Theoretical Study on Inverse Sandwich Complexes[E-C5−nH5−nNn-E]+and [E-C5−nH5−nPn-E]+(n=1,2,3;E=Al,Ga,In,Tl)
Nan-nan Liu,Yi-hong Ding
a.Chemistry Center,College of Food Engineering,Harbin University of Commerce,Haerbin 150028, China
b.State Key Laboratory of Theoreticaland ComputationalChemistry,Institute of TheoreticalChemistry, Jilin University,Changchun 130012,China
(Dated:Received on April 6,2015;Accepted on September 21,2015)
Theoretical Study on Inverse Sandwich Complexes[E-C5−nH5−nNn-E]+and [E-C5−nH5−nPn-E]+(n=1,2,3;E=Al,Ga,In,Tl)
Nan-nan Liua∗,Yi-hong Dingb∗
a.Chemistry Center,College of Food Engineering,Harbin University of Commerce,Haerbin 150028, China
b.State Key Laboratory of Theoreticaland ComputationalChemistry,Institute of TheoreticalChemistry, Jilin University,Changchun 130012,China
(Dated:Received on April 6,2015;Accepted on September 21,2015)
The inverse sandwiches[E-C5−nH5−nNn-E]+and[E-C5−nH5−nPn-E]+(n=1,2,3;E=Al, Ga,In,Tl)with low-valent boron group elements are studied.The(η5,η5)coordinated inverse sandwich[E-C5−nH5−nNn-E]+is unstable in energy or nonexistent.However,the (η5,η5)coordinated[E-C5−nH5−nPn-E]+is not only stable in energy,but also stable against dissociation.The dissoction stability[E-C5−nH5−nPn-E]+with the same E element decreases as the number n increases,while for the certain n number,the dissociation energies with different E elements are close to each other.[E-C4H4P-E]+has similar dissocition stability to the well-known[E-C5H5-E]+.The inteaction between C5−nH5−nPnand lowvalent E element is mainly ionic.Since lone pairs of electrons locate on both E and P atoms,the(η5,η5)coordinated inverse sandwich[E-C5−nH5−nPn-E]+would act as multi electron-donors.
Low-valent,Boron group elements,Electron-donor,Organometallic,Heterocyclic
I.INTRODUCTION
Low-valent boron group elements complexes have drawn a wide range of interest in the fields of coordination chemistry and organometallic chemistry in the past decade[1–3].The accessibility of low-valent boron group elements in ER,ECp∗etc.(E=boron group elements,Cp∗=C5Me5,R=C(SiMe3)3)makes them potential electron-richσ-donor ligands in materials chemistry[4–6].The assembly between poly ECp∗and transition metals can produce the multi-core sandwiches [Ma(ECp∗)b](M=Pb,Pt)[7,8].Aiming at the type [M(ECp∗)n]m+(M=transition metals),the[Ga-Cp∗-Ga]+cation with a special inverse sandwich structure was surprisingly synthesized[9].As a nearly“naked”Ga+ion,[Ga-Cp∗-Ga]+was proposed to be a potential selective source for highly reactive Ga+.
The important discovery inspired theoretical studies on the inverse sandwiches[E-Cp-E]+and E-C4H4-E (E=B,Al,Ga,In,Tl,Cp=C5H5)[10,11].For[E-Cp-E]+,[B-Cp-B]+dissociates via the loss of the neutral B atom,while heavier[E-Cp-E]+(E=B,Al,Ga,In, Tl)dissociates via the loss of the charged E+.For EC4H4-E,inverse sandwich B-C4H4-B is not available, the E-C4H4-E(E=Al,Ga,In,Tl)dissociates via the loss of the neutral E atom.Obviously,different ligands bring different properties for inverse sandwiches.However,the cases of low-boron group inverse sandwiches with other aromatic ligand are still seldom known so far.
Cp anion is a widely used aromatic ligand in organometallic and coordination chemistry[12–16]. Heterocyclic polyphospholyl(CH)5−nPn−have similar aromaticity to that of Cp−,various researches focused on the coordination mode of(CH)5−nPn−with metals.Generally theη5-coordination is common for these heterocyclic rings,such as the ferrocene-like[Fe(η5-Since the coordination mode of(CH)5−nPn−is similar to Cp−,the inverse sandwich[E-C5−nH5−nPn-E]+might exist like[E-Cp-E]+.Although the low-valent boron group elements generally act as electron-donors, the vacant p-orbitals also make them potential acceptors to form donor-acceptor bond with P atom.Therefore,it is of interest whether the inverse sandwiches [E-C5−nH5−nPn-E]+are available or not.Herein,a series of inverse sandwiches[E-C5−nH5−nNn-E]+and[EC5−nH5−nPn-E]+(E=Al,Ga,In,Tl;n=1,2,3)are theoretically considered.
