First-Principles Study of La Doping Effects on the Electronic Structures and Photocatalytic Properties of Anatase TiO2
Ping Huang,Bo Shang,Ling-jie Li,Jing-lei Lei
School of Chemistry and Chemical Engineering,Chongqing University,Chongqing 400044,China
(Dated:Received on March 12,2015;Accepted on June 16,2015)
First-Principles Study of La Doping Effects on the Electronic Structures and Photocatalytic Properties of Anatase TiO2
Ping Huang,Bo Shang∗,Ling-jie Li,Jing-lei Lei
School of Chemistry and Chemical Engineering,Chongqing University,Chongqing 400044,China
(Dated:Received on March 12,2015;Accepted on June 16,2015)
The effects of doping concentration,position and oxygen vacancy defect on the stability, electronic and optical properties of La-doped anatase TiO2have been investigated based on DFT+U method.The calculations indicated that the doping concentration and sites of La affected the stability and band gap of La-doped TiO2significantly due to the lattice distortion,which obey the ionic Pauling’s rules and crystal field theories;moreover,the simulated adsorption spectrum shows an obviously increase in the photocatalysis properties, which are in good agreement with recently experimentalmeasurements.The oxygen vacancy defect will enhance the structural stability and the adsorption of visible light in La-doped TiO2system,which is important in photocatalytic application.
Density functional theory,La doping,Anatase TiO2,Oxygen vacancy
I.INTRODUCTION
Titanium dioxide(TiO2)has been found to be a promising material for photocatalytic degradation of harmful organic compounds due to its excellent properties such as biological and chemical inertness,stability to corrosion,non-toxicity,and relatively low cost[1, 2].However,the use of TiO2is impaired by its wide band gap(3.2 eV for anatase),which requires ultraviolet irradiation for photocatalysis.For increased utility of TiO2,it is highly recommended to shift its absorption edge towards visible light,which constitutes the major part(about 50%)of solar energy[3].Among the various methods,doping of foreign atoms in the TiO2lattice is found to be a promising approach to shift its absorption edge towards visible light and improve the photocatalytic activity of TiO2[4,5].
Metal and nonmetal doping in TiO2has been widely studied for improving its visible-light photocatalytic activity[6,7].Nitrogen(N)doping in TiO2has been widely studied via experimental[8,9]and theoretical [10−13]techniques,and found to be a successfulmethod for extending the absorption edge of TiO2towards the visible region.Dai et al.found that the dopant location and the dopant concentration will affect the band structure ofthe defective TiO2system and play a major role in obtaining better electronic and opticalproperties [12,13].Among the metals,the rare earth ions can offer the advantage of transitions in the visible region,many studies have focused on the luminescence properties of rare earth elements hosted in crystalline matrices[14, 15],especially in TiO2[16−27].Jing et al.synthesized the La doped TiO2nanoparticles by sol-gelmethod with the concentrations ranging from 0.5mol%to 3mol%and found that the La-TiO2with 1mol%(873 K)molar ratio gave the best activity[19].Similar results were also found by Zhang et al.while investigation of the photoelectric conversion efficiency in the dye-sensitized solar cells was fabricated from La-doped TiO2[20].
In theory,much work has been reported recently in this field[28−33].Zhao et al.investigated the lanthanide doping TiO2with density functional theory (DFT)and indicated that lanthanide doping could remarkably improve the photocatalytic activity of TiO2[28].Gao et al.showed that La and Y doping could enhance the redox potential of TiO2dramatically and shift the absorption edges of doped TiO2toward the ultraviolet region[29].However,the effects of doping concentration,position and O vacancy have not been investigated,which will help to understand the mechanism of band gap narrowing.
In this work,the first principles calculations were performed to get the geometrical,electronic,and optical properties of La-doped anatase TiO2by considering the effect of doping concentration,sites and O vacancy.It is hoped that this study will provide the theoretical rationale and guidance to the design and construction of new effective photocatalyst in the practical future.
II.COMPUTATIONAL DETAILS
All the calculations were performed with the Vienna ab initio simulation package(VASP)[34,35]. Both the generalized gradient approximation(GGA) and DFT+U methods have been employed,and in both cases the PAW-PBE method was adopted to describethe interaction between electrons and ions[36−39].For higher accuracy,the O(2s22p4),Ti(3s23p63d24s2)and La(5s25p65d16s2)electrons were considered as valence states,and a kinetic cutoff energy of 450 eV was chosen. For the crystalcalculation,the Monkhorst-Park scheme [40]k-point grids sampling was set at 5×5×3 in the supercell.The structures were relaxed using a conjugate gradient minimization algorithm until the magnitude of residual Hellman-Feynman force on each atom was less than 0.05 eV/˚A[41].
