Fox,3
1Irvine School of Medicine, University of California, 1001 Health Sciences Road, 252 Irvine Hall, Irvine, CA 92697, USA
2Gritman Medical Center, 700 S Main Street, Moscow, ID, 83843, USA
3Department of Emergency Medicine, Irvine Medical Center, University of California, 101 The City Drive, Orange, CA, 92868, USA
Original Article
Ultrasound diagnosis of malaria: examination of the spleen, liver, and optic nerve sheath diameter
Yuanting Zha1, Michelle Zhou1, Anjali Hari1, Bradley Jacobsen1, Neha Mitragotri1, Bianca Rivas1, Olga Gabriela Ventura1, Janice Boughton2, John Christian Fox1,3
1Irvine School of Medicine, University of California, 1001 Health Sciences Road, 252 Irvine Hall, Irvine, CA 92697, USA
2Gritman Medical Center, 700 S Main Street, Moscow, ID, 83843, USA
3Department of Emergency Medicine, Irvine Medical Center, University of California, 101 The City Drive, Orange, CA, 92868, USA
BACKGROUND:Over 90% of all cases of malaria worldwide occur in Africa. Current methods of diagnosis are time and labor intensive, and could lead to delayed treatment.
METHODS:In this study we investigated the effectiveness of measurements of spleen, liver, and optic nerve sheath diameter (ONSD) in identifying patients with malaria or severe malaria through the use of hand-held ultrasound devices. We recruited 40 adult patients with malaria and 16 adult control subjects at two hospitals in Mwanza, Tanzania. Ultrasonographic diagnosis was compared with rapid antigen diagnostic test and peripheral blood smear as the gold standards. An receiver operating characteristic curve test was performed to determine the most optimal diagnostic threshold for malaria and severe malaria, using each of the measurements for liver size, spleen size, and ONSD. The thresholds were determined to be >12 cm for spleen length and >15.1 cm for liver length, whereas ONSD was not signif cant in this study.
RESULTS:The sensitivities for malaria diagnosis were 66.7% and 58.3% for liver and spleen length respectively, suggesting that these measurements may not be suitable for identifying patients with severe malaria. However, the high specificity of 90.9% for spleen length and the acceptable specif city of 75.0% for liver length suggest that these measurements can be used as a method to eliminate false-positive diagnoses (i.e. patients who do not have severe malaria but are classif ed as having it by a test with a high sensitivity), giving a high positive predictive value.
CONCLUSIONS:We report a high specif city for spleen size and a moderate specif city for liver size in the ultrasonographic diagnosis of severe malaria. Thus when paired with a highly sensitive method of malaria diagnosis, ultrasonographic measurement of spleen and liver size is promising as part of a diagnostic algorithm for malaria. It could be used to stratify risk in patients diagnosed with malaria and assist in their triage. If no sensitive tests are available, ultrasound might be useful to suggest malaria as a cause of a patient's constellation of clinical symptoms.
