Antireflective and Self-cleaning Properties of SiO2/TiO2Double-Layer Films Prepared by Cost-Effective Sol-Gel Process
Hui Zhang,Duo-wang Fan,Tian-zhi Yu,Cheng-long Wang
Key Laboratory of Opto-Electronic Technology and Intelligent Control,Lanzhou Jiaotong University, Lanzhou 730070,China
(Dated:Received on April 16,2015;Accepted on May 13,2015)
Antireflective and Self-cleaning Properties of SiO2/TiO2Double-Layer Films Prepared by Cost-Effective Sol-Gel Process
Hui Zhang,Duo-wang Fan∗,Tian-zhi Yu,Cheng-long Wang
Key Laboratory of Opto-Electronic Technology and Intelligent Control,Lanzhou Jiaotong University, Lanzhou 730070,China
(Dated:Received on April 16,2015;Accepted on May 13,2015)
SiO2/TiO2double-layer films with antireflective and self-cleaning properties were prepared by dip-coating glass substrate into cost-effective SiO2and TiO2sol successively and subsequently being calcined at 500◦C.The optical and structural properties of films have been investigated by UV-visible spectrophotometer and field emission scanning electron microscope, respectively.At the same time,self-cleaning property generated from superhydrophilicity and photocatalysis was obtained.The results indicated that the as-prepared SiO2/TiO2double-layer films show maximum transmittance of 95%and self-cleaning property.
SiO2/TiO2double-layer films,Antireflective,Self-cleaning,Sol-gel process
I.INTRODUCTION
Antireflective(AR)films are widely used in various optical applications such as display panels,optical lenses,greenhouses,solar collectors,solar cells and so on[1,2].SiO2film with homogenous pore distribution is used as AR film on glass in order to enhance the light transmittance of glass due to its low reflective index and low surface scattering[3,4].The SiO2coated glass used in solar collectors can maximize the sunlight through the glass and improve the utilization rate of solar energy[5].
For outdoor application,the single-layer AR film is exposed to various environmental influences,and cannot maintain antireflectivity for a long time,because the pollution and water condensation can decrease the transmittance of the cover glass significantly[6].The decrease is attributed to filled pores of the thin film, which lead to an increase in the refractive index and hence to a higher reflectance[7].Therefore,not only high transmittance for visible light but also self-cleaning and antifogging properties of the film on solar collector are desirable[8],because self-cleaning film could save a lot of time and cost for maintenance.
TiO2was shown in the last decade to be the best candidate for self-cleaning application.The self-cleaning property of TiO2film derives from its two phenomena: photocatalysis and superhydrophilicity.TiO2can decompose organic contaminant or kill bacteria adhering to the surface of TiO2under illumination of ultraviolet (UV)light.Moreover,the superhydrophilic TiO2film favors the fast and complete spreading of water droplets on the surface(e.g.rainwater),which can wash off the contaminant and dust[9,10].
For the preparation of SiO2and TiO2films the sol-gel method is often used.It is thought that sol-gel method has the advantage of simpler,faster and lower cost, compared to vacuum evaporation and chemical vapor deposition[11,12].But in traditional sol-gel methods,tetraethoxysilane(Si(OEt)4,TEOS)and tetrabutylorthotitanate(Ti(OC4H9)4,TBOT)were always used as precursors of SiO2soland TiO2solrespectively, and anhydrous alcohol was used as solvent.These materials are expensive and unsuitable for continuous mass production techniques in solar-power industry.
In this work,SiO2and TiO2colloidal solution were used to substitute TEOS and TBOT respectively for preparing corresponding metal oxide films,and industrial alcohol was used to substitute anhydrous alcohol as solvent.We deposited TiO2nanoparticles on singlelayer SiO2film to fabricate self-cleaning and antireflective SiO2/TiO2double-layer films.To verify the efficiency of the prepared double-layer films,light transmittance,surface morphology,superhydrophilicity and photocatalysis were evaluated.
II.MATERIAL AND METHODS
A.Materials and reagents
Aqueous SiO2colloidal solution(pH=8−9,20wt%) was obtained from Qingdao Jiyida Silica Reagent Factory.Aqueous TiO2colloidal solution(anatase, pH=6−8,10wt%)was purchased from Hangzhou Wanjing New MaterialCo.Ltd.Other reagents and solvents were commercially available and used without furtherpurification.