The DFT+U method has been employed in order to accurately describe the electronic structure and the strong on-site Coulomb repulsion among the localized transition/rare earth metal d/f electrons.The choice of U is,however,ambiguous and it is not trivial to determine the value,though there are attempts to extract it from standard First-principles calculations.Hence,U is often fitted to reproduce a certain set of experimental data,such as band gaps and structural properties.We used the approach formulated by Dudarev et al.[42]. The function is shown in the following
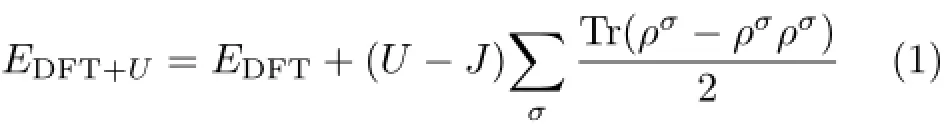
whereρσdenotes the spinσpolarized on-site density matrix.The spherically averaged Hubbard parameter U describes the increase in energy caused by placing an extra electron at a particular site,and the parameter J (1 eV)represents the screened exchange energy.The effective Hubbard parameter,Ueff=U−J,which accounts for the on-site Coulomb repulsion for each affected orbital,is the only external parameter required in this approach.In this work,the U of8.2 eV for Ti3d was selected from previous work about metal oxides[43−46]. Suitable Hubbard U parameters used in the calculations were determined by the band gap of the system.We found that the band gap of the system with U=8.2 eV added to Ti agrees with the previous calculation[47] and the experimental results[49]much better.Moreover,the lattice parameters of pure anatase TiO2were optimized to be a=b=3.776˚A and c=9.486˚A,which are in good agreement with the experimental values (a=b=3.785˚A,c=9.512˚A)[50],as well as theoretical results with PBE,a=b=3.800˚A,c=9.670˚A[51].This implies that the DFT+U approach with adopted parameters in our calculations may produce reasonable results.
The spectra were simulated based on the electric dipole approximation.From the view point of quantum mechanics,the interaction of a photon with the electrons in the system is described in terms of time dependent perturbations of the electronic ground states. The straight forward transition rates between occupied and unoccupied states,which are caused by photon absorption are determined by the electronic structures. Therefore,according to Fermi’s golden rule,the imaginary part of the dielectric functionε2can be calculated as the following formula: whereµis the vector defining the polarization of the incident electric field,C and V represent the conduction band(CB)and the valence band(VB),respectively,K represents the reciprocal lattice vector,is the matrix of momentum transi-represents the intrinsic level in CB and VB.Since the dielectric function describes a causal response,its real partε1and imaginary partε2are connected with a Kramers-Kronig transform,andε2is related to the absorption coefficientαby
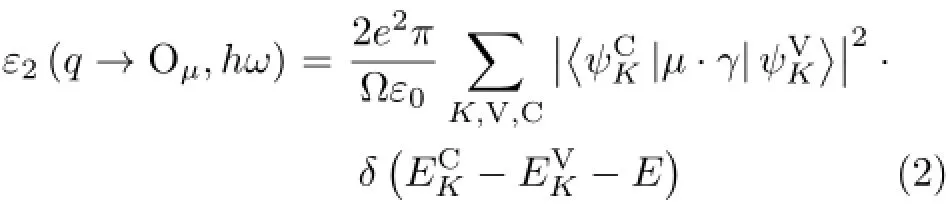

where c is light velocity in vacuum.
The structures of La-doped anatase TiO2are modeled by replacing Ti with La in a 48 atoms 2×2×1 supercell.The species that n Ti atoms are replaced by n La atoms are named as n-TiO2(Fig.1(a)).For the different substituting position of La,all models are built based on the 2-TiO2system,named as(1,2)-,(1,3)-, (1,7)-,and(3,5)-TiO2.
III.RESULTS AND DISCUSSION
A.Different concentrations of La-doped TiO2
We initiated the investigation with the concentration effect.All the six types of models are based on the pure 48 atoms 2×2×1 anatase supercell;where 16−n (n=1−6)Ti atoms are replaced with La,as shown in Fig.1(a).The corresponding atomic concentrations of impurity are 2.08%,4.17%,6.25%,8.33%,10.42%,and 12.5%(atom fraction)in total.In addition,the doping sites effect is not taken into account,which will be discussed in later section.