The geometries of all structures were fully optimized using the density functional theory methods PBEPBE, B3LYP and MPW91PW91 with the def2-TZVP basis set by mean of Gaussian 09 program packages[19–27]. Bonding analyses are performed using NBO 3.0,Multi-wfn and Visual Molecular Dynamics(VMD)programs [28,29].
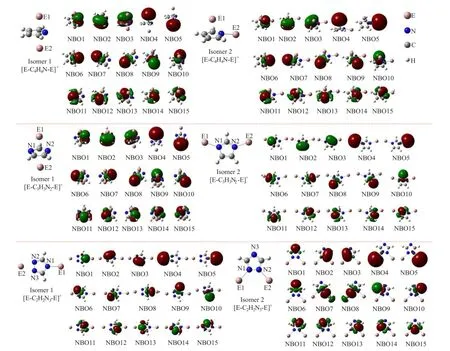
FIG.1 The structures and NBOs of[E-C5−nH5−nNn-E]+.
II.RESULTS AND DISCUSSION
A.Geometries and bonding of[E-C5−nH5−nNn-E]+(E=Al,Ga,In,Tl;n=1,2,3)
The structures and the natural bond orbitals(NBOs) of[E-C5−nH5−nNn-E]+isomers are shown in Fig.1.Table I shows the relative energies of[E-C5−nH5−nNn-E]+or[E-C5−nH5−nPn-E]+(E=Al,Ga,In,Tl)isomers.
[E-C4H4N-E]+has two isomers,isomer 1 is a typical inverse sandwich structure with the(η5,η5)coordination,isomer 2 is with the(η5,η1)coordination.Isomer 1 of[E-C4H4N-E]+is about 2.7−10.8 kcal/mol higher in energy than the isomer 2.Noted that the inverse sandwich isomer 1 of[Al-C4H4N-Al]+, which has an imaginary frequency of−93.6 cm−1at PBEPBE/TZVP,is not a local minimum.Thus,(η5, η5)coordinated isomer 1 of[Al-C4H4N-Al]+would not exist.As the element grows heavier from Ga to Tl, the stability of isomer 1 is increased.The bonding is studied by NBO analysis at PBEPBE/def2-TZVP level.Since the NBO orbitals for[E-C5−nH5−nNn-E]+or[E-C5−nH5−nPn-E]+with different E are similar,we only take[Ga-C5−nH5−nNn-Ga]+or[Ga-C5−nH5−nPn-Ga]+as an example for illustration.For isomer 1,the vertical distances Ga−C4H4N,In−C4H4N,Tl−C4H4N are 2.320,2.548 and 2.629˚A,respectively.As shown in Fig.1,NBOs 1−3 correspond to the delocalizedbond of C4H4N,NBOs 4 and 5 correspond to the nGa(s) LPs,NBO 6 for the nN(sp2)lone-pair(LP),NBOs 7−10 for the fourσC−Hbonds,NBOs 11−15 for the fiveσC−CandσC−Nbonds.The NBO numbers are arranged in anascending order according to the orbital energies.The NBO charge distributed on each Ga atom is high to 0.857,the Ga-(η5-C4H4N)interactions are mainly ionic. The sum of the Mayer bond orders for Ga-(η5-C4H4N) interaction is 0.68,where the N−Ga contribution is 0.22 (32.4%).
