Effects of Traditional Chinese Herbal Medicine on the Structure of Duodenal Mucosa of Chickens under Heat Stress
Guisheng GAO,Juan CHEN,Huan LIU,Chao XING,Qiumei SHI,Ping SHEN,Guangping GAO,Yanying ZHANG
Hebei Normal University of Science and Technology/Key Laboratory of Preventive Veterinary Medicine of Hebei Province,Qinghuangdao 066004,China
Responsible editor:Xiaohui FAN Responsible proofreader:Xiaoyan WU
H eat stress is an important factor restricting the development of intensive poultry industry.Due to the impacts of heat stress,chickens exhibit less feed intake,weight reduction,and slow growth,thus increasing feeding costs,causing greater losses to chicken rearing industry.Therefore,in summer,appropriate measures should be adopted in chicken production to reduce the economic losses caused by heat stress.Traditional Chinese herbal medicine contains abundant vitamins,trace elements,minerals,amino acids and other nutrients,which can improve the production performance and immune function of chickens,reduce and eliminate the impacts of heat stress on the physiological function of chickens.Thus,in poultry production,traditional Chinese herbal medicine can be applied for clearing away summer-heat,detoxification and sterilization,invigorating spleen and eliminating dampness.For instance,adding 1.0%traditional Chinese herbal medicine into hen diets in summer can improve feed reward by 5.45%-13.2%[1].Herbal antiheat stress additive can not only mitigate the impacts of heat stress on chickens and improve production performance,but also reduce drug tolerance and antibiotic usage to avoid drug residues in poultry and eggs,thereby reducing the harm to human.Therefore,traditional Chinese herbal medicine is an ideal antiheat stress additive with good application prospects[2].
In this study,a traditional Chinese herbal compound agent was prepared with gypsum,patchouli,mint,dried rehamnnia root,Rhizoma atractylodis and Rhodiola rosea L.for clearing away heat and eliminating dampness;Isa Brown chickens were administrated continuously with the prepared compound agent for a week under certain heat stress conditions; subsequently,duodenal villus length,crypt depth and mucosal thickness of chickens were measured,to investigate the protective effects of traditional Chinese herbal medicine on intestinal mucosa of chickens under heat stress.
Materials and Methods
Materials
Experimental animalsOne hundred and twenty 88-day-old Isa Brown chickens were purchased from the chicken farm of Hebei Normal University of Science and Technology.
InstrumentsOlympus biological microscope (BH2); Motic pathological image processing system;Yidi paraffin slicing machine.
ReagentsHematoxylin,eosin,alcohol,xylene,formaldehyde,paraffin,etc.
Traditional Chinese herbal compound agentThe traditional Chinese herbal compound agent was prepared with gypsum,patchouli,mint,dried rehamnnia root,Rhizoma atractylodis and Rhodiola rosea L.
Grouping and management of experimental animals
Normal temperature control group:14-25 ℃,without administration of any drug; other five groups were treated at 28-39 ℃.High temperature control group:without administration of any drug; high temperature Vc control group:each chicken was administrated with 5.0 ml of 0.67 mg/ml Vc solution; high-dose administration group:each chicken was administrated with 7.5 ml of traditional Chinese herbal compound agent;moderate-dose administration group:each chicken was administrated with 5.0 ml of traditional Chinese herbal compound agent; low-dose administration group:each chicken was administrated with 2.5 ml of traditional Chinese herbal compound agent.
Sampling and preparation of tissue slices
SamplingFive experimental chickens in each group were euthanized at 1,4,8 and 10 d post-treatment,respectively.Approximately 2 cm long mid duodenum was collected,rinsed with saline,and fixed promptly with 4%formaldehyde solution for 24 h before paraffin embedding,slicing and hematoxylin-eosin(HE)staining.
Preparation of tissue slicesTissue slices were prepared with the conventional method,involving six steps:trimming and washing,dehydration and decolorization,wax impregnation and paraffin embedding,slicing,unfolding and adhesion.
Staining
Tissue slices were stained with hematoxylin-eosin.Dried slices were dewaxed in xylene I,II for 10 min respectively,soaked into 100%alcohol I,II for 5 min respectively,placed into 95%,90%,80%,70%alcohol for 4 min respectively,washed with distilled water,stained with hematoxylin for about 10 min,separated in 0.5% hydrochloric acid-ethanol solution,blued with running water for 20 min,soaked into 1% eosin solution for 10 min,rinsed three times with 70%,80%,90%alcohol respectively,placed into 95% alcohol I,II and 100%alcohol I,II for 5 min respectively,and decolorized with xylene I,II for 10 min respectively;finally,excess xylene was wiped off and the slides were sealed rapidly with gum.
