Fraction Distribution and Risk Assessment of Heavy Metals in Soils around the Mining Area in Zhijin
Yaqi JIA,Zhen YANG,Dan GENG,Qin DENG,DiWU
Guizhou Provincial Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment,Guizhou Normal University,Guiyang 550001,China
1 Introduction
Coal resource is the second big resource after the oil resources to the survival of people's life,butalong with the human exploitation of coal resources,especially unreasonable exploitation,it has caused a lot of damage to the surrounding vegetation and hydrological conditions as well as the pollution of the atmosphere,water,soil,and the soil heavy metal pollution has become widespread concern.The heavy metal pollution of soil around the coal mining are a mainly comes from the migration and deposition of the dust or is under the power of wind,coal dust in the surrounding soil redistribution and through leaching osmosis into the soil,causing the soil to be polluted by heavy metal[1].Once the heavy metals take part in a chemical reaction entering into environment,the process is often irreversible[2].Heavy metal pollutants in soil are stable and have the biological accumulation,thus affecting human health through drinking water and food chain[3].It is widely known that metals in soil are in different chemical forms which influence their reactivity and hence their mobility and bioavil ability.Assessing metal pollution of soils on the basis of total metal content gives little information on the mobility and bioavil ability of heavy metals,thus providing poor guidance for the selection of appropriate remediation strategies for polluted soil.In this study,BCR sequential method was used to extract the chemical forms of heavy metals,and it becomes popular in recent years from a variety of sequential extraction procedures.This method can provide very useful information on metal speciation when assessing the availability of potentially toxic elements in soil[4].The evaluation of heavy metal pollution in soil is very important.In order to assess the pollution degree effectively,the index of geoaccumulation(Igeo)and risk assessment code(RAC)were used in this paper.In this paper,the heavy metal pollution in agricultural soil around coal mining area in Zhijin of Guizhou Province was studied.The concentrations of Cd,Cr,Cu,Ni,Hg and Zn were determined to study the pollution levels of heavy metals.The geological evaluation of the cumulative index was used to evaluate the pollution levels of Cd,Cr,Cu,Ni,Hg and Zn in the soil.Moreover,the sequential extraction was performed for the fraction of heavy metals consisting of the weak acid soluble,reducible,oxidizable and residual fractions.At the same time,risk assessment code(RAC)was used to evaluate the potential ecological risk of heavy metals in soil and provide a scientific basis for heavy metal pollution control.
2 Materials and methods
2.1 Sample collection and preparationThe geography coordinates of the survey site are longitude 105°36′E-106°43′E,latitude 26°21′N-27°46′N,and it is located at Zhijin,Bijie,Guizhou Province,China.Study area is located in the vicinity of a coal mining area.Soil sampling was carried out in September 2013.The location of sampling area is shown in Fig.1.A total of 41 topsoil samples were collected from the sampling area.Samples were collected from a depth of5-20 cm.Each sample was picked out from a mixture of 3-5 subsamples.The collected samples were neatly packed in polyethylene bags and transported to the laboratory.At the laboratory,any foreign adhesive material was manually removed.All samples were dried at room temperature,disaggregated and sieved through 2mm sieve for subsequent analysis.
2.2 Measuring method of total heavy metal concentrations and pHAll glass bottles were filled with 10%nitric acid(G.R.)for 12 h,and then washed with ionized water.To ensure the accuracy of data and measurement,standard soil samples(GBW0-7403)and black soil were tested in the process of dissipation.Sample quality control and deviation is within the scope of the specified requirements,and relative deviation of parallel determination content is within 10%.The content of heavy metals(Cr,Cd,Ni,Cu,Zn,Hg)was analyzed by Atomic Absorption Spectrometry(AAS)and Atomic Fluorescence Spectrometry(AFS).For the determination of pH,2 g soil was taken in a clean and dry beaker(25 mL).Then 10 mL distilled water was added to the beaker and was thoroughly stirred with a glass rod and stewed for 30min.The pH of the suspension was determined with an electrical digital pH meter[5].
2.3 Evaluation of heavy metal contamination in soilsElement contamination in the soil samples was evaluated via the index of geoaccumulation proposed by Muller for bottom sediments[6]and is suitable for use in soil contamination assessment[7-9].The Igeoassesses contamination by comparing current and previous soils.The Igeoindex is calculated using following equation:
where Ciis the measured concentration of element i in soil;Biis the geochemical background value of the element.