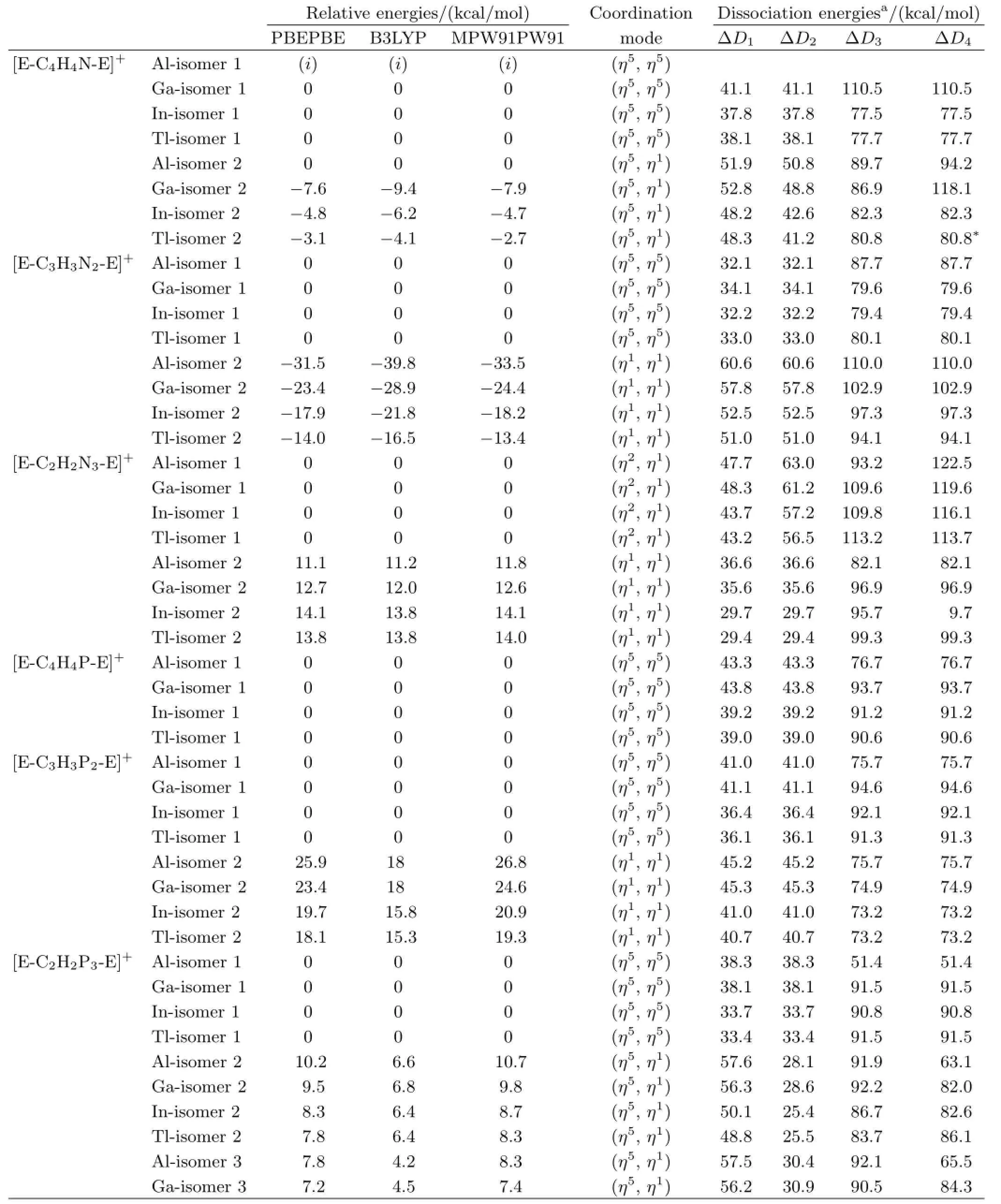
TABLE I The relative energies,coordination mode and dissociation energies of[E-C5−nH5−nNn-E]+and[E-C5−nH5−nPn-E]+,the isomer 1 of[Al-C4H4N-Al]+marked by(i)is not a local minimum.

TABLE I continued.
For isomer 2 of[E-C4H4N-E]+,the vertical distances Al1−C4H4N,Ga1−C4H4N,In1−C4H4N,Tl1−C4H4N are 2.252,2.383,2.524,and 2.615˚A;the distances N−Al2,N−Ga2,N−In2 and N−Tl2 are 2.021,2.146, 2.369,2.466˚A,respectively.NBOs 4 and 5 correspond to the nGa(s)LPs,NBO 6 for the nN(sp2)LP.The NBO charges on Ga1 and Ga2 atoms are 0.754 and 0.912.The sum of the Mayer bond orders for Ga1-(η5-C4H4N)is 0.81,where the Ga1−N contribution is 0.11 (13.6%).By second order perturbation theory analysis,(sp26.9dO.5)interaction represents the donor-acceptor N−Ga2 bond,of which the Mayer bond order is 0.53(see Fig.2).
[E-C3H3N2-E]+has two isomers,isomer 1 is a(η5, η5)coordinated inverse sandwich structure,isomer 2 is(η1,η1)coordinated.Isomer 2 of[E-C3H3N2-E]+is much lower in energy(13.4−39.8 kcal/mol)than isomer 1.For isomer 1,the vertical distances Al−C3H3N2, Ga−C3H3N2,In−C3H3N2,and Tl−C3H3N2are 2.324, 2.371,2.599,and 2.666˚A,respectively.NBOs 6 and 7 correspond to the nN(sp1.6O,sp1.58)LPs,NBOs 4 and 5 for the nGa(s)LPs.The NBO charge on each Ga atom is 0.874.The sum of the Mayer bond orders for Ga-(η5-C3H3N2)is 0.51,where each N−Ga contribution is 0.15 (29.4%).