To examine the efficiency of doping level,we define the relative binding energyΔEbcompared with pure anatase TiO2according to the following equation[30]:
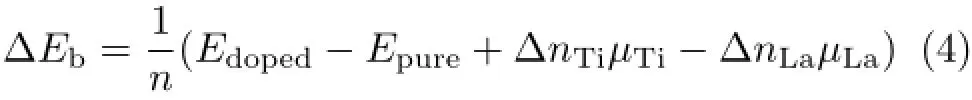
where Edopedand Epureare the total energy of doped-TiO2and pure TiO2in the same supercell,µTiandµLarepresent the chemical potential of La and Ti atom, which is generated from the bulk metal,respectively.n refers to the number of atoms per supercell andΔn is the change atoms due to the doping(nTi=nLa).According to the results,which are listed in Table I,ΔEbfor all doped-TiO2systems are above zero(the lowest one is 3.74 eV for 1-TiO2system),which meansthat stability will decrease after La doping.For the 3-TiO2and 5-TiO2,ΔEbare 3.81 and 3.89 eV,which are greater than that of the neighborhood.This oddeven phenomenon might be caused by doping symmetry and doping sites,which willbe discussed in the next part.
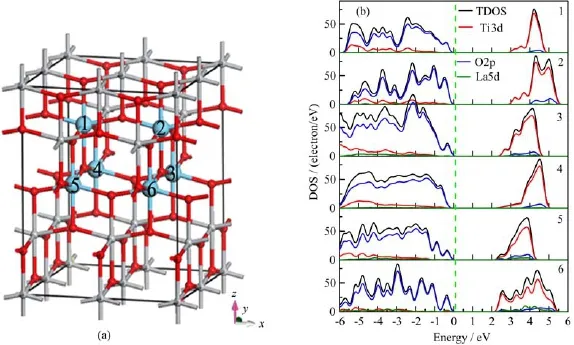
FIG.1(a)Calculated model of different La doping level systems.Gray spheres and red spheres represent the Ti and O atoms,respectively.The blue spheres are the La atom,where the digitals mean the name of La atoms which will be replaced. The species that n Ti atoms are replaced by n La atoms are named as n-TiO2(n=1−6).(b)Total/projected density of states(TDOS/PDOS).Black line denotes the total DOS,red line denotes the Ti3d states,blue line denotes the O2p states, and green line denotes the La5d states.
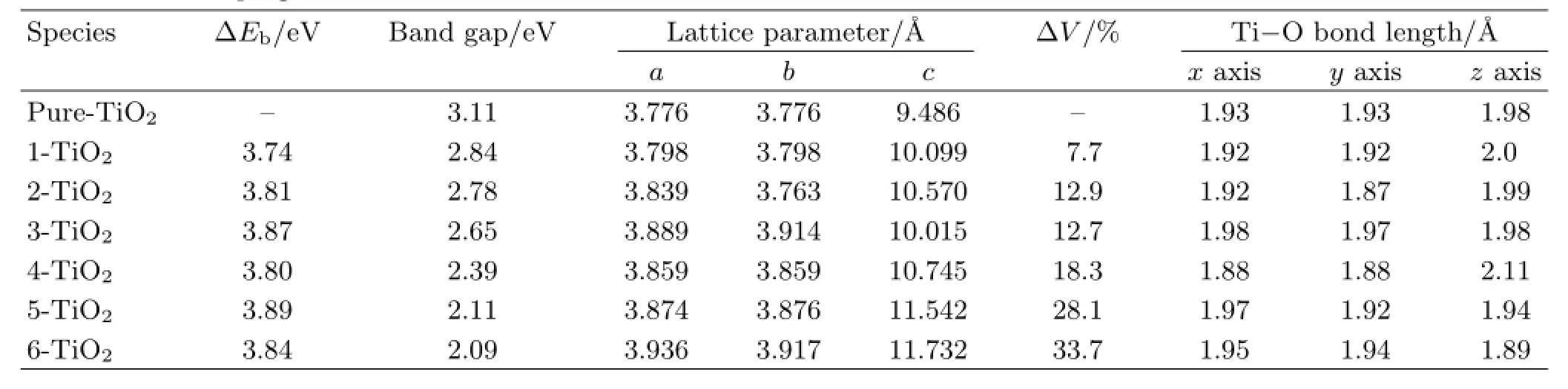
TABLE I The relative binding energyΔEb,band gap,lattice parameters,and average bond-length in three axial directions at different La doping level.
