Synthesis,Characterization,and Fluorescence Sensor Property of Polyurethane/Ag2S Nanostrips
Shan Wang,Yi Huang,Jian-she Yue
Schoolof Chemistry and Chemical Engineering of Xianyang Normal University,Xianyang 712000,China
(Dated:Received on February 11,2015;Accepted on May 14,2015)
Synthesis,Characterization,and Fluorescence Sensor Property of Polyurethane/Ag2S Nanostrips
Shan Wang∗,Yi Huang,Jian-she Yue
Schoolof Chemistry and Chemical Engineering of Xianyang Normal University,Xianyang 712000,China
(Dated:Received on February 11,2015;Accepted on May 14,2015)
Apolyurethane/silver sulfide nanocomposite film was synthesized by a biomineralization simulated method.The effect of the Ag2S nanoparticles on the physical properties of the composite was studied by Fourier transform infrared,differential scanning calorimetry(DSC), scanning electron microscopy.The thermalstability of the composite was measured by DSC. The fluorescence emission of the nanocomposite films was found to be very sensitive to Ni(II) ions,with a small amount of Ni(II)ions making the emissions increase dramatically.The films are predicted to have the potentialto be developed into excellent sensing films of Ni(II) ions in the water.
Polyurethane,Ag2S nanostrip,Fluorescence sensor,Ni(II)ion sensor
I.INTRODUCTION
Nanomaterials have interesting properties and useful functionalities that often substantially differ from those of their bulk counterparts.Nanosemiconductors exhibit favorable electronic,optical,magnetic,and other properties that make them suitable for various applications[1].These characteristics significantly depend on nanoparticals size and shape[2,3].Given the good intrinsic properties and narrow band gap of Ag2S,it is used to manufacture photovoltaic cells,photoconductors,and IR detectors.However,Ag2S is difficult to synthesize because it easily aggregates.Numerous methods of synthesizing Ag2S nanocrystals,including those using silica zeolite thin films[4,5]and polymers[6−12],have been proposed.Recently,Gao et al.used dodecylthiol as a structure-directing agent to prepare an assembly of Ag2S nanostructures[13].Stabilized nanostructured Ag2S in polymer solution has also been reported[14].Polyvinylpyrrolidone(PVP), a good stabilization agent,was used to prepare PVPAg2S nanocomposites[10,11].Polyurethane(PU)and starch biopolymer[12]have been less successfully used to synthesize Ag2S nanoparticles.
In this work,we report a pollution-free method of synthesizing PU and an in situ preparation of Ag2S/PU nanocomposites by simulating a biomineralization process.This polymer was used in our previous study as a matrix for CdS nanoparticles[15].Here,it can be successfully applied to controlthe growth of Ag2S nanoparticles,and the inorganic/organic polymer nanocomposite film can serve as a sensor for Ni(II).
II.EXPERIMENTS
A.Materials
Thioacetaminde(TAA),N,N-dimethylformamide (DMF)and silver nitrate(AgNO3)were supplied by Sigma-Aldrich(USA)and used as received. H2N(CH2)6NH2,AlCl3,OHCH2CH2OH,epoxy resins were purchased from Shanghai Sanpu Chemical Co., Ltd.,China.DMF was dried over 4A molecular sieves and used without further purification.All others were purchased commercially with A.R.grade.
B.Synthesis of PU containing Ag+
A 150 mL three-neck boiling flask was equipped with a mechanical stirrer and a nitrogen inlet.In the flask, 11.6 g of H2N(CH2)6NH2were dissolved into 41.3 mL dimethly carbonate.And 0.7 g CH3OH and 0.7424 g AgNO3were added in it.Under condition of normal temperature and pressure,the suspension was stirred with a mechanical stirrer for 8 h.Finally,the mixture of products was dried at room temperature.The products were then dissolved in 3.5 mL OHCH2CH2OH in the 150 mL three-neck flask.With AlCl3and epoxy resins catalyst,the mixture was stirred vigorously under a nitrogen atmosphere at 160◦C for 1.5 h.
C.The preparation of nano-composite films
1.0043 g polyurethane which contained Ag+was first diluted with 10 mL DMF.The mixture was added tothe solution which contained TAA(CTAA=0.1 mmol/L, pH=10),then dispersed by ultrasonic for 15 min.The compound slide on the activation of the quartz plates (1 inch×3 inch)[15]was used in a series of controllable experiments.The solid was dried in vacuum at room temperature for 6 h and kept for further characterization[16].