For isomer 2 of[E-C3H3N2-E]+,the distances N1−Al1(N2−E2),N1−Ga1,N1−In1,and N1−Tl1 are 2.012,2.118,2.348,and 2.443˚A,respectively.NBOs 4 and 5 correspond to the nGa(s)LPs,NBOs 6 and 7 for the nN(sp1.95)LPs.The NBO charge on Ga1 atom is 0.892.The two nN1(sp1.95)tions represent the two donor-acceptor N−Ga bonds in isomer 2 with the Mayer bond order of 0.57(Fig.2).
[E-C2H2N3-E]+has two isomers,isomer 1 is a (η2,η1)coordinated structure,isomer 2 is a(η1, η1)coordinated structure.Isomer 1 is 11.1−14.1 kcal/mol lower in energy than isomer 2.For isomer 1,the distances N2−Al2(N3−E2),N2−Ga2, N2−In2,and N2−Tl2 are 2.178,2.284,2.482,and 2.575˚A;the distances N1−Al1,N1−Ga1,N1−In1,and N1−Tl1 are 2.066,2.167,2.387,and 2.480˚A,respectively.NBOs 6 and 7 correspond to the nN(sp1.6) LPs,NBO 8 for the nN(sp1.9)LP,NBOs 4 and 5 for the nGa(s)LPs.The NBO charges on Ga1 and Ga2 atom are 0.902 and 0.873.The two donoracceptor interactions of nN2(sp1.6)(sp49.6dO.7)form one three-center/ two-electron bo nd 3c/2e(1),the interactions ofother three-center/two-electron bond 3c/2e(2).The total Mayer order for N2−Ga2 and N3−Ga2 is 0.60(each is 0.30).interaction indicates the donor-acceptor N1−Ga1 bond with the Mayer bond order of 0.52(Fig.2).
For isomer 2 of[E-C2H2N3-E]+,the distances N1−Al1(N2−E2),N1−Ga1,N1−In1,and N1−Tl1 are 1.997,2.146,2.355,and 2.478˚A,respectively.NBOs 4 and 5 correspond to the nGa(s)LPs,NBO 6 for the nN(sp1.6)LP,NBOs 7 and 8 for the non-Lewis N−Ga bonds ofσN−Ga=0.976(sp3.38)N+0.217(sp34.3dO.8)Ga. The natural ionicity parameter iN−Gacould be defined as:
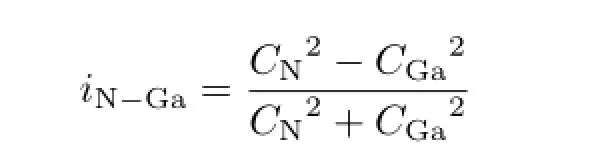
The value of iN−Gais 0 for a pure covalent bond,−1 and 1 are for a pure ionic bond.As the value for N1−Ga1 is 0.905,the bond is mainly ionic and strongly polarized toward N atom.
In addition,the(η5,η5)coordinated inverse sandwich[E-C5−nH5−nNn-E]+is unstable in energy compared with other isomers.C5−nH5−nNnrings prefer to form direct N−E bonds with E elements.The high NBO charges on E atoms(Ga as an example)state the E-C5−nH5−nNninteractions are mainly ionic.
B.Geometries and bonding of[E-C5−nH5−nPn-E]+

FIG.2 Donor-acceptor bonds in[E-C5−nH5−nNn-E]+.

FIG.3 The structures and natural bond orbitals(NBOs)of[E-C5−nH5−nPn-E]+.

FIG.4 Donor-acceptor bonds in[E-C5−nH5−nPn-E]+.
[E-C4H4P-E]+has only one inverse sandwich structure with the(η5,η5)coordination(Fig.3).The vertical distances Al−C4H4P,Ga−C4H4P,In−C4H4P,and Tl−C4H4P are 2.301,2.351,2.596,and 2.672˚A,respectively.NBOs 1−3 correspond to the delocalizedπ56bond of C4H4P,NBOs 4 and 5 for the nGa(s)LPs,NBO 6 for the nP(spO.5)LP,NBOs 7−10 for theσC−Hbonds and NBOs 11−15 for theσC−CandσC−Pbonds.The NBO charge on Ga atom is 0.792,which is lower than 0.857 in[Ga-C4H4N-Ga]+.The sum of the Mayer bond orders for Ga-(η5-C4H4P)interaction is 0.79,where the P-Ga contribution is 0.28(35.4%).The NBO charges indicate the Ga-(η5-C4H4P)interaction is mainly ionic, but more covalent compared with the Ga-(η5-C4H4N) interaction.