The band gap is described as in Table I.With the increase of the doping level,the band gap decreased dramatically,from 3.11 eV for pure TiO2to 2.09 eV for 6-TiO2.The band gap narrowing will cause an enhancement of visible-light absorption and photocatalytic performance,which agree quite well with experimental results[21−23].The lattice volume of pure TiO2is 135.25˚A3and the band gap is 3.11 eV.For the 1-TiO2,lattice volume increases by 7.7%compared with pure TiO2,and the band gap drops to 2.84 eV.For the 2-TiO2and 6-TiO2,lattice volume increases by 12.9% and 33.7%,while the band gap is 2.78 and 2.09 eV,respectively.As the ionic radius of La3+(1.04˚A)is much larger than that of Ti4+(0.61˚A)[24,25],after high level doping,the lattice volume increased significantly, which will cause phase transformation and will not be considered in this work.However,with a lower doping level,the band gap will decrease and the stability can also be guaranteed.Hence,a proper doping level will be selected in the application.
In order to explore the band gap narrowing mechanism,we present the projected density of states(PDOS) for all atoms in Fig.1(b).In order to compare the DOS/PDOS of different systems directly,we have made some adjustment on the DOS/PDOS figure.All the DOS/PDOS figures in this work,the 0 position refers to the valence band maximum but not Fermi level.The PDOS show that the most contribution of the valenceband edge(VBE)is O2p states and the conduction band edge(CBE)is Ti3d states,which are the same as those obtained from pure bulk TiO2[31].PDOS further confirm that the La doping states are delocalized and the band gap narrowing is caused by the move down of unoccupied Ti3d orbital.Interestingly,the bottom of the conduction band is occupied by Ti3d states for alldoped systems.
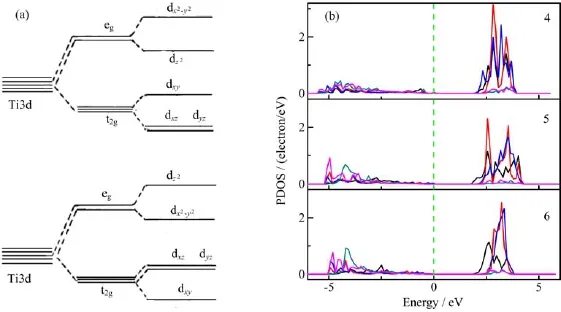
FIG.2(a)Schematic representations of energy levels splitting by distortion to anatase TiO2with octahedron elongated along the z-axis and squashed octahedron along the z-axis,(b)PDOS of Ti3d states for different concentration systems. Black,red,blue,green,and pink lines denote Ti dxy,dxz,dyz,and dx2−y2,respectively.
The relationship between the movement of the Ti unoccupied 3d states and the distortion can be interpreted with the crystal field theory(CFT)[52].The Ti−O bond lengthes of doped systems are also listed in Table I.The Ti−O in pure TiO2is 1.93˚A in x and y axis and 1.98˚A to z axis,which are in good agreement with the present results[32].The Ti−O bond length in all doped systems is changed compared to that of pure TiO2.Two kinds of change can be found,e.g.,in the 4-TiO2,the bond length in the z axis is stretched to 2.11˚A but shortened to 1.88˚A in x and y axis,which give rise to an elongated octahedral pyramid,while in 6-TiO2system,the average bond length is shortened to 1.89˚A in z axis but elongated to 1.99 and 1.94˚A in x and y axis,which brought about a squashed octahedral pyramid.As a six-coordinated octahedral structure,the d orbital energy splitting is set out in Fig.2(a). Without distortion,d orbital spitted into two degeneracy groups,with the symmetry,eg(dx2−y2,dz2)and t2g(dxz,dyz,dxy).In the elongated octahedron along the z-axis,the energy of dx2−y2orbital is higher than the dz2orbital and the energies of dxzand dyzorbital are lower than that of dxy.Otherwise,in squashed octahedron along the z-axis,the dxz,dyz,and dz2 energies increase and the others decrease.The CFT inference is confirmed by our PDOS results,as shown in Fig.2(b). For elongated 4-TiO2model,the bottom of the conduction band is mainly composed of Ti3dxzstate while in the 5-TiO2and 6-TiO2they are the Ti3dxystate.
It is shown that the narrowing of band gap mainly contributes to the splitting of Ti3d orbital which roots in the structural distortion.The structural distortion is increased with the doping level.However,the relationship between the stability and band gap with the doping level is not an ordinary linear relationship,as the doping level increases,the structure distorts significantly,which will lead to a narrowing of the band gap but a decreasing of the stability,illustrating that the distortions also depend on the doping sites;that is why further investigation of different La doping sites will be extremely necessary.