Diagnostic ultrasound; Malaria; International health; Sub-Saharan Africa; Tanzania
INTRODUCTION
Diagnosis of malaria remains a major problem in endemic regions especially in the absence of a gold standard.[1–3]Optical microscopy and rapid diagnostic test (RDT) are the most commonly used methods. Optical microscopy entails an examination of a Giemsastained peripheral blood smear. This method requires the skill of a trained medical professional to identify signs of a parasite through its life cycle and density in the bloodstream. Despite its high eff cacy and low cost,microscopy is a time-intensive method that requires dedicated equipment and experienced technicians.[4,5]Malaria RDTs detect specific antigens produced by malaria parasites in the infected individual. Some RDTs are specific for the different species of Plasmodium as well. The accepted level of sensitivity for rapid diagnostic tests is 95%, with sensitivities ranging from 77 % to 98% and specificities ranging from 83% to 98% for P. falciparum.[6]Various RDTs are allowed for diagnosis of malaria in the absence of trained technicians or laboratory equipment. However, temperature and humidity can affect the performance of RDTs.[7–9]Additionally, the optimal accuracy for RDTs and microscopy is often not achieved in low-resource settings due to lack of quality control measures.[10]Furthermore, RDTs only provide qualitative results and cannot determine parasite load, which is important in diagnosing malaria and classifying the severity of the disease.[11,12]
Several studies have examined the effectiveness of using single or a combination of clinical signs to diagnose malaria. Redd et al[3]showed that symptoms of the nail bed pallor, splenomegaly, and fever history have a sensitivity of 85%, but a specificity of 41% in identifying children with P. falciparum parasitemia. Chandramohan et al[13]reported that in urban areas of India with a low malaria endemic, a palpable spleen plus fever in the absence of cough or vomiting was 99.8% specific in detecting malaria although the sensitivity was only 0.6%. Maroushek et al[14]reported a moderate sensitivity of 64% and a high specificity of 94% for splenomegaly on physical examination detecting malaria in Liberian refugee children. When splenomegaly was evaluated alongside thrombocytopenia and fever, the sensitivity rose to 71% and the specif city was 88%.
Furthermore, ultrasound can detect splenic rupture, a complication of massive splenomegaly that can be seen in malaria patients.[15]
In our study, we investigated the utility of several clinical findings for the diagnosis of malaria in the endemic region of Mwanza, Tanzania. The findings included hepatomegaly, splenomegaly and widened optical nerve sheath diameter (ONSD), which were reported to be associated with malaria.[16,17]As cerebral malaria becomes more severe, the intracranial pressure rises, resulting in papilledema on physical examination and widening of the ONSD on ultrasound.[18]
In contrast to previous studies, we used ultrasound instead of palpation and fundoscopy as a main diagnostic tool, since low-cost ultrasound units are increasingly available in low resource health clinics.[19,20]Ultrasonography has proven more accurate than palpation in identifying hepatosplenomegaly.[21–23]
METHODS
We measured sonographic dimensions of the liver, spleen, and ONSD for patients in Mwanza, Tanzania.
Study population
Adult patients who had undergone peripheral blood smear test or RDT at Mwananchi Hospital or Buzuruga Clinic in Mwanza, Tanzania were divided into three groups: patients with uncomplicated or non-severe malaria; patients with complicated or severe malaria; and controls who had negative test results. All patients with suspected malaria underwent initial screening with RDT. In the patients with positive RDT results, those who manifested clinical symptoms of severe malaria were tested with peripheral blood smear under optic microscopy. Symptoms of severe malaria that warranted further evaluation with blood smear included high fever greater than 40 °C, altered mental status, convulsions, prostration, jaundice, hypoglycemia with blood glucose less than 40 mg/dL (2.2 mmol/L), or severe anemia with hemoglobin less than 50 g/dL or hematocrit less than 15%. A diagnosis of severe malaria was made if there were over 2 000 parasites per 200 white blood cells. Researchers were not involved in the diagnosis of patients. Patients with trauma to the eye, liver, or spleen were excluded.
The study protocol was approved by the Institutional Review Board of the University of California Irvine, as well as the Ethics Approval Committee of the Tandabui Institute of Health Science and Technology, which served as the ethics approval board for the two hospitals. Informed consent was received from the subjects who were English-speaking patients aged 18 and older. The Declaration of Helsinki was followed.
Methods of imaging
Trained researchers performed ultrasound using Sonosite Nanomaxx battery-powered, hand-carried ultrasound machines (Bothell, WA). A 5–10 MHz linear transducer was used to measure ONSD, and a 3.5–5 MHz curvilinear transducer was used to measure the liver and spleen, with the patient in the supine position. As shown in Figure 1, we measured the longitudinal dimension of the spleen from the most superomedial to most inferolateral borders with the transducer in the coronalplane on the patient's left midaxillary line. We measured the longitudinal liver size along the right mid-clavicular line in the sagittal plane (Figure 2). We obtained bilateral ONSD measurements, 3 mm posterior to the retina, with the linear transducer on the patient's closed eyelid in the transverse view of the optic nerve (Figure 3).