B.Preparations of sols and SiO2/TiO2double-layer films
3.3 g SiO2colloidal solution and 40 g industrial alcohol were added into the mixture of 3.0 g industrial alcohol and 2.7 g HCl(2 mol/L)with stirring.The SiO2sol was obtained,sealed and aged at room temperature for 21 h before dip-coating.TiO2colloidal solution was used directly without any treatment.
High borosilicate glass plates(75 mm×25 mm× 2 mm,refractive index 1.52)were used as substrates and cleaned ultrasonically in ethanol.The washed substrates were dipped in the aged SiO2solfor 1 min(withdrawal rate:1 mm/s).After the wet film dried at room temperature,TiO2particles were deposited onto the single SiO2layer by dipping the SiO2-coated glass substrates into TiO2colloidal solution for 30 s or 1 min (withdrawal rate:1 mm/s).Finally,the SiO2/TiO2double-layer films were calcined at 500◦C under atmospheric environment for 2 h to remove any organic substances and reinforce the films.
C.Photocatalytic experiment
The photocatalytic activity was evaluated in terms of the photodegradation rate of methyl orange(MO) aqueous solution under UV irradiation at room temperature.The extent of MO photodegradation was determined by measuring the absorbance at its maximum absorption wavelength of 464 nm using UV-2550 spectrophotometer.The height of the absorbance peak of MO indicates the concentration of MOin solution,that decreases with increasing irradiation duration as a result of photochemical reaction.
Three glass plates with deposited films were settled in photoreactor containing 100 mL MO solution (10 mg/L).The reactor was exposed to 250 W superhigh pressure mercury lamp.Through solution layer, UV light vertically irradiated the surface of films,where photocatalytic degradation took place.The distance between lamp and the surface of the photocatalytic films was 25 cm,and the intensity of the lamp was set to 192−194µW/cm2.For comparison,a blank sample solution that contained only MO and no glass sample in it, was subjected to irradiation together with other samples.During irradiation,the solution was stirred and the temperature was kept constantly at 20◦C.A small amount of sample solution was withdrawn at regular intervals in order to measure the absorbance variations of MO solution.
Prior to illumination with UV,the MO solution was magnetically stirred in contact with the SiO2/TiO2double-layer films under test for 30 min in darkness to establish the adsorption-desorption equilibrium [13−15].The degradation rate(D)could be calculated as the following equation[16]:

where,cOand ctare the concentrations of MO solution before and after irradiation,respectively.
D.Other measurements
Light transmission spectra of film-coated glass samples under normally incident light were recorded on spectrophotometer(Shimadzu,UV-2550)in the wavelength region of 300−800 nm without using integrating sphere.Morphologies of the films coated on silicon wafers were investigated by field emission scanning electron microscope(FESEM)(JSM-6701F)at operating voltage of 5 kV.The water contact angles of films on glass substrates were measured with contact angle meter(KRUSSU DSA100)in the sessile mode at room temperature.The layer thicknesses were determined by Generation Stylus Profiling System(Bruker,Dektak XT 10th).
III.RESULTS AND DISCUSSION
A.Light transmittance
Figure 1 shows the transmittance spectra of some samples.The transmittance of blank glass substrate in the wavelength range of 400−800 nm is about 92% (Fig.1(a)).After being coated by single SiO2film on both sides,the glass sample increases the transmittance in the whole region.The transmittance of SiO2-coated glass is 96.6%at 749 nm(Fig.1(b)).If the SiO2-coated glass is dip-coated in TiO2colloidal solution and the dipping time is 30 s,the average transmittance of this sample can reach 95%(Fig.1(c)).If the dipping time in TiO2colloidal solution is 1 min,the transmittance of the sample is about 93%(Fig.1(d)).It is found that increased dipping time in TiO2colloidalsolution increases the amount of TiO2nanoparticles and decreases the transmittance of films because of the absorption,scattering and large refractive index[17,18]of the TiO2nanoparticles.
For practical applications,high transmittance is important critically,therefore TiO2amount should be kept as low as possible.So the second layer of TiO2film with 30 s dipping time will be used.
B.Surface wettability
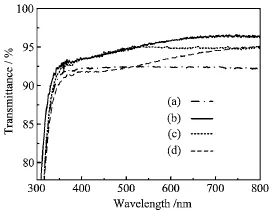
FIG.1 Transmission spectra of(a)blank glass,(b)SiO2-coated glass,(c)SiO2/TiO2double-layer films on glass with dipping time of 30 s in TiO2colloidal solution,and (d)SiO2/TiO2double-layer films on glass with dipping time of 1 min in TiO2colloidal solution.