The constant 1.5 allows analysis of both natural fluctuations in the content of a given substance in the environment and small anthropogenic influence[6]has defined seven classes of Igeo,as shown in Table 1.
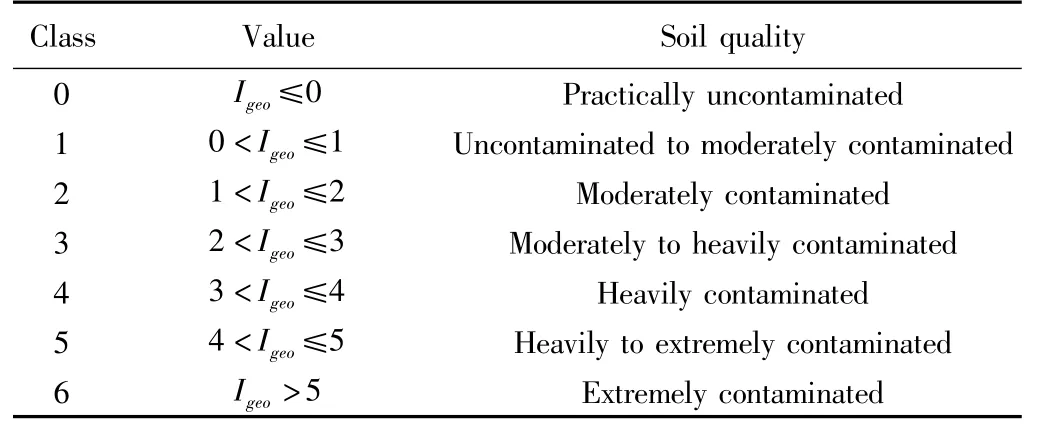
Table 1 Seven classes of Igeo index
2.4 Sequential extraction procedure
2.4.1 Weak acid soluble fraction(F1).1.000 g soil sample was treated with 40mL of0.11mol/LHAc solution.This mixture was shaken in a mechanical shaker at(22±5)℃ for 16 h.The extraction was separated from the soil residue by centrifugation for 30 min.The supernatant was decanted,collected and stored in bottles for analysis.The residue was washed with 20mL deionized water,shaken and centrifuged.The super matant was decanted and discarded,taking care not to discard any of the solid residues.
2.4.2 Reducible fraction(F2).40mL of0.5mol/LNH2OH·HCl solution was added to the residue from the first step.The pH value was kept at2.0 by 0.05 mol/L HNO3.The extraction and the residue were treated as the previous step.
2.4.3 Oxidizable fraction(F3).10 mL of 30%H2O2solution was added to the residue from the second step.The mixture was digested at(22±5)℃ for 1 h and at(85±2)℃ for 1 h,and the volume of liquid was reduced to less than 3mL.A second aliquot of 10 mL of30%H2O2 solution was added,the mixture was digested at(85±2)℃ for 1 h,and the volume of liquid was reduced to less than 1mL.Finally,50mL of1mol/LNH4Ac(pH was adjusted to 2.0)solution was added.The extraction and the residue were treated as the previous step.
2.4.4 Residual fraction(F4).The residual fraction was calculated by the difference between the total content and all other fraction content.The concentration of Cr,Cd,Ni,Cu,Zn,Hg in the various extracts was determined by AAS and AFS.
2.5 Assessment about environmental risk of heavy metals
The risk assessment code(RAC)was used to determine the risk of each metal to environment.The RAC is determined on the basis of a percentage of the total concentration of heavy metals in the weak acid soluble fraction.The higher the percentage of the metal in this part,the higher the probability of releasing metal from the solid phase to liquid phase[10].The RAC classification introduced by Perin et al.[11](Table 2)was employed for the present study.According to the classification,when the weak acid soluble fraction is less than 1%,the metals in the sediment pose no risk to the environment.The percentage of this part at 1 to 10%,11 to 30%,31 to 50%and over 50%indicates low risk,moderate risk,high risk and very high risk,respectively,and indicates that metal can easily enter the food chain[11].