Method of analysis
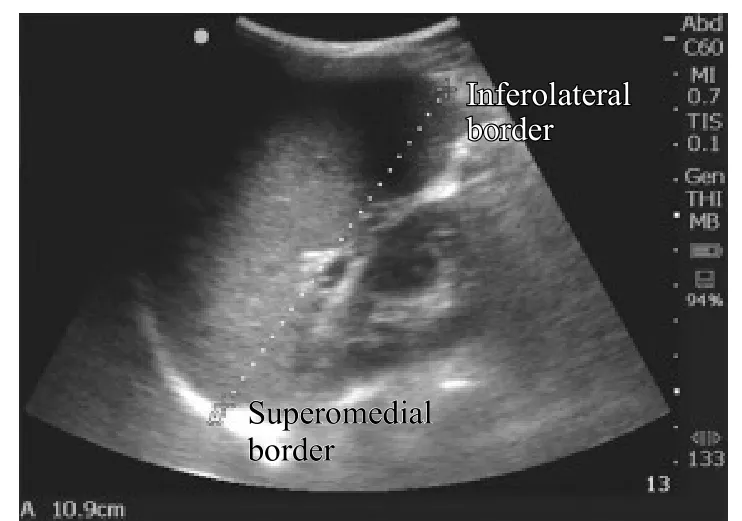
Figure 1. Longitudinal measurement of spleen dimension in the coronal plane at the midaxillary line.
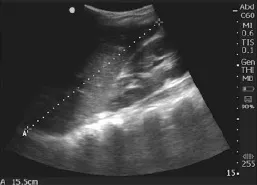
Figure 2. Longitudinal measurement of liver dimension in the sagittal plane at the midclavicular line.
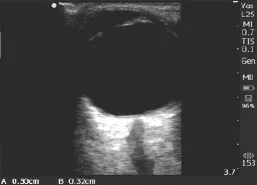
Figure 3. Measurement of optic nerve sheath diameter. Arrows indicate the retina and ONSD.
We calculated the average ONSD, liver and spleen sizes for patients in the non-severe malaria, severe malaria, and control groups, and Student's t tests were performed to evaluate the significance of these measurements in the different groups. Because the populations in malaria-endemic regions exhibit larger than average liver and spleen sizes, we did not use the standard diagnostic criteria (liver >15 cm and spleen >11 cm) to classify hepatosplenomegaly.[24,25]Instead, we adjusted these measurements by using receiver operating characteristic (ROC) curves. Using these curves we were able to f nd the best cut-off values to maximize the sensitivity and specif city of diagnosing either malaria or severe malaria. This allowed us to appropriately assess the diagnostic use of ultrasound in diagnosing malaria in populations from malaria-endemic regions. Using this information, we could select the most optimal model for diagnostic criteria and design an appropriate diagnostic algorithm (Figure 4).
RESULTS
Table 1 shows the average measurements of hepatic dimensions, splenic dimensions and ONSD in the severe malaria, non-severe malaria and control groups. Based on the results of Student's t test, the liver and spleen sizes of the patients with severe malaria were significantly larger than those of the controls (P=0.02), whereas the ONSD was not signif cantly larger (P=0.44). In contrast to the control group, the non-severe malaria group had significantly larger liver sizes (P=0.03), but the spleenand ONSD measurements were not significantly larger (P=0.48, P=0.36, respectively).