B.Different La substituting sites
The simplest two atoms doping system has been studied.There are four possible nonequivalent sties in 2×2×1 supercell with the doping concentration of 4%, namely(1,2)-,(1,3)-,(1,7)-,and(3,5)-TiO2.The La octahedrons have been drawn as shown in Fig.3(a).For the(1,7)-TiO2,the cell parameter has been changed from(a=b=3.776˚A,c=9.486˚A)to(a=b=3.932˚A, c=10.266˚A)after full optimization,which means the doping willincrease the volume ofthe cellby 15%.This result coincides with that of the current date in the experiment[26].
The stability of systems with different doping sites can be judged byΔEb,as shown in Table II.For the (1,7)-TiO2and(1,3)-TiO2,ΔEbis very similar and a little lower than the other two systems.It states that stabilities are closely bound up to the distances of the most neighboring doping atoms.For the(1,7)-TiO2,the nearest distance of two La atoms is 5.443˚A.While in the(1,2)-TiO2,the distance is 3.776˚A,as a result, the(1,7)-TiO2has a lower relative binding energy.The stability of these different doping sites can be interpreted with the ionic Pauling’s Rules[53].For anatase TiO2,structure is based on octahedrons which share four O−O edges,while doped with La,some of the Ti octahedrons are replaced by La octahedron.Based on the Pauling’s Rules of three and four,in a crystal containing different cations,those of high value and small coordination number don’t often share polyhedron elements with one another.Different kinds of doping types willslightly change the polyhedron elements sharing.In the(1,2)-TiO2,the La octahedron share the vertex,and thus with a higher energy.For the other three systems, the sharing of O−O edge between octahedrons of TiO2will decrease,which will enhance the stability.
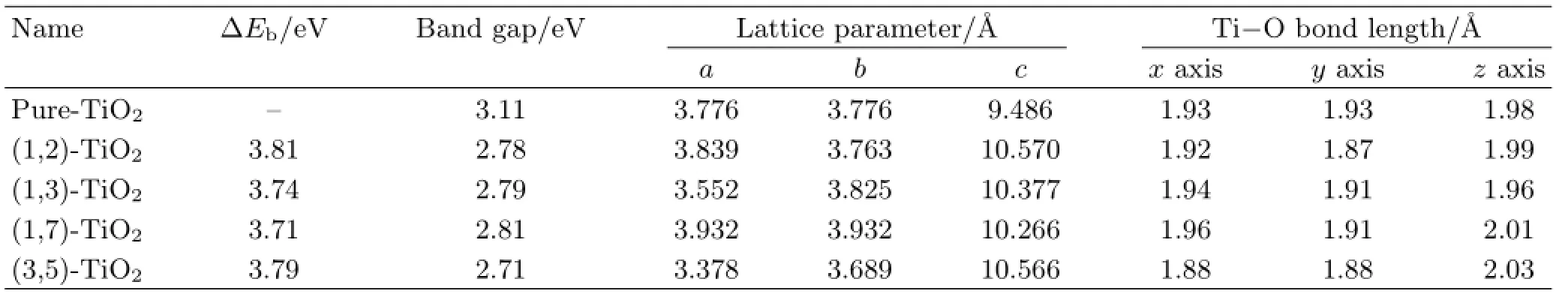
TABLE II The relative binding energyΔEb,band gap,lattice parameters,and average bond length of the supercell in axial directions at different doping sites.
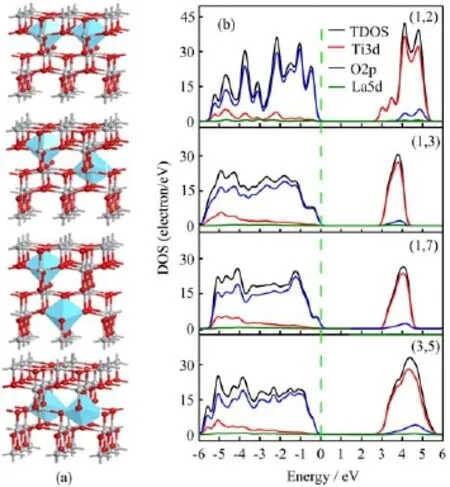
FIG.3(a)Calculated model of different La substitute position systems.Gray spheres and red spheres are the Tiand O atoms,respectively.The blue octahedrons are the La atoms. (b)Total/projected density of states(TDOS/PDOS).Black line denotes the total DOS,red line denotes the Ti3d state, blue line denotes the O2p state,and green line denotes the La5d states.