D.Characterizations
Ultraviolet-visible(UV-Vis)absorption spectra were taken with a Perkin Elmer LAMBDA EZ-221 spectrometer(USA)with the scan range of 300−500 nm using DMF as solvent.Fourier transform infrared(FTIR) spectra were recorded on a Nicolet-nexus670 spectrometer(USA).The samples were ground with KBr crystals,and the mixture was pressed into a flake for FTIR measurement.Thermal analysis experiments were performed using a differential scanning calorimetry(DSC) apparatus operated in the conventional DSC mode at the heating rate of 10.0◦C/min to simultaneously determine the correlation of temperature and heat flowing in a nitrogen atmosphere.SEM was obtained on JSM-6380(Japan)at an accelerating voltage of 100 kV.Fluorescence measurements were performed at room temperature on a Instruments RF-5301PC(Shimadzu Instruments Inc,Japan)fluorescence spectrometer.
III.RESULTS AND DISCUSSION
A.Spectral data
Figure 1 shows the FTIR spectra of pure PU and PU/Ag2S.The absorption peaks at 1750 cm−1(ν(C=O))show that abundant hydroxyl groups are tethered onto the surface of Ag2S nanocrystals.The absorption peaks at 1250 cm−1(ν(C−O−C))as wellas 3200 and 1440 cm−1(ν(N−H))indicate the existence of a PU intermediate.However,the carbonyl group of urethane is shown at 1925 cm−1.The PU/Ag2S spectrum shows that the characteristic peaks of pure PU and Ag2S were maintained,whereas the pure Ag2S spectrum showsν(OH)stretching and bending bands at 3423 cm−1.This result proves that the structure of PU was affected by the presence of Ag2S,indicating that Ag2S does not react with the PU molecules.
B.UV characteristics

FIG.1 FTIR spectra of PU and PU/Ag2S nanostrip.
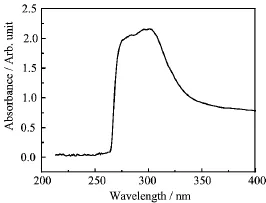
FIG.2 UV-Vis spectrum of the hybrid film.
The synthesis of amido-coated Ag2S nanocrystals involves the reaction of silver with sulfur ions in the presence of amido-group-containing ligands as the organic ligands.The electron-deficient atoms of silver on the semiconductor surface serve as binding sites onto which organic ligands anchor and hinder further growth of crystal grains.Consequently,nanosized crystals form. Tang et al.reported that this reaction has to be performed at elevated temperatures for more than 10 h [17].However,in the present study,the preparation of Ag2S nanocrystals by silver nitrate(AgNO3),TAA, and PU(as the ligand)was performed within only 15 min at room temperature.The UV-Vis spectrum in Fig.2 shows that the maximum absorption band of Ag2S nanocrystals occured at 311 nm.This observation is consistent with the quantum confinement effects induced by the particle size.The strong absorption peak is assigned to the opticaltransition of the first excitonic state of the Ag2S nanoparticles.The average crystallite size was smaller than 20 nm[18].This size is attributed to the band gap absorption and related to the bulk because of the quantum size confinement effect.
C.Microstructure
Figure 3 shows the scanning electron microscopy (SEM)image of a PU/Ag2S nanostrip film,which provide direct evidence for the formation of a true nanocomposite.The micrographs confirm that the Ag2S nanostrips were well-dispersed in the PU matrix. The average width of the Ag2S nanostrips ranges from about 90 nm to 1.8µm.Polymer chains can be bridgedby connecting to the same nanostrip,and a multiplicity of such bridged chains and nanostrips can lead to nanostrip clustering[19].The nanostrips are observed to be dispersed in PU matrix at the nanoscale,which indicates the formation of a nanoparticle.
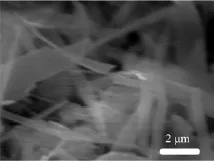
FIG.3 SEM of the hybrid film of PU/Ag2S.

FIG.4 DSC of the hybrid PU/Ag2S film.
D.Thermal properties
In the DSC curve(Fig.4)of the PU/Ag2S,the endothermic peak at about 101.62 and 108.47◦C were attributed to the volatilization of organic solvent.The exothermic peak at 255.02◦C is related to the polymer decomposition.This result indicates a strong and uniform interaction between PU and nanoparticles[20]. Meanwhile,the few small exothermic peaks observed from 95.55◦C to 255◦C are due to the heat effect of the oxidation combustion of organic substances[21].