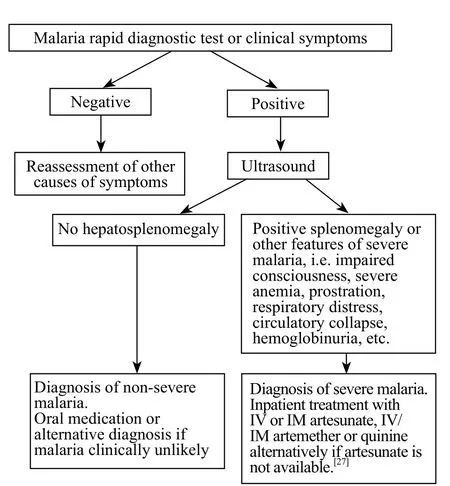
Figure 4. Algorithm for malaria diagnosis using ultrasound. Because splenomegaly >12 cm had a high specificity of 90.9% and hepatomegaly >15.1 cm had a moderate specif city of 75% for severe malaria, we used hepatosplenomegaly via ultrasound as one of the features to determine severe malaria and subsequent treatment.
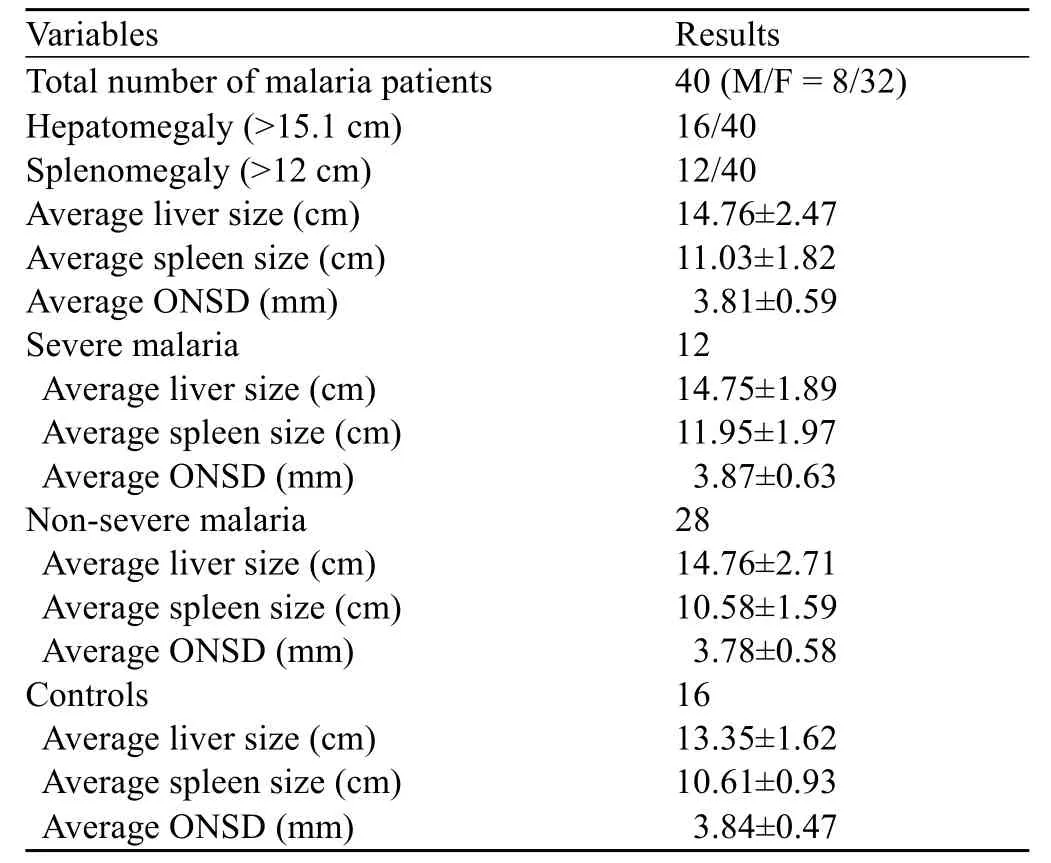
Table 1. The average liver size, spleen size and ONSD of the patients in the three groups