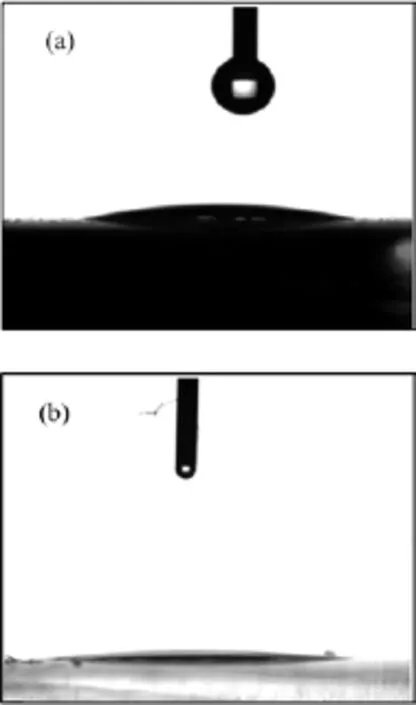
FIG.2 Water contact angles of 5µL water on(a)SiO2film and(b)SiO2/TiO2double-layer films.
The excellent surface wettability is crucialfor the selfcleaning function of the film.Surface hydrophilicity of the single SiO2film and SiO2/TiO2double-layer films were quantified from water contact angle measurements. Figure 2 shows the contact angles of 5µL water on SiO2film and SiO2/TiO2double-layer films.The water contact angle on SiO2film is 14◦(Fig.2(a)).After the SiO2film is coated by TiO2particles,the water contact angle on SiO2/TiO2double-layer films decreases.The SiO2/TiO2double-layer films show superhydrophilicity with only 6◦contact angle(Fig.2(b)).Obviously,the TiO2film plays an important role in the remarkable surface wettability of the films.
C.Microstructure
SiO2film and SiO2/TiO2double-layer films were subjected to FESEM analysis in order to gain information about the surface morphology.As shown in Fig.3,the obtained films are uniform and no cracks can be observed.The observation indicates that the experimental procedure employed is proven to be appropriate for attaining crack-free films.It can be seen in Fig.3(a)that the size of SiO2particles of SiO2film is about 30 nm, and the SiO2particles are closely and stably packed on the substrate.

FIG.3 Field emission scanning electron microscope micrographs of(a)SiO2film and(b)SiO2/TiO2double-layer films.
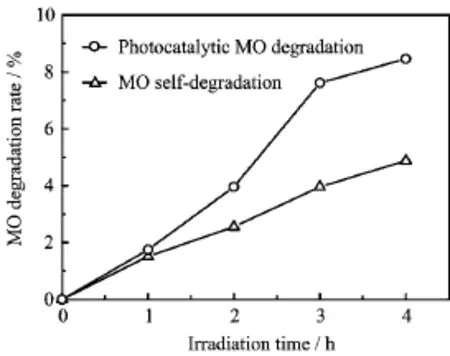
FIG.4 The photocatalytic efficiency of SiO2/TiO2double-layer films toward MO solution.UV irradiation: 192−194µW/cm2.
After being dip-coated by TiO2colloidalsolution,the surface of SiO2film was covered with a layer of TiO2particles with size of about 25 nm.The surface of the double-layer films is not as uniform as that of SiO2film, which can be observed in Fig.3(b).Few TiO2particles can form larger aggregates with size of 50−62 nm. Thicknesses of SiO2film and SiO2/TiO2double-layer films are about 100 and 230 nm respectively,according to Generation Stylus Profiling System.
D.Photocatalytic property
The photocatalytic efficiency of SiO2/TiO2doublelayer films was tested by the degradation of MO under UV light illumination for 4 h.The results displayed in Fig.4 show that the photodegradation rate of MO is 8% for 4 h,while the rate of MO self-degradation is about 5%within 4 h.It is evidenced that deposition of TiO2particles on SiO2film results in the concentration ofMO decreased with the extension of the exposure time. The enhanced photocatalytic activity is attributed to the existence of TiO2particles.The efficiency of the photocatalytic activity is directly proportional to the total surface area of the TiO2particles.The degradation efficiency is not very strong because of the small amount of TiO2.