Table 2 Classification of risk assessment
3 Results and discussions
3.1 Heavy metal concentrations in soilsThe pH value of soil ranged between 4.09 and 5.86 and the average pH of soil was 5.05,which indicated that the soil samples were slightly acid.The heavy metal concentration of soil samples is shown in Table3.Total concentrations of heavy metals ranged as follows:Cd 0.08-2.08 mg/kg,Cr 144.2-664.20 mg/kg,Cu 91.63-187.5 mg/kg,Ni46.61-104.47 mg/kg,Zn 134.65-272.4 mg/kg and Hg 0.14-2.60mg/kg.The obtained results show that the maximum concentrations of Cu and Ni in soil samples exceeded their corresponding limits of the National Soil Environmental Quality StandardⅡ,which indicated that the concentrations of Cu and Ni in soil were high.Results of statistical analysis indicated that the average concentrations of heavy metals were higher than their corresponding limits of the National Soil Environmental Quality StandardⅡ.Especially for Cd,its concentration was 3.2 times that of the National Soil Environmental Quality Standard.This results reflected that the long-term mining and smelting activities led to significant accumulations of this6 elements in soils.
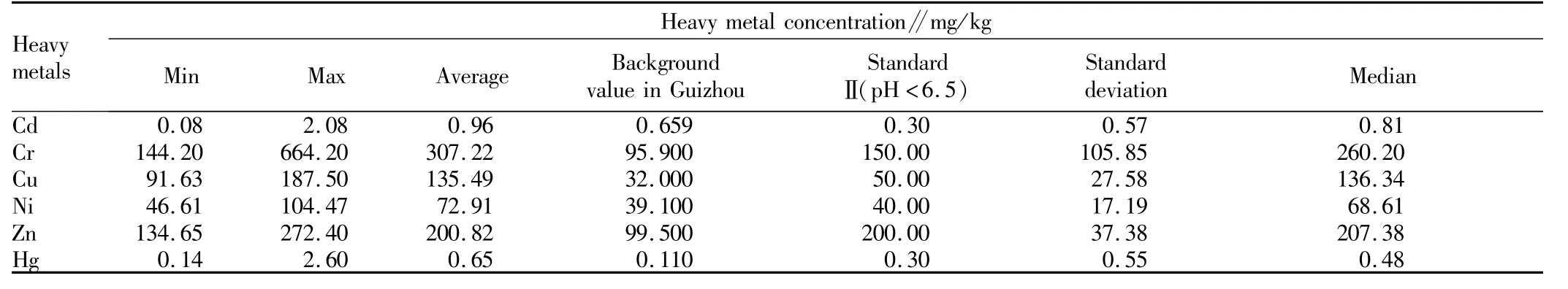
Table 3 Mean and standard deviation for element concentration in soils
3.2 Evaluation of heavy metal contamination in soilsTo evaluate heavy metal contamination of soils in the mining area,the background values for soils in Guizhou Province were obtained from existing literature,as shown in Table 3.The Igeomethod was applied to evaluate the levels and overall range of contamination.The background values for Guizhou were chosen were the geochemical background values(Bi),as indicated in Eq.(1).According to Eq.(1),the percentage of samples in Muller class is shown in Fig.2.It can be found from Fig.2 that the most serious polluted element was Hg,for 26.83%of Hg was moderately or heavily contaminated and 7.32%of Hg was heavily contaminated.As for Cu,Cr,Cd,92.68%,41.46%,7.32%were moderately contaminated,respectively.As for Zn and Ni,most samples were uncontaminated or moderately contaminated.The Igeovalues were calculated by the average concentrations of heavy metals in soil samples.The average values of Igeofor each metal and their pollution levels are shown in Table4.The results indicate that the soils of the study area can be categorized as follows:uncontaminated or moderately contaminated with Ni and Zn;moderately contaminated with Cr,Cu and Hg;practically uncontaminated with Cd.The assessment results show that the contamination degree from strong to weak in soil is Hg>Cu>Cr>Zn>Ni>Cd.