[E-C3H3P2-E]+has two isomers,isomer 1 is(η5,η5) coordinated,isomer 2 is(η1,η1)coordinated.Isomer 1 is more stable(15.8−26.8 kcal/mol)than isomer 2.For isomer 1,the vertical distances Al−C3H3P2, Ga−C3H3P2,In−C3H3P2,and Tl-C3H3P2are 2.330, 2.380,2.625,and 2.696˚A,respectively.NBOs 4 and 5 correspond to the nGa(s)LPs,NBOs 6 and 7 for the nP(spO.5)LP.The NBO charge on each Ga atom is 0.764.The sum of the Mayer bond orders for Ga-(η5-C3H3P2)is 0.87,where the each Ga-P contribution is 0.25(28.7%).
For isomer 2 of[E-C3H3P2-E]+,the distances P1−Al1(P2−E2),P1−Ga1,P1−In1,and P1−Tl1 are 2.601,2.626,2.827,2.884˚A,respectively.NBOs 4 and 5 correspond to the nGa(s)LPs,NBOs 6 and 7 correspond to the nP(spO.8)LPs.The NBO charge on Ga1 atom is 0.762.The twointeractions make known the two donor-acceptor P−Ga bonds with the Mayer bond order of 0.5(Fig.4).
[E-C2H2P3-E]+has four isomers,isomer 1 is(η5, η5)coordinated,isomer 2 and isomer 3 are(η5,η1) coordinated,isomer 4 is(η2,η1)coordinated.Isomer 1 is the lowest energy structure,while isomer 4 is the highest.For isomer 1,the vertical distances Al−C4H4P,Ga−C4H4P,In−C4H4P,and Tl−C4H4P are 2.359,2.400,2.646,and 2.719˚A,respectively.NBOs 4 and 5 correspond to the nGa(s)LPs,NBO 6 for the P−P bond(0.707(sp7.2)P+0.707(sp7.2)P),NBO 7,8 and 9 for the nP(spO.5for P1,spO.4for P2 and P3)LPs. The NBO charge on each Ga atom is 0.742.The sum of the Mayer bond orders for Ga-(η5-C2H2P3)is 0.91, where P1−Ga contribution is 0.21(23.1%),P2−Ga and P3−Ga contributions are both 0.22(24.2%).
For isomer 2 of[E-C2H2P3-E]+,the vertical distances Al1−C2H2P3,Ga1−C2H2P3,In1−C2H2P3,and Tl1−C2H2P3are 2.283,2.336,2.589,and 2.666˚A;the distances P1−Al2,P1−Ga2,P1−In2 and P1−Tl2 are 2.653,2.741,2.900,2.953˚A,respectively.NBOs 4 and 6 correspond to the nGa(s)LPs,NBO 5 for the P−P bond (0.707(sp7.3)P+0.707(sp7.3)P),NBO 6,7 and 8 for the nP(spO.8for P1,spO.4for P2 and P3)LPs.The NBO charges on Ga1 and Ga2 atom are 0.618 and 0.830.The sum of the Mayer bond orders for Ga1-(η5-C4H4N)is 1.06,where the P1−Ga1,P2−Ga1,and P3−Ga1 contribution are 0.26(24.5%),0.27(25.5%),and 0.27(25.5%),
respectively.The nP1(sp84.Od1.2)interaction represents the donor-acceptor P1−Ga2 bond,of which the Mayer bond order is 0.40(Fig.4).
For isomer 3 of[E-C2H2P3-E]+,the vertical distances Al1−C2H2P3,Ga1−C2H2P3,In1−C2H2P˚3,Tl1−C2H2P3are 2.300,2.337,2.597 and 2.673A; the distances P2−Al2,P2−Ga2,P2−In2,and P2−Tl2 are 2.623,2.695,2.876,and 2.929˚A,respectively. NBOs 4 and 5 correspond to the nGa(s)LPs,NBO 6,7 and 9 for the nP(spO.5for P1,spO.4for P2 and spO.6for P3)LPs,NBO 8 for the P−P bond(0.707(sp7.5dO.1)P+0.707(sp7.5dO.1)P).The NBO charges on Ga1 and Ga2 atom are 0.641 and 0.852.The sum of the Mayer bond orders for Ga1-(η5-C4H4N)is 1.09,where the P1−Ga1,P2−Ga1,and P3−Ga1 contribution are 0.13(11.9%),0.24(22.0%),and 0.26(23.9%),tion represent the donor-acceptor P3−Ga2 bond with the Mayer bond order of 0.48(Fig.4).