Determination of major indicators
The morphological structure of duodenal mucosa of chickens was observed under a light microscope.By using Motic pathological image processing system,duodenal villus length,mucosal thickness and crypt depth were measured.Five slices of each duodenal tissue were collected;two longest duodenal villus lengths,deepest crypt depths and thickest mucosal thicknesses on each slice were selected for data analysis using SPSS statistical software.The experimental results were represented with X+SD(mean±standard deviation).
Results and Analysis
Changes of mucosal thickness
Changes of duodenal mucosal thickness in each experimental group were shown in Table1.
As could be seen from Table1,with the increase of experimental duration,duodenal mucosal thickness in normal temperature control group remained at a stable level,while that in three different high-temperature administration groups was reduced.Especially,duodenal mucosal thickness in normal temperature control group at 8 and 10 d post-treatment varied extremely significantly(P<0.01);duodenal mucosal thickness in three different high-temperature administration groups at 8 and 10 d post-treatment was higher than that in high temperature control group and high temperature Vc control group,exhibiting extremely significant differences (P <0.01).Duodenal mucosal thickness in high-dose administration group,moderate-dose administration group and low-dose administration group declined successively; to be specific,duodenal mucosal thickness in low dose administration group exhibited extremely significant differences (P<0.01)compared with high dose administration group and exhibited significant differences (P <0.05) compared with moderate dose administration group; duodenal mucosal thickness varied significantly (P<0.05) between moderate dose administration group and high dose administration group.
According to the microscopic pictures of tissue slices (Fig.1,Fig.2,Fig.3,Fig.4 and Fig.5),duodenal villi in high temperature control group were relatively scarce and thin with shedding and breakage,which were improved in high-temperature administration groups.
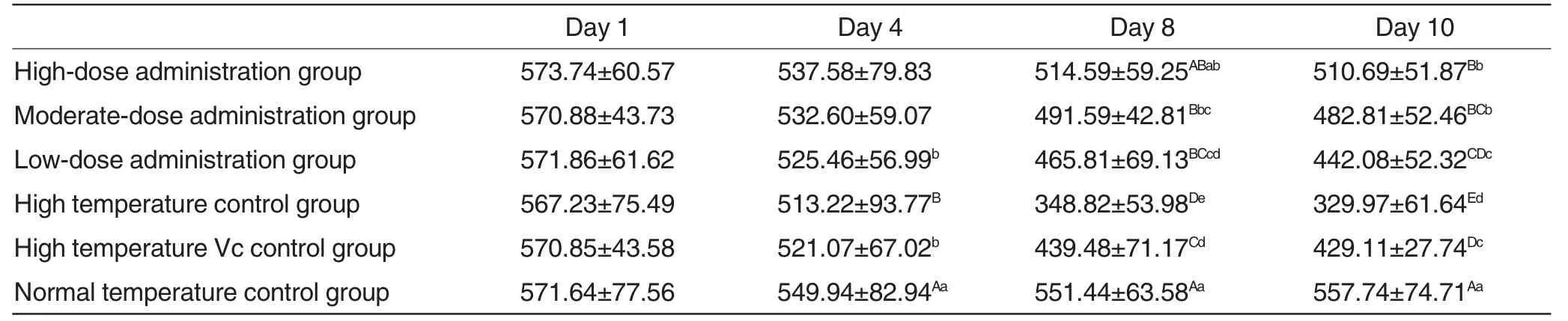
Table1 Changes of duodenal mucosal thickness in each experimental group μm
Changes of villus length
Changes of duodenal villus length in each experimental group were shown in Table2.
As could be seen from Table2,with the increase of experimental duration,duodenal villus length in normal temperature control group remained at a stable level,which exhibited extremely significant differences (P <0.01) at 4,8 and 10 d compared with moderate-dose administration group,low-dose administration group,high temperature control group and high temperature Vc control group and exhibited significant differences(P<0.05)compared with high-dose administration group.Moreover,duodenal villus length in high-dose administration group at 4,8 and 10 d was higher compared with high temperature control group and high temperature Vc control group,exhibiting extremely significant differences (P<0.01);duodenal villus length in high-dose administration group,moderate-dose administration group and low-dose administration group declined successively; to be specific,duodenal villus length varied significantly (P<0.05) among highdose administration group,moderatedose administration group and lowdose administration group at 4 d;duodenal villus length varied extremely significantly (P <0.01) among highdose administration group,moderatedose administration group and lowdose administration group at 8 d; furthermore,at 10 d post-treatment,duodenal villus length in high-dose administration group varied extremely significantly (P<0.01) compared with moderate-dose administration group and low-dose administration group;duodenal villus length varied significantly(P<0.05) between moderate-dose administration group and low-dose administration group.