IV.CONCLUSION
In this work,SiO2/TiO2double-layer films with high transmittance and self-cleaning properties have been prepared from cost-effective SiO2and TiO2sol by solgel dip-coating method,which is suitable for large-area coating in solar-power industry.The bottom SiO2layer displays AR effect and the top TiO2layer acts as selfcleaning coating.Because of the high refractive index of TiO2nanoparticles,the SiO2/TiO2double-layer films improve the maximum transmittance of the glass substrate to 95%.The superhydrophilicity and photocatalysis of SiO2/TiO2double-layer films favor the selfcleaning function greatly.The improvement of scratch resistance of films on glass substrate is the subject of future study.
V.ACKNOWLEDGMENTS
This work was supported by the National Key Basic Research Program of China(No.2012CB626805) and the Science and Technology Project of Lanzhou (No.2013-1-4).
[1]S.Y.Lien,D.S.Wuu,W.C.Yeh,and J.C.Liu,Sol. Energy Mater.Sol.Cells 90,2710(2006).
[2]Y.D.Xu,C.Peng,C.F.Xin,and J.Q Wu,Mater. Lett.94,89(2013).
[3]¨O.Kesmez,H.E.C¸amurlu,E.Burunkaya,and E. Arpa¸c,Sol.Energy Mater.Sol.Cells 93,1833(2009).
[4]L.Q.Ye,Y.L.Zhang,X.X.Zhang,T.Hua,R.Ji,B. Ding,and B.Jiang,Sol.Energy Mater.Sol.Cells 111, 160(2013).
[5]Y.Zhang,F.M.Gao,L.H.Gao,L.Hou,and Y.F.Jia, J.Sol-Gel Sci.Technol.62,134(2012).
[6]L.Miao,L.F.Su,S.Tanemura,C.A.J.Fisher,L.L. Zhao,Q.Liang,and G.Xu,Appl.Energy 112,1198 (2013).
[7]G.Helsch and J.Deubener,Sol.Energy 86,831(2012).
[8]K.Miyashita,S.Kuroda,T.Ubukata,T.Ozawa,and H.Kubota,J.Mater.Sci.36,3877(2001).
[9]Z.Y.Liu,X.T.Zhang,T.Murakami,and A.Fujishima, Sol.Energy Mater.Sol.Cells 92,1434(2008).
[10]X.T.Zhang,O.Sato,M.Taguchi,Y.Einaga,T. Murakami,and A.Fujishima,Chem.Mater.17,696 (2005).
[11]M.Vishwas,K.Narasimha Rao,K.V.Arjuna Gowda, and R.P.S Chakradhar,Spectrochim.Acta PT A 83, 614(2011).
[12]N.H.Arabi,A.Iratni,H.E.Hamzaoui,B.Capoen,M. Bouazaoui,M.Halbwax,J.P.Vilcot,and S.Bastide, J.Sol-Gel Sci.Technol.62,24(2012).
[13]P.Cheng,C.S.Deng,M.Y.Gu,and W.F.Shangguan, J.Mater.Sci.42,9239(2007).
[14]H.M.Zhang,X.Quan,S.Chen,H.M.Zhao,and Y. Z.Zhao,Sep.Purif.Technol.50,147(2006).
[15]J.Zita,J.Maixner,and J.Kr´ysa,J.Photochem.Photobio.A Chem.216,194(2010).
[16]Q.Z.Luo,X.Y.Li,D.S.Wang,Y.H.Wang,and J. An,J.Mater.Sci.46,1646(2011).
[17]J.J.Wang,D.S.Wang,J.Wang,W.L.Zhao,and C. W.Wang,Surf.Coat.Technol.205,3596(2011).
[18]S.H.Lin,Y.N.Wu,Y.C.Lin,and Y.M.Yang,J. Taiwan Inst.Chem.Eng.42,852(2011).
∗Author to whom correspondence should be addressed.E-mail: fanduowang@lzdctc.com,Tel.:+86-931-4956021,FAX:+86-931-4938756
 CHINESE JOURNAL OF CHEMICAL PHYSICS2015年6期
CHINESE JOURNAL OF CHEMICAL PHYSICS2015年6期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Importance of Metal Cations and Water for Stability of MnO2Crystals
- Elastic Low-Energy Electron Collisions with Methanethiol
- Effects of Sm Co-doping on Luminescent Properties of Sr4Al14O25:M (M=Mn4+,Cr3+)Phosphors
- Pyrene Derivate Functionalized with Acetylene for Organic Field Effect Transistors
- Miniature Boat Fabrication with Striking Loading Capacity in Seawater from Hydrophobic Steel Mesh
- High-Sensitive Glucose Biosensor Based on Ionic Liquid Doped Polyaniline/Prussian Blue Composite Film