For isomer 4 of[E-C2H2P3-E]+,the distances P1−Al1,P1−Ga1,P1−In1,and P1−Tl1 are 2.662, 2.689,2.883,and 2.980˚A,the distances P2−Al2 (P3−Al2),P2−Ga2(P3−Ga2),P2−In2(P3−In2), and P2−Tl2(P3−Tl2)are 2.655,2.686,2.922,and 2.959˚A,respectively.NBOs 4 and 5 correspondto the nGa(s)LPs,NBOs 6−8 for the nP(spO.9for P1,spO.6for P2 and P3)LPs.The NBO charges on Ga1 and Ga2 atom are 0.782 and 0.758.form one 3c/2e(1)bond,form another 3c/2e(2)bond(Fig.4).The total Mayer order for N1−Ga1 and N2−Ga1 is 0.84(each 0.44).Theinteraction represents the donor-acceptor N1−Ga1 bond with the bond order of 0.50.
The(η5,η5)coordinated inverse sandwich [E-C5−nH5−nPn-E]+is stable in energy than other isomers.Unlike direct N−Ebonds in[E-C5−nH5−nNn-E]+which is favorable,C5−nH5−nPnbinds with E elements by E-(η5-C5−nH5−nPn)interactions.The NBO charges distributed on E atoms in C5−nH5−nPnare generally lower than those in C5−nH5−nNn.Therefore,the [E-C5−nH5−nPn-E]+interactions are mainly ionic,but more covalent compared with[E-C5−nH5−nNn-E]+interactions.Since there are LPs of electrons on both E and P atoms,the inverse sandwich[E-C5−nH5−nPn-E]+would be multielectron-donors(Fig.5).
C.The dissociation stability of[E-C5−nH5−nNn-E]+and [E-C5−nH5−nPn-E]+
The first-step dissociation reactions of[EC5−nH5−nNn-E]+or[E-C5−nH5−nPn-E]+respect to the loss of a neutral or charged E are represented as follows:
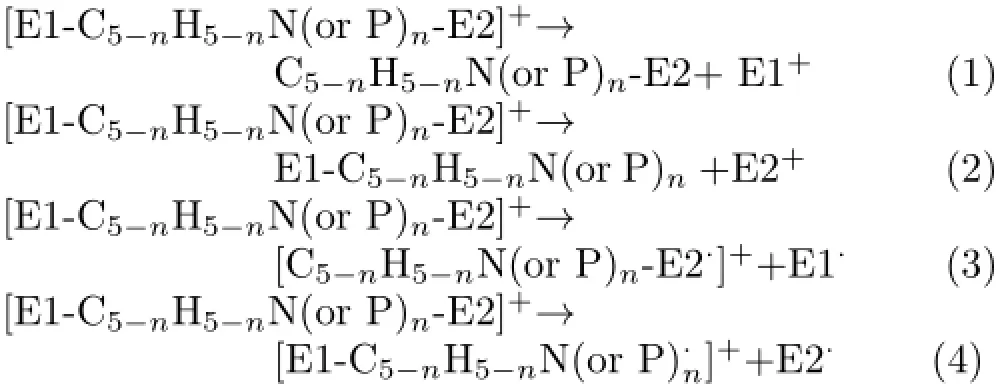
The dissociation energies for reactions(1)−(4)represent asΔD1,ΔD2,ΔD3,andΔD4are listed in Table II.Similar to[E-Cp-E]+,[E-C5−nH5−nN(or P)n-E]+dissociates via loss of the charged E+.
For the(η5,η5)coordinated[E-C5−nH5−nN(or P)n-E]+,the structures dissociate via dividing into the charged E+cation and the half-sandwich E-(η5-C5−nH5−nN(or P)n).TheΔD1(orΔD2)values for isomer 1 of[E-C4H4N-E]+,[E-C3H3N2-E]+, [E-C4H4P-E]+,[E-C3H3P2-E]+,and[E-C2H2P3-E]+are 37.8−41.1,32.1−34.1,39.0−43.3,36.1−41.1,and 33.4−38.3 kcal/mol.The dissociation energies of the inverse sandwich[E-C4H4P-E]+are very close to those of[E-C5H5-E]+[10,11].As the number of N or P increases,the dissociation stability of the(η5, η5)coordinated[E-C5−nH5−nN(or P)n-E]+decreases. The dissociation energies of[E-C5−nH5−nN(or P)n-E]+with different E are close to each other,while[Ga-C5−nH5−nN(or P)n-Ga]+is the most stable one among them.The(η5,η5)coordinated[E-C5−nH5−nPn-E]+is relatively stable both in energy and dissociation reaction,meanwhile,the(η5,η5)coordinated[EC5−nH5−nNn-E]+is just the other way round.