To find the effects of doping position on the electronic properties of La-doped TiO2,the total DOS and PDOS have been established(Fig.3(b)).The calculated band gaps of TiO2with distinct sites of La doping are 2.78, 2.79,2.81,and 2.71 eV,respectively.Band gap differences between separate doping sites are not significant in comparasion with that of different doping concentration.The conduction bands are wildly extended in the PDOS of(1,2)-TiO2and(3,5)-TiO2while localized at a higher peak in the(1,3)-TiO2and(1,7)-TiO2.The results of the band gap and the lattice distortion coincide, as shown in Table II.In the(1,3)-TiO2,the bond length of Ti−O is slightly changed from pure anatase and its band gap is 2.79 eV,and in the(3,5)-TiO2,bond length of Ti−O change much bigger,which results in the band gap of 2.71 eV.
From the simulated spectra in Fig.4,it is noted that the absorption spectrum is cut offat~440 nm,the band gap is narrowed to 2.81 eV and the absorbance intensity in the wavelength range from 380 nm to 440 nm is increased obviously.Such light absorbance enhancement in the visible light range corresponds roughly to values obtained in the experiment[33].
From the doping sites effect on the stability and electronic properties,it is found that the stabilities of the system are mainly affected by the distance oftwo neighboring doping atoms in the supercell,which obeys the ionic Pauling’s Rules.Doping sites prefer to spread separately to gain higher stability,which will result in less band gap narrowing.However,sites reduced band gap narrowing is not as significant as the concentration effect.Finally,we found that in the(1,7)-TiO2type material,the 4%is the just doping level,which can decrease the band gap from UV to blue light and stillobey the ionic Pauling’s rules.The calculated band gap of this system is 2.81 eV and absorption shifts from UV-light region to visible-light region,which makes it applicable to practice.
C.Oxygen vacancy defect

FIG.4 Optical absorption curves of pure TiO2,(1,7)-TiO2, and(1,7)-TiO2with O vacancy.The black line denotes the pure TiO2,red line denotes(1,7)-TiO2,and blue line denotes (1,7)-TiO2with O vacancy.
In the study of La-doped TiO2system,charge compensation needs to be considered while Ti4+is substituted by impurities with La3+.To meet the electronic neutrality of the entire system,oxygen vacancies are inevitable when a high-valence host ion is substituted by a low-valence guest ion.In order to investigate the effect of oxygen vacancy on electronic structures of La-TiO2,we calculated the electronic structures of La doped TiO2with oxygen vacancy defect.We investigated two La dopants incorporated into the TiO2and the formation of an oxygen vacancy since this system retains electronic neutrality.In the previous section,we found that the(1,7)-TiO2was the most stable models with two La atoms doping.
For the(1,7)-TiO2with O vacancies,ΔEbis 3.67 eV, which is 0.04 eV lower than that of(1,7)-TiO2,explaining that O vacancies will enhance stability of La doping TiO2.Two types of models with O vacancies have been considered,as shown in Fig.4.The DOS of two La atoms doped TiO2with and without oxygen vacancy defect is shown in Fig.5.Figure 5(b)shows a tiny change of the band gap in comparison with the DOS of La-doped TiO2without O vacancy.The top of the valence band is occupied by O vacancy states, and the band gap of(1,7)-TiO2with O vacancy is 2.87 eV which is slightly larger than that without O vacancy system(2.81 eV).Doping with two La atoms in combination with an O vacancy does not introduce any defect level in the band gap,since the excess electrons induced by the La are compensated by the O vacancy. The Ti3d level is located above the valance band that is expected to hybridize with oxygen defect states and then enhance the adsorption of the UV light.In all, the results indicated that oxygen vacancy defect could not only make the structure of(1,7)-TiO2more stable but also enhance the adsorption of UV light while in La-doped TiO2system,this result is in agreement with the report in Ref.[27].

FIG.5 DOS of(1,7)-TiO2system(a)with an oxygen vacancy defect and(b)without oxygen vacancy defect.Black line denotes the total DOS,red line denotes the Ti3d state, blue line denotes the O2p state,and green line denotes the La5d state.
It also clearly shows that the absorbance enhanced in UV region after the introduced of O vacancy(Fig.4). The photocatalysis is totally affected by the band gap, electron-hole recovery rate,and the photon adsorption effect.From the electronic and optical calculations,we found that La doping will enhance the photocatalysis effect via band gap engineering and the improvement of adsorption efficiency,which illustrate the agreement with experiment.The presence of O vacancy in La doped TiO2will slow down the recombination rate of electron-hole pairs due to the cooperation of dopants and vacancy states,which could enhance the photocatalytic efficiency of doped TiO2.