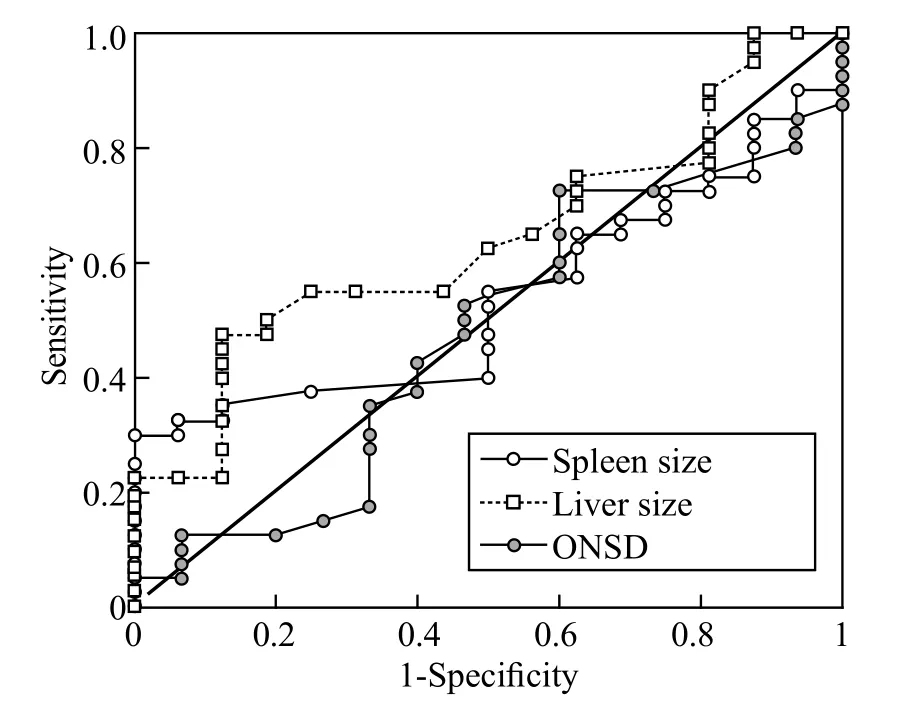
Figure 5. The ROC of spleen size, liver size and ONSD in the diagnosis of malaria. Area under curve for spleen size=0.5359, liver size=0.6383, ONSD=0.4742.
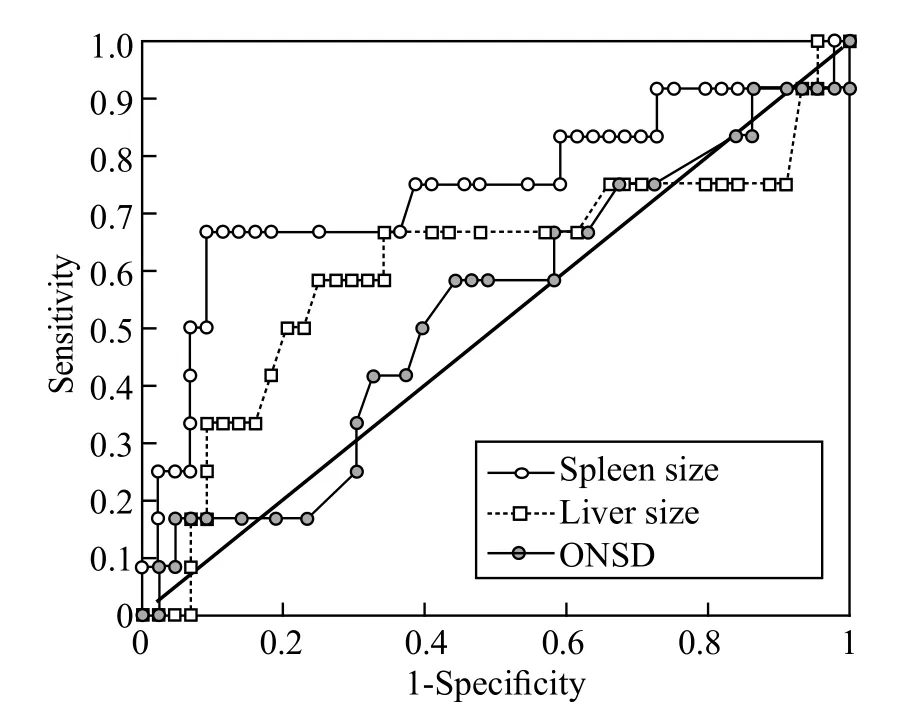
Figure 6. The ROC curve of spleen size, liver size and ONSD in the diagnosis of severe malaria. Area under curve for spleen size=0.7415, liver size=0.6089, ONSD=0.5310. Spleen size showed the highest accuracy of the three measurements.