FIG.5 The orbitals of Ga LPs(in blue)and E LPs(in red) in[E-C5−nH5−nPn-E]+.
For the(η5,η1)coordinated structures,the dissociation energiesΔD2are lower thanΔD1.The isomer 2 of[E-C4H4N-E]+,isomer 2 of[E-C2H2P3-E]+and isomer 3 of[E-C2H2P3-E]+dissociate via dividing into the charged E+cation and the half-sandwich E-(η5-C5−nH5−nN(or P)n).
The(η2,η1)and(η1,η1)coordinated structures include at least two N−E(or P−E)bonds.The former dissociates into the E-(η2-C5−nH5−nN(or P)n) and E+cation,the latter dissociates into the E-(η1-C5−nH5−nN(or P)n)and E+cation.The dissociation energies indicate stronger N−E interaction than P−E.
III.CONCLUSION
In this work,inverse sandwiches[E-C5−nH5−nNn-E]+and[E-C5−nH5−nPn-E]+(n=1,2,3;E=Al,Ga,In, Tl)with low-valent boron group elements are theoretically studied.The(η5,η5)coordinated inverse sandwiches[E-C4H4N-E]+and[E-C3H3N2-E]+are unstable in energy,the(η5,η5)coordinated[E-C2H2N3-E]+could not be obtained during the optimization.However,the(η5,η5)coordinated inverse sandwiches[EC5−nH5−nPn-E]+are not only stable in energy,but also stable against dissociation.Similar to the well-known [E-Cp-E]+,the(η5,η5)coordinated[E-C5−nH5−nPn-E]+dissociates via the division of the half-sandwich E-(η5-C5−nH5−nPn)and the charged E+cation.For the(η5,η5)coordinated structures with the same E element,the dissociation stability of[E-C5−nH5−nPn-E]+decreases as the number n increases;for the structures with the certain n number,the dissociation energies with different E are close to each other,while [Ga-C5−nH5−nP-Ga]+is generally the most stable one among them.The dissociation energies of the(η5,η5) coordinated[E-C4H4P-E]+are very close to those of [E-C5H5-E]+,thus,the former might exist like the latter.The high NBO charges on E atoms indicate the E-C5−nH5−nNnand E-C5−nH5−nPninteractions aremainly ionic,but the former is more covalent than the latter.For the relatively stable(η5,η5)coordinated[EC5−nH5−nPn-E]+,since both E and P atoms possess lone pairs of electrons,the inverse sandwiches would be potential multielectron-donors for further application.
IV.ACKNOWLEDGMENTS
This work was supported by the the Natural Science Foundation of Heilongjiang Province of China (No.B201409),the National Natural Science Foundation of China(No.21273093,No.21301041),and the Doctoral Scientific Research Foundation of Harbin University of Commerce(No.13DL019).
[1]A.H.Cowley,Chem.Commun.2369(2004).
[2]G.Merino,H.I.Beltran,and A.Vela,Inorg.Chem.45, 1091(2006).
[3]G.G.Sandra,T.Bollermann,R.A.Fischer,and R. Murugavel,Chem.Rev.112,3136(2012).
[4]G.Linti,J.Organomet.Chem.520,107(1996).
[5]P.Jutzi,B.Neumann,G.Reumann,and H.G.Stammler,Organometallics 17,1305(1998).
[6]P.Jutzi and L.O.Schebaum,J.Organomet.Chem. 654,176(2002).
[7]C.Gemel,T.Steinke,M.Cokoja,A.Kempter,and R. A.Fischer,Eur.J.Inorg.Chem.4161(2004).
[8]T.Steinke,C.Gemel,M.Winter,and R.A.Fischer, Chem.Eur.J.11,1636(2005).
[9]B.B.Buchin,C.Gemel,T.Cadenbach,R.Schmid,and R.A.Fischer,Angew.Chem.Int.Ed.45,1074(2006).
[10]I.I.Fern´andez,E.Cerpa,G.Merino,and G.Frenking, Organometallics 27,1106(2008).
[11]N.N.Liu,J.Xu,and Y.H.Ding,Int.J.Quantum. Chem.113,1018(2013).