IV.CONCLUSION
The doping concentration and position effects on the stability,electronic and optical properties of La-doped anatase TiO2have been investigated based on firstprinciples density functional theory.The calculation shows that the structural stability and the band gap of La-doped TiO2are mainly controlled by the concentration of La.Structural stability decreased and the band gap narrowed with the increase of La doping concentration.The band gap narrowed as a consequence of Ti3d orbit splitting root in the lattice distortion due to the doping concentration and sites.Increases in photocatalysis of La-doping TiO2mainly contribute to the band gap narrowing and enhancement of adsorption.The results ofthe electronic structures oftwo La atoms doping TiO2systems with an O vacancy indicated that the O vacancy should make the structure more stable and alter the light absorption of TiO2in the UV light region. The O vacancy is important in the unbiased charged doping,which could not be omitted.Finally,based on the ionic Pauling’s rules,a proper density of La doping has been suggested,which has a remarkable stability and a proper band gap,and might be appropriate for photocatalysis.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.21273293 and No.21373281),the Program for New Century Excellent Talents in University(No.NCET-12-0587 and No.NCET-13-0633),the National Science Foundation Project of CQ CSTC(No.020005303100),and the Fundamental Research Funds for the Central Universities.
[1]K.G.Grigorov,I.C.Oliveira,H.S.Maciel,M.Massi, J.M.S.Oliveira,J.Amorim,and C.A.Cunha,Surf. Sci.605,775(2011).
[2]Y.Ma,J.Zhang,B.Tian,F.L.Chen,and J.Wang,J. Hazard.Mater.82,386(2010).
[3]Y.Su,Y.Xiao,Y.Li,Y.Du,and Y.Zhang,Mater. Chem.Phys.126,761(2011).
[4]C.He,Y.Yu,X.Hu,and A.Larbot,Appl.Surf.Sci. 200,239(2012).
[5]I.E.Saliby,L.Erdei,H.K.Shon,and J.H.Kim,J. Ind.Eng.Chem.17,358(2011).
[6]V.C.Stengland S.Bakardjieva,J.Phys.Chem.C 114, 19308(2010).
[7]X.Yu,C.Li,H.Tang,Y.Ling,T.A.Tang,and Q.Wu, J.Kong,Comput.Mater.Sci.49,430(2010).
[8]F.Peng,Y.Liu,H.J.Wang,H.Yu,and J.Yang,Chin. J.Chem.Phys.23,437(2010).
[9]F.Spadavecchia,G.Cappelletti,S.Ardizzone,M. Ceotto,and L.Falciola,J.Phys.Chem.C 115,6381 (2011).
[10]X.Han and G.Shao,J.Phys.Chem.C 115,8274 (2011).
[11]L.Jia,C.Wu,S.Han,N.Yao,Y.Li,Z.Li,B.Chi,J. Pu,and L.Jian,J.Alloys Compd.509,6067(2011).
[12]K.Yang,Y.Dai,B.Huang,and S.Han,J.Phys.Chem. B 110,24011(2006).
[13]K.Yang,Y.Dai,and B.Huang,J.Phys.Chem.C 111, 12086(2007).
[14]J.Zhu,K.Zhu,and L.Chen,J.Non-Cryst.Sol.352, 150(2006).
[15]J.C.Gacon,K.Horchani,A.Jouini,C.Dujardin,and I.Kamenskikh,Opt.Mater.28,14(2006).
[16]C.Y.Huang,L.C.Zhang,and X.H.Li,Chin.J.Catal. 29,163(2008).
[17]K.M.Parida and N.Sahu,J.Mol.Catal.A 287,151 (2008).
[18]L.J.Li,Z.Q.Zhou,J.L.Lei,J.X.He,P.P.Liu,and F.S.Pan,Mater.Lett.79,252(2012).
[19]L.Q.Jing,X.J.Sun,B.F.Xin,B.Q.Wang,W.M.Cai, and H.G.Fu,J.Sol.State Chem.177,3375(2004).
[20]J.Y.Zhang,Z.Y.Zhao,X.Y.Wang,T.Yu,J.Guan, Z.T.Yu,Z.S.Li,and Z.G.Zou,J.Phys.Chem.C 114,18396(2010).
[21]C.Liang and F.Li,Dyes Pigments 76,477(2008).
[22]W.Zhou,Y.H.Zheng,and G.H.Wu,Appl.Surf.Sci. 253,1387(2006).
[23]Y.Q.Wang,H.M.Cheng,Y.Z.Hao,J.M.Ma,W.H. Li,and S.M.Cai,Thin Solid Films 349,120(1999).
[24]Y.H.Zhang,H.X.Zhang,Y.X.Xu,and Y.G.Wang, J.Sol.State Chem.177,3490(2004).
[25]Y.H.Zhang and A.Reller,Mater.Lett.57,4108 (2003).