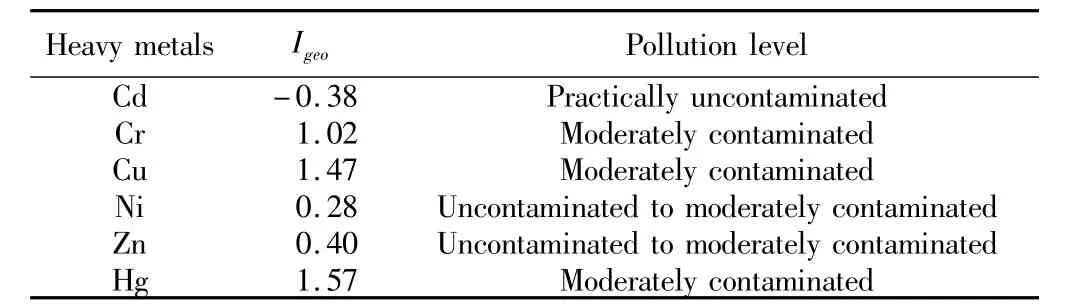
Table4 Average values of for each metal
3.3 Fraction distribution of heavy metalsTo assess the mobility and bioavail ability of heavy metals and determine the geochemical distribution of Cu,Cd,Zn,Ni,Cr and Hg in the mining area,20 soil samples with relatively high heavy metal concentrations were selected from 41 topsoils to conduct BCR sequential extraction procedure.Data were expressed as different fractions with respect to the total amount in the soil.BCR results of 6 elements are shown in Fig.3.It can be seen form Fig.3 and Table 5 that the order of average percentage about weak acid soluble fraction(F1)is that Cd>Ni>Zn>Cu>Hg>Cr;the order of average percentage about(F2)is that Cd>Cu>Zn>Ni>Hg>Cr;the order of average percentage about(F3)is that Ni>Cu>Zn>Hg>Cd>Cr;the order of average percentage about(F4)is that Cr>Hg>Zn>Ni>Cd>Cu.Generally,the value of(F1+F2+F3)is considered the availability[12-13].In conclusion,the order of 6 heavy metals(F1+F2+F3)is that Cu(56.89)>Cd(50.95)>Ni(41.52)>Zn(35.06)>Hg(23.04)>Cr(4.88).It is widely accepted that the weak acid soluble fraction is generally absorbed by soil and humus.This fraction is sensitive to environment and it is easy to transfer and move,directly leading to toxicity in the plants.In the available fraction,the highest percentage in the weak acid soluble fraction was related to Cd,indicating that Cd in soil was unstable and easily released by dissolution.Additionally,the toxicity of Cd is relatively high.It may be a serious threat to the surrounding ecosystem.At the same time,Ni and Zn deserve much attention.In the available fractions,Cu,Cd,Ni were mainly presented in the reducible and oxidizable fraction.The reducible fraction is the combination of element in soil and carbonate,this fraction is sensitive to pH value,and when the pH decreases,it will be released.Oxidizable fraction is another main occurrence speciation besides the residual for most elements.It is absorbed by Fe-Mn oxides or a part of precipitation of hydroxide itself and it is more stable,but can release when external conditions are changed,such as the pH and redox potential.So,the potential ecological risk of Cu,Cd,Nialso deserves much attention.At the same time,the high reducible and oxidizable fraction may be influenced by strong acid of local soil(average pH value=5.05).Cr was mainly presented in the residual fraction,with content ranging from 89.23%to96.64%,so its status in the soil was considered to be stable.

Table 5 Chemical fractionation of heavy metals in soil(%)
3.4 Risk assessment code of heavy metalsAfter determining the available fraction of 6 heavy metals,the risk assessment code is used to evaluate the ecological risk.The result is shown in Fig.4.It can be seen that Cd inmost part of soil samples was at a low or moderate risk to the environment.And its reducible and oxidizable fraction is also a high percentage,and it is easily released to environment when pH and redox potential are changed.From environmental point of view,it is notable that Cd posesa serious risk to surrounding ecosystems.In the studied soils,Zn,Cu,Ni,Hg are at a low risk or no risk,and their reducible and oxidizable fraction is relatively high,thus,once the pH and redox potential change,this fraction of heavy metals is unstable and easily released by dissolution.So,the environmental risk from Zn,Cu,Ni,Hg can not be ignored.Fig.4 shows that Crwas strongly associated with the residual fraction,with content ranging from 89.23 to 96.64,so its risk in the environment was considered to be safe.The assessment results show that the risk degree from strong to weak in soil is Cd>Ni>Zn>Cu>Hg>Cr.We can find that the two assessment methods show different results.It is because metals in soil are presented in different chemical forms which influence their reactivity and hence their mobility and bioavail ability.