E.Sensing properties of the prepared film to Ni(II)
Toxic heavy metals,such as nickel,mercury,and zinc, from aqueous environments have received considerable attention in recent years because of their toxicity and carcinogenicity[22].Nickel ions are non-biodegradable toxic heavy metals[23].According to the World Health Organization,the maximum permissible concentration of nickel in effluents from the electroplating process of waste water is 4.1 mg/L,whereas that in drinking water should be less than 0.1 mg/L[24].Different methods have been used to remove nickelions from water,including adsorption,chemical precipitation,ion exchange, filtration,membrane separation,and reverse osmosis. Sensor technique development can benefit heavy metal analysis.An ion sensor must be highly selective for a particular heavy metal so that it can be used for analysis.Accordingly,the effects of a series of Ni(II)on the fluorescence mission of our films were examined.
In the experiment the film was laid on one inner side of a rectangle quartz cell with a volume of ca. 3.5 cm3.Then,a solvent with a volume of 2.5 cm3was added to the cell,and the spectrum was recorded when the fluorescence intensity was stable after injecting the ion solution into the cell.The fluorescence of the film was remarkably enhanced upon the addition of Ni(II), whereas no obvious enhancement was observed for other familiar ions including Cu2+,Cr2+,Hg2+,Ba2+,Sr2+, Mn2+,Mg2+,Al3+,and Pb2+(Fig.S1 in supplementary materials).These results demonstrate that the film has high selectivity for Ni(II)over other metal ions.Figure 5(a)shows the fluorescence emission spectra(λex=311 nm)of the film as a function of the Ni(II) concentration.The emission of the film significantly increases with increased Ni(II)concentration(CNi(II)), which ranged from 0 to 320µmol/L.The fluorescence intensity(λex=311 nm,λem=401 nm)of the Ag2S/PU film was found to depend on the Ni(II)concentration. The correlation equation was as follows:

The increasing trend could last for as long as 5 h. Considering that some minerals exist in tap water,ions such as Cu2+,Cr2+,Hg2+,Ba2+,Sr2+,Mn2+,Mg2+, etc.were used to test the sensitivity of the fluorescence of the films to different ions.The results show that the common ions of all added salts do not affect the fluorescence behavior of the Ni(II)(Fig.5(b)).
F.Reversibility of the response of the film to Ni(II)
The reversibility of the film to Ni(II)was examined using a standard method.The film was exposed to an aqueous solution of the analyte,the maximum emission intensity of the film was recorded,and the appropriate amount of Ni(II)solution was added to the solution. Finally,the emission intensity of the film was measured every 3 min five times.The film was then washed with pure water several times and the emission was measured.The measurement was repeated five times at the same analyte concentration.The response of the film to the same concentration of Ni(II)was observed to be fully reversible(Fig.S2 in supplementary materials). The time needed for the response to reach equilibrium is less than 3 min,which is a rather fast response.
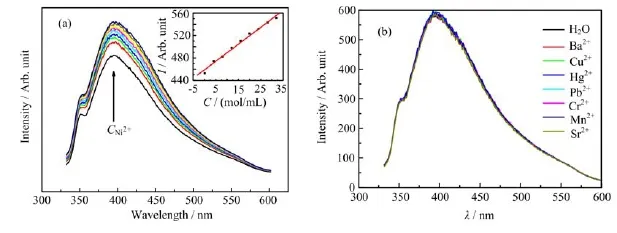
FIG.5(a)Fluorescence of PU/Ag2S film as a function of Ni(II)with various concentrations of 0,40,80,120,160,200,240, 280,320µmol/L as arrow shows and(b)effect of various familiar ions on the detection of Ni(II).
IV.CONCLUSION
A novelsensor can be easily constructed for the detection of Ni(II).The Ni(II)sensor exhibits good selectivity and sensitivity,and shows a linear response within the range of 0−320µmol/L Ni(II).Ni(II)is much more efficient and sensitive to the emission of the film than other familiar ions.This result is ascribed to the hindrance effect induced by Ag2S particles.As a film sensor that is highly sensitive to Ni(II),PU/Ag2S film can be used to monitor the Ni(II)level in water and soil.
Supplementary materials:Figure S1 shows the fluorescence of the film was remarkably enhanced upon the addition of Ni(II),whereas no obvious enhancement was observed for other familiar ions.These results demonstrate that the film has high selectivity for Ni(II)over other metal ions.Figure S2 shows the response of the film to the same concentration of Ni(II) was observed to be fully reversible.