[26]L.Q.Jing,X.J.Sun,B.F.Xin,B.Q.Wang,W.M.Cai, and H.G.Fu,J.Sol.State Chem.177,3375(2004).
[27]Y.N.Huo,J.Zhu,J.X.Li,G.S.Li,and H.X.Li,J. Catal.A 278,237(2007)
[28]Z.Y.Zhao and Q.J.Liu,J.Phys.D 41,085417(2008). [29]H.T.Gao,B.Lu,X.H.Li,W.C.Liu,and G.J.Liu, J.Comput.Theor.Nanos.10,1503(2013).
[30]H.C.Wu,S.W.Lin,and J.S.Wu,J.Alloys Compd. 522,46(2012).
[31]R.Asahi,T.Taga,W.Mannstadt,and A.J.Freeman, Phys.Rev.B 61,7459(2000).
[32]K.S.Yang,Y.Dai,B.B.Huang,and M.H.Whangbo, J.Phys.Chem.C 113,2624(2009).
[33]H.Y.Wei,Y.S.Wu,N.Lun,and F.Zhao,J.Mater. Sci.39,1305(2004).
[34]G.Kresse and J.Furthmuller,Phys.Rev.B 54,11169 (1996).
[35]G.Kresse and J.Furthmuller,Comput.Mater.Sci.6, 15(1996).
[36]P.E.Blo¨ochl,Phys.Rev.B 50,17953(1994).
[37]G.Kresse and D.Joubert,Phys.Rev.B 59,1758 (1999).
[38]J.P.Perdew,K.Burke,and M.Ernzerhof,Phys.Rev. Lett.77,3865(1996).
[39]J.P.Perdew,A.Ruzsinszky,G.I.Csonka,O.A.Vydrov,G.E.Scuseria,L.A.Constantin,X.L.Zhou,and K.Burke,Phys.Rev.Lett.100,136406(2008).
[40]H.Q.Sun,Y.Bai,H.J.Liu,W.Q.Jin,N.P.Xu,G. J.Chen,and B.Q.Xu,J.Phys.Chem.C 112,13304 (2008).
[41]X.C.Zhang,L.J.Zhao,C.M.Fan,Z.H.Liang,and P.D.Han,J.Phys.B 407,4416(2012).
[42]S.L.Dudarev,G.A.Botton,S.Y.Savrasov,C.J. Humphreys,and A.P.Sutton,Phys.Rev.B 57,1505 (1998).
[43]M.E.A.Dompablo,A.M.Garcia,and M.Taravillo, J.Chem.Phys.135,054503(2011).
[44]W.G.Chen,P.F.Yuan,S.Zhang,Q.Sun,E.J.Liang, and Y.Jia,Phys.B 407,1038(2012).
[45]K.K.Ghuman and C.V.Sinqh,J.Phys.25,085501 (2013).
[46]Q.S.Meng,T.Wang,E.Z.Liu,X.B.Ma,Q.F.Ge, and J.L.Gong,Phys.Chem.Chem.Phys.15,9549 (2013).
[47]R.Long and N.English,J.Chem.Phys.Lett.478,175 (2009).
[48]A.Heller,Acc.Chem.Res.28,503(1995).
[49]R.Shirley and M.Kraft,Phys.Rev.B 81,075111 (2010).
[50]Z.Y.Zhao and Q.J.Liu,J.Catal.Lett.124,111 (2008).
[51]J.K.Burdett,T.Hughbandks,G.J.Miller,and J.V. Smith,J.Am.Chem.Soc.109,3639(1987).
[52]K.B.Jeremy,D.P.Geoffrey,and L.P.Sarah,J.Am. Chem.Soc.104,92(1982).
[53]L.Pauling,J.Am.Chem.Soc.51,1010(1929).
∗Author to whom correspondence should be addressed.E-mail: bshang@cqu.edu.cn,Tel.:+86-13983858642
 CHINESE JOURNAL OF CHEMICAL PHYSICS2015年6期
CHINESE JOURNAL OF CHEMICAL PHYSICS2015年6期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Importance of Metal Cations and Water for Stability of MnO2Crystals
- Elastic Low-Energy Electron Collisions with Methanethiol
- Antireflective and Self-cleaning Properties of SiO2/TiO2Double-Layer Films Prepared by Cost-Effective Sol-Gel Process
- Effects of Sm Co-doping on Luminescent Properties of Sr4Al14O25:M (M=Mn4+,Cr3+)Phosphors
- Pyrene Derivate Functionalized with Acetylene for Organic Field Effect Transistors
- Miniature Boat Fabrication with Striking Loading Capacity in Seawater from Hydrophobic Steel Mesh