ROC plots were constructed to evaluate the effectiveness of using these clinical symptoms to diagnose malaria (Figure 5) and severe malaria (Figure 6). The result of ROC analysis revealed that none of the indicators (liver size, spleen size and ONSD) was sensitive or specific enough for the diagnosis of nonsevere malaria. However, for severe malaria, the results of ROC analysis showed promising sensitivity vs. specif city performances, especially for spleen and liver lengths. We found the most optimal combination of sensitivity (58.3%) and specificity (75.0%) in patients with severe malaria with liver length >15.1 cm as the indicator for hepatomegaly. The sensitivity and specificity in all patients with malaria were 40% and 87.5% respectively with 15.1 cm as the cut off value. For spleen size, we chose a cut-off value of 12 cm to indicate splenomegaly in patients with severe malaria because of its optimal sensitivity of 66.7% and specif city of 90.9%. However, the increased sample size could improve the accuracy of diagnosis.
DISCUSSION
In our study, we examined the effectiveness of sonographic measurements of the spleen, liver, and ONSD in the diagnosis of malaria and differentiation of patients with severe malaria from those with uncomplicated malaria. Hand-held ultrasound devices were used to quantify these clinical manifestations because of the application of ultrasonography in areas with low resources. Statistical analysis revealed that spleen and liver dimensions are significantly larger in patients with the severe malaria group compared with the control group. Liver dimension was significantly higher in the uncomplicated malaria group than in the control group. There was no statistically significant difference in ONSD in the severe malaria group or the non-severe malaria group as compared with the control group.
ROC test was performed to find the most optimal diagnostic threshold for the malaria and severe malaria groups using the measurements of liver size, spleen size, and ONSD. These thresholds were determined to be >12 cm for spleen length and >15.1 cm for liver length. The low sensitivities (66.7% and 58.3%, respectively) suggested that spleen and liver length might not be suitable to screen patients with severe malaria. However, the high specificities (90.9% for the spleen and 75.0% for the liver) suggested that spleen andliver dimension can be used to filter out false-positive diagnoses with a high positive predictive value. Because splenomegaly >12 cm has a high specificity of 90.9% for severe malaria, we conclude that splenomegaly via ultrasound is useful for the confirmation of severe malaria. Hepatomegaly >15.1 cm may also be useful, since it procurs a specif city of 75%. Again, the accuracy of the method may be diminished because of our limited sample size. Currently, severe malaria is diagnosed in malaria patients presenting with signs of altered consciousness, severe anemia, respiratory distress, prostration, convulsions, circulatory collapse or shock, clinical jaundice, hemoglobinuria, abnormal spontaneous bleeding, and pulmonary edema. Laboratory findings of severe malaria include hypoglycemia, metabolic acidosis, severe normocytic anemia, hyperlactatemia, and renal impairment.[26,27]While these are sensitive for the diagnosis of severe malaria, they are also present in other illnesses.[2,28,29,30]Thus when paired with a highly sensitive method of malaria diagnosis, ultrasonographic measurement of spleen and liver size is promising as part of the diagnostic algorithm for malaria.[2]
Over-diagnosis of severe malaria not only induces unnecessary economical burden for the patients and healthcare providers, but also leads to the failure in treating other life-threatening diseases. Sicuri et al[31]indicated that on average the cost for the treatment of severe cases of malaria, which requires hospitalization, is at least an order of magnitude higher than the treatment cost of uncomplicated malaria. Furthermore, 70% of the treatment costs were assumed by the household. Therefore, having an effective and easily accessible method such as the ultrasonographic measurement of the spleen and liver size is extremely valuable, particularly in low-resource communities.