[12]X.J.Li and Z.Z.Zeng,Chem.Res.Chin.U.22,6 (2006).
[13]W.G.Lu,J.X.Tao,X.Y.Li,and Y.Z.Wang,Acta Phys.Chim.Sin.17,836(2001).
[14]F.Yang,P.Yua,J.Shi,J.Zhao,X.He,and J.Wang, Chin.J.Chem.Phys.26,721(2013).
[15]P.Ding,E.J.Liang,M.J.Huang,X.Y.Guo,and J. W.Zhang,Chin.J.Chem.Phys.18,268(2005).
[16]N.N.Liu and Y.H.Ding,Chem.J.Chin.Univ.35, 1720(2014).
[17]G.Frison,F.Mathey,and A.Sevin,J.Phys.Chem.A 106,5653(2002).
[18]E.D.V.Bruce and W.R.Rocha,Organometallics 23, 5308(2004).
[19]A.D.Becke,Phys.Rev.A 38,3098(1988).
[20]C.Lee,W.Yang,and R.G.Parr,Phys.Rev.B 37,785 (1988).
[21]J.P.Perdew,K.Burke,and M.Ernzerhof,Phys.Rev. Lett.77,3865(1996).
[22]J.P.Perdew,K.Burke,and M.Ernzerhof,Phys.Rev. Lett.78,1396(1997).
[23]C.Adamo and V.V.Barone,J.Chem.Phys.108,664 (1998).
[24]J.P.Perdew,K.Burke,and Y.Wang,Phys.Rev.B 54,16533(1996).
[25]D.Feller,J.Comp.Chem.17,1571(1996).
[26]K.L.Schuchardt,B.T.Didier,T.Elsethagen,L.Sun, V.Guru-moorthi,J.Chase,J.Li,and T.L.Windus,J. Chem.Inf.Model.47,1045(2007).
[27]M.J.Frisch,G.W.Trucks,H.B.Schlegel,G.E. Scuseria,M.A.Robb,J.R.Cheeseman,G.Scalmani, V.Barone,B.Mennucci,G.A.Petersson,H.Nakatsuji,M.Caricato,X.Li,H.P.Hratchian,A.F.Izmaylov,J.Bloino,G.Zheng,J.L.Sonnenberg,M. Hada,M.Ehara,K.Toyota,R.Fukuda,J.Hasegawa, M.Ishida,T.Nakajima,Y.Honda,O.Kitao,H.Nakai, T.Vreven,J.A.Jr.Montgomery,J.E.Peralta,F. Ogliaro,M.Bearpark,J.J.Heyd,E.Brothers,K.N. Kudin,V.N.Staroverov,R.Kobayashi,J.Normand, K.Raghavachari,A.Rendell,J.C.Burant,S.S.Iyengar,J.Tomasi,M.Cossi,N.Rega,J.M.Millam,M. Klene,J.E.Knox,J.B.Cross,V.Bakken,C.Adamo, J.Jaramillo,R.Gomperts,R.E.Stratmann,O.Yazyev, A.J.Austin,R.Cammi,C.Pomelli,J.W.Ochterski, R.L.Martin,K.Morokuma,V.G.Zakrzewski,G.A. Voth,P.Salvador,J.J.Dannenberg,S.Dapprich,A. D.Daniels,¨O.Farkas,J.B.Foresman,J.V.Ortiz,J. Cioslowski,and D.J.Fox,Gaussian 09,Revision A.1, Wallingford CT:Gaussian,Inc.,(2009).
[28]T.Lu and F.Chen,J.Comp.Chem.33,580(2012).
[29]W.Humphrey,A.Dalke,and K.Schulten,J.Molec. Graphics.14,33(1996).
∗Authors to whom correspondence should be addressed.E-mail: liunann.yl@gmail.com,yhdd@jlu.edu.cn
 CHINESE JOURNAL OF CHEMICAL PHYSICS2015年6期
CHINESE JOURNAL OF CHEMICAL PHYSICS2015年6期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Importance of Metal Cations and Water for Stability of MnO2Crystals
- Elastic Low-Energy Electron Collisions with Methanethiol
- Antireflective and Self-cleaning Properties of SiO2/TiO2Double-Layer Films Prepared by Cost-Effective Sol-Gel Process
- Effects of Sm Co-doping on Luminescent Properties of Sr4Al14O25:M (M=Mn4+,Cr3+)Phosphors
- Pyrene Derivate Functionalized with Acetylene for Organic Field Effect Transistors
- Miniature Boat Fabrication with Striking Loading Capacity in Seawater from Hydrophobic Steel Mesh