V.ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of Shaanxi Province(No.2014JQ2077),the Special Foundation of the Education Department of Shaanxi Province(No.2013JK0643),and the Special Research Fund of Xianyang Normal University for Talent Introduction(No.13XSYK021).
[1]A.P.Alivisatos,Science 271,933(1996).
[2]A.C.Balazs,T.Emrick,and T.P.Russell,Science 314,1107(2006).
[3]V.Djokovi´c,T.Radhakrishnan,P.S.Nair,M.I. ˇComor,and J.M.Nedeljkovi´c,Semiconductor Polymer Nanocomposites,Netherlands:Brill Academic Publishers,227(2009).
[4]D.Bruhwiler,C.Leiggener,S.Glaus,and G.Calzaferri, J.Phys.Chem.B 106,3770(2002).
[5]C.Leiggener and G.Calzaferri,Chem.Eur.J.11,7191 (2005).
[6]K.Akamatsu,S.Takei,M.Mizuhata,A.Kajinami,S. Deki,S.Takeoka,and K.Yamamoto,Thin Solid Films 359,55(2000).
[7]T.G.Schaaff and A.J.Rodinone,J.Phys.Chem.B 107,10416(2003).
[8]R.V.Kumar,O.Palchik,Y.Koltypin,Y.Diamant, and A.Gedanken,Ultrason Sonochem.9,65(2002).
[9]X.F.Qian,J.Yin,S.Feng,S.H.Liu,and Z.K.Zhu, J.Mater.Chem.11,2504(2001).
[10]L.Xiao,L.Lin,Z.Wan,and W.Ce,Nanotechnology 16,2233(2005).
[11]J.F.Zhu,Y.J.Zhu,M.G.Ma,L.X.Yang,and L. Gao,J.Phys.Chem.C 111,3920(2007).
[12]D.K.Bozanic,V.Djokovic,J.Blanusa,P.S.Nair,M. K.Georges,and T.Radhakrishnan,Eur.Phys.J.22, 51(2007).
[13]F.Gao,Q.Y.Lu,and D.Y.Zhao,Nano Lett.3,85 (2003).
[14]R.Chen,N.T.Nuhfer,L.Moussa,H.R.Morris,and P.M.Whitmore,Nanotechnology 19,455604(2008).
[15]S.Wang,D.Yu,Y.Huang,J.Guo,and Y.Wei,Mater. Sci.Eng.B 176,873(2011).
[16]S.Wang and M.Zheng,Chin.J.Chem.Phys.28,370 (2013).
[17]C.Lv,Z.Wang,P.Wang,and X.Tang,Langmuir 28, 2012(9387).
[18]S.Wang,D.Yu,Y.Huang,J.Guo,and Y.Wei,Mater. Sci.Eng.B 176,271(2011).
[19]W.Tang,R.J.Farries,W.J.Macknight,and C.D. Eisenbach,Macromolecules 27,2814(1994).
[20]L.Shaopu,L.J.Zhi K.Ling,and L.Qi,Sci.China Ser. B 32,554(2002).
[21]R.Premachandran,S.Banerjee,V.T.John,G.L. McPherson,and J.A.Akkara,Chem.Mater.9,1342 (1997).
[22]Q.G.Zhang,F.Z.Y.Huang,and Li,Colloids Surf.A 497,257(2005).
[23]S.C.Jiang,X.L.Ji,L.J.An,and B.Z.Jiang,Acta Polym.Sin.4,452(2000).
∗Author to whom correspondence should be addressed.E-mail: shanwang2005@163.com
 CHINESE JOURNAL OF CHEMICAL PHYSICS2015年6期
CHINESE JOURNAL OF CHEMICAL PHYSICS2015年6期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Importance of Metal Cations and Water for Stability of MnO2Crystals
- Elastic Low-Energy Electron Collisions with Methanethiol
- Antireflective and Self-cleaning Properties of SiO2/TiO2Double-Layer Films Prepared by Cost-Effective Sol-Gel Process
- Effects of Sm Co-doping on Luminescent Properties of Sr4Al14O25:M (M=Mn4+,Cr3+)Phosphors
- Pyrene Derivate Functionalized with Acetylene for Organic Field Effect Transistors
- Miniature Boat Fabrication with Striking Loading Capacity in Seawater from Hydrophobic Steel Mesh