ACKNOWLDDGEMENTS
The authors wish to thank the Kay Family Foundation International Ultrasound Initiative and the John Tu Grant for the iMed Initiative at UC Irvine for their financial support for this study. Additionally, we would like to acknowledge the following individuals and institutions for their assistance: Dr. Joseph Kavit of the Tandabui Institute of Health Science and Technology, Eric Oguta, Dr. Sarah Murphy of the Massachusetts General Hospital, Muzi Na, and Jie Zhang of the Johns Hopkins University for assistance with statistical analysis.
Funding:The study was supported by grants from Kay International Ultrasound Initiative, John Tu Grant for the iMed Initiative, New Universal Health Technologies Scholarship.
Ethical approval:This study was approved by the Institutional Review Board of the University of California, Irvine and the Ethics Approval Committee of the Tandabui Institute of Health Science and Technology in Mwanza, Tanzania.
Conf icts of interest:The above authors have declared that there are no competing interests or financial disclosures regarding this manuscript.
Contributors:YZ participated in study design, performed ultrasound for data collection, performed statistical analysis, and drafted the manuscript. MZ participated in study design , performed ultrasound for data collection, assisted in statistical analysis and manuscript writing. AH, BJ, NM, BR, OGV all performed ultrasound for data collection and aided in study design and manuscript revision. JB aided in ultrasonographic data collection and image interpretation. JCF aided in image interpretation, manuscript revision and provided general guidance for the study. All authors read and approved the submission of this f nal manuscript.
1 WHO. The Health of the People: The African Regional Health Report 2006. Geneva, Switzerland: World Health Organization.
2 Anstey NM, Price RN. Improving case definitions for severe malaria. PLOS Med 2007; 4: 1291–1292.
3 Redd SC, Kazembe PN, Luby SP, Nwanyanwu O, Hightower AW, Ziba C, et al. Clinical algorithm for treatment of Plasmodium falciparum malaria in children. The Lancet 1996; 347: 223–227.
4 Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg 2007; 77: 119–127.
5 Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J 2008; 7: 221.
6 Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev 2002; 15: 66–78.
7 Murray CK, Gasser RA, Magill AJ, Miller RS. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev 2008; 21: 97–110.
8 Chiodini PL, Bowers K, Jorgensen P, Barnwell JW, Grady KK, Luchavez J, et al. The heat stability of Plasmodium lactate dehydrogenase-based and histidine-rich protein 2-based malaria rapid diagnostic tests. Trans R Soc Trop Med Hyg 2007; 101: 331–337.
9 Moonasar D, Goga AE, Frean J, Kruger P, Chandramohan D. An exploratory study of factors that affect the performance and usage of rapid diagnostic tests for malaria in the Limpopo Province, South Africa. Malar J 2007; 6: 74.
10 McMorrow ML, Masanja MI, Abdulla SM, Kahigwa E, Kachur SP. Challenges in routine implementation and quality control for malaria –Rufiji District, Tanzania. Am J Trop Med Hyg 2008; 79: 385–390.
11 Mouatcho JC, Goldring JP. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol 2013; 62: 1491–1505.
12 WHO. The use of malaria rapid diagnostic tests 2004. Geneva, Switzerland: World Health Organization.
13 Chandramohan D, Carneiro I, Kavishwar A, Brugha R, Desai V, Greenwood B. A clinical algorithm for the diagnosis of malaria: results of an evaluation in an area of low endemicity. Trop Med Int Health 2001; 6: 505–510.
14 Maroushek SR, Aguilar EF, Stauffer W, Abd-Alla MD. Malaria among refugee children at arrival in the United States. Pediatr Infect Dis J 2005; 24: 450–452.