4 Conclusions
(i)Soil samples are slightly acid.After long-term mining activity,the soil around mining area was polluted by6 elements to different degrees.Especially for Cd,its concentration was3.2 times that of the National Soil Environmental Quality StandardⅡ.(ii)The Igeovalues suggest that the soils of the study area can be categorized as follows:uncontaminated or moderately contaminated with Ni and Zn;moderately contaminated with Cr,Cu and Hg;practically uncontaminated with Cd.The assessment results show that the contamination degree from strong to weak in soil is Hg>Cu>Cr>Zn>Ni>Cd.(iii)BCR sequential extraction results show that the order of average percentage about weak acid soluble fraction(F1)is that Cd>Ni>Zn>Cu>Hg>Cr,and the order of6 heavy metal available fraction(F1+F2+F3)is that Cu(56.89)>Cd(50.95)>Ni(41.52)>Zn(35.06)>Hg(23.04)>Cr(4.88).(iv)The RAC results show that the risk degree from strong to weak in soil is Cd>Ni>Zn>Cu>Hg>Cr.Ni,Zn,Cu and Hg are the potential elements with risk,and especially for Cd,it poses a serious risk to surrounding ecosystems.
[1]WANG L,WANG I,WANGM,et al.Contaminate characteristic of heavy metals in soils in Shenmumining area[J].Ecology and Environmental Sciences,2011,20(8-9):1343-1347.
[2]GUOWJ,JIANGXW,CHENXJ,et al.Research on soil remediation technology of heavy metal pollution abandoned land in metal mine[J].Journal of Anhui Agricultural Science,2010,38(22):11954-11956.
[3]LIAO GL,ZHOU YD,WU C.Forecast models of heavy metal contamination near tailing dam and their application[J].Journal of Central South U-niversity Technology:Science and Technology,2004,35(6):1009-1013.
[4]TESSIR A,CAMPBELLPGC.BISSONM.Sequential extraction procedure for the speciation of particulate trace metals[J].Analytical Chemistry,1979,51(7):844-851.
[5]XU Y,LUO F,XU RY,et al.Research on determination methods for pH value of agricultural soil in Sanya District[J].Journal of Changjiang Vegetables,2011(24):50-51.
[6]MULLERG.Index of geoaccumulation in sediments of the Rhine River[J].Geojournal,1969(2):108-118.
[7]JIYQ,FENG YC,WU JH,et al.Using geoaccumulation index to study source profiles of soil dust in China[J].Journal of Environmental Sciences,2008,20(5):571-578.
[8]LOSKA K,WIECHULA D,KORUS I.Metal contamination of farming soils affected by industry[J].Environment International,2004,30(2):159-165.
[9]WEI BG,YANG LS.A review of heavy metal contaminations in urban soils,urban road dusts and agricultural soils from China.Microchemical Journal,2010(94):99-107.
[10]Safoura Javan,Amir Hessam Hassani,Ahmad Gholamalizadeh,et al.Fractionation of heavy metals in bottom sediments in Chahnimeh 1,Zabol,Iran[J].Environmental Monitoring Assessment,2015(187):340-351.
[11]Perin G,Craboledda L,Lucchese M,etal.Heavy metal speciation in the sediments of Northern Adriatic sea.A new approach for environmental toxicity determination[C].Heavy Metals in the Environment,1985(2):454-456.
[12]GAN GJ,LIUW,QIU YQ,et al.Heavy metal pollution and ecological risk assessment of the paddy soils in a smelting area in Central Hunan[J].Environmental Chemistry,2013,23(1):132-138.
[13]GUOWH,LIU XB,LIU ZG,et al.Pollution and potential ecological risk evaluation of heavy metals in the sediments around Dongjiang Harbor,Tianjin[J].Procedia Environmental Science,2010(2):729-736.
 Asian Agricultural Research2015年12期
Asian Agricultural Research2015年12期
- Asian Agricultural Research的其它文章
- Coupling Path of China's Modern Service Industry and Agriculture Based on Improved Entropy Method
- Bayesian Analysis of Small Multi-frequency Investment of Agricultural Products
- Effort Levels of Capital-constrained Retailer under Bank Financing
- Frontier and Evolution of Marketing Discip line Based on Scientific Metrological Analysis of Top 4 Marketing Periodicals in 2009-2013
- Changes in Net Barter Term s of Trade for Sino-Australian Agricultural Products after China's Accession to the WTO
- Systematic Analysis and Innovation for Development Policies of Beijing Seed Industry at Transformation Stage