15 Benter T, Klühs L, Teichgräber U. Sonography of the spleen. J Ultrasound Med 2011; 30: 1281–1293.
16 Murphy S, Cserti-Gazdewich C, Dhabangi A, Musoke C, Nabukeera-Barungi N, Price D, et al. Ultrasound findings in Plasmodium falciparum malaria: a pilot study. Pediatr Crit Care Med 2011; 12: e58–e63.
17 Le A, Hoehn ME, Smith ME, Spentzas T, Schlappy D, Pershad J. Bedside sonographic measurement of optic nerve sheath diameter as a predictor of increased intracranial pressure in children. Ann Emerg Med 2009; 53: 785–791.
18 Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med 2008; 15: 201–204.
19 Adler D, Mgalula K, Price D, Taylor O. Introduction of a portable ultrasound unit into the health services of the Lugufu refugee camp, Kigoma District, Tanzania. Int J Emerg Med 2008; 1: 261–266.
20 Sippel S, Muruganandan K, Levine A, Shah S. Review article: Use of ultrasound in the developing world. Int J Emerg Med 2011; 4: 1–11.
21 Doehring-Schwerdtfeger E, Kaiser C, Schlake J, Abdel-Rahim IM, Mohamed-Ali Q, Richter J, et al. Ultrasound versus clinical examination as indication for Schistosoma mansoni associated morbidity in children. Trop Med Parasitol 1992; 43: 245–248.
22 Cyr J, Johnston DL. Accuracy of physical examination versus ultrasound in the detection of hepatosplenomegaly at diagnosis of pediatric leukemia. J Hematol Malig 2013; 3: 24–27.
23 Picardi M, Vincenzo M, Ciancia R, Soscia E, Morante R, Sodano A, et al. Measurement of spleen volume by ultrasound scanning in patients with thrombocytosis: a prospective study. Blood 2002; 99: 4228–4230.
24 Ehimwenma O, Tagbo MT. Determination of normal dimension of the spleen by ultrasound in an endemic tropical environment. Niger Med J 2011; 52: 198.
25 Kratzer W, Fritz V, Mason RA, Haenle MM, Kaechele V, Roemerstein Study Group. Factors affecting liver size: a sonographic survey of 2080 subjects. J Ultrasound Med 2003; 22: 1155–1161.
26 World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg 2000; 94 (Supplement 1): 1–90.
27 WHO: Guidelines for the treatment of malaria (2e) 2nd edition 2010. Geneva, Switzerland: World Health Organization.
28 Gwer S, Newton C RJC, Berkley JA. Over-diagnosis and comorbidity of severe malaria in African children: a guide for clinicians. Am J Trop Med Hyg 2007; 77 (6 Suppl): 6–13.
29 Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ 2004; 329: 1212.
30 Chandler CI, Mwangi R, Mbakilwa H, Olomi R, Whitty CJ, Reyburn H. Malaria overdiagnosis: is patient pressure the problem?. Health Policy Plan 2008; 23: 170–178.
31 Sicuri E, Vieta A, Lindner L, Constenla D, Sauboin C. The economic costs of malaria in children in three sub-Saharan countries: Ghana, Tanzania and Kenya. Malar J 2013; 12: 307.
Received April 9, 2014
Accepted after revision November 28, 2014
Yuanting Zha, Email: yuantinz@uci.edu
World J Emerg Med 2015;6(1):10–15
10.5847/wjem.j.1920–8642.2015.01.002
 World journal of emergency medicine2015年1期
World journal of emergency medicine2015年1期
- World journal of emergency medicine的其它文章
- The role of regulatory T cells in immune dysfunction during sepsis
- Instructions for Authors
- Lingual angioedema after alteplase treatment in a patient with acute ischemic stroke
- Regulatory effects of hydrogen sulf de on alveolar epithelial cell endoplasmic reticulum stress in rats with acute lung injury
- Relationship between intubation rate and continuous positive airway pressure therapy in the prehospital setting
- Acute intoxication cases admitted to the emergency department of a university hospital