Changes of villus length to crypt depth ratio
Changes of duodenal villus length to crypt depth ratio in each experimental group were shown in Table3.
As could be seen from Table3,at 8 d post-treatment,villus length to crypt depth ratio in high temperature control group varied extremely significantly (P<0.01) compared with other five groups;villus length to crypt depth ratio in high-dose administration group was higher than that in moderate-dose administration group,low-dose administration group and high temperature Vc control group,exhibiting extremely significant differences (P<0.01);villus length to crypt depth ratio varied extremely significantly (P<0.01) between moderate-dose administration group and high temperature Vc control group; villus length to crypt depth ratio exhibited no significant differences(P>0.05)between low-dose administration group and high temperature Vc control group.At 10 d post-treatment,villus length to crypt depth ratio in high-dose administration group was higher than that in other five groups,exhibiting extremely significant differences (P <0.01); villus length to crypt depth ratioin high temperature control group varied extremely significantly (P <0.01)compared with three administration groups and varied significantly (P <0.05) compared with high temperature Vc control group; moreover,villus length to crypt depth ratio in three administration groups varied extremely significantly (P<0.01) compared with high temperature Vc control group.
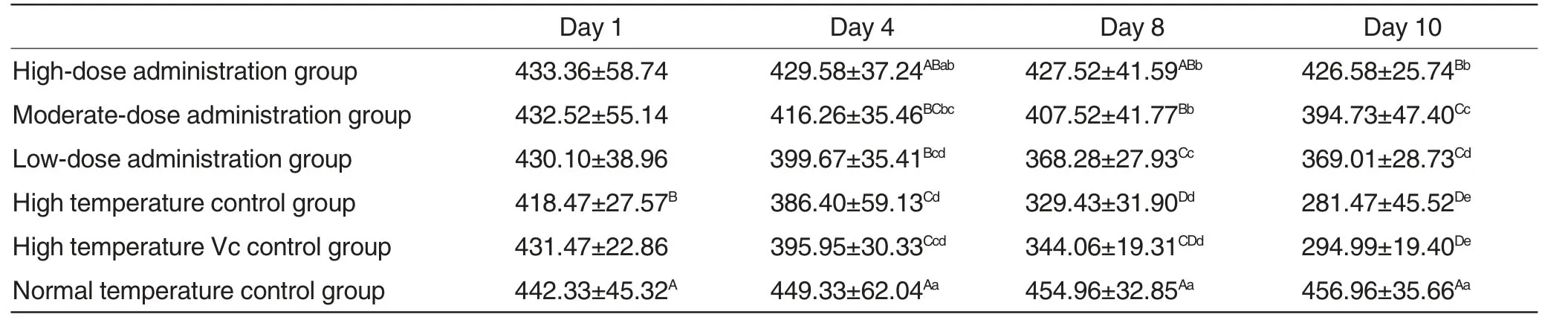
Table2 Changes of duodenal villus length in each experimental group μm
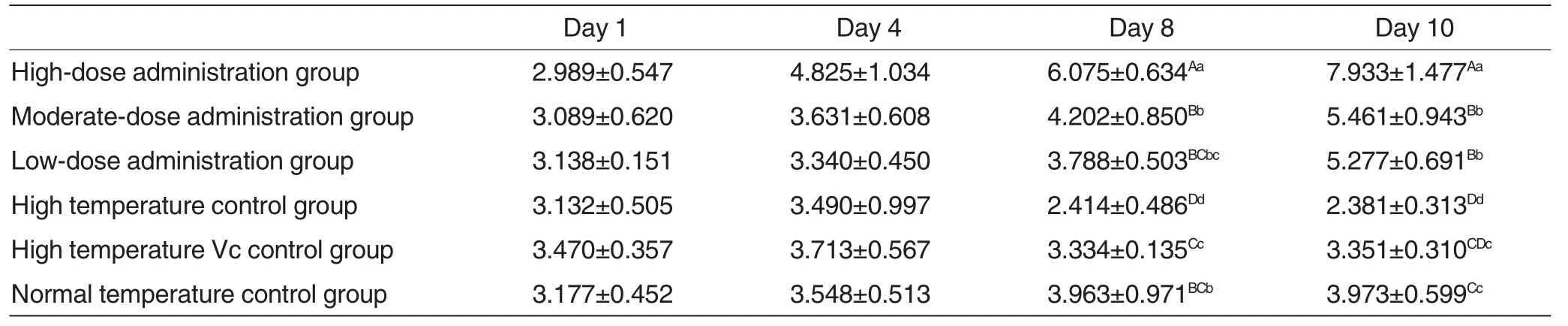
Table3 Changes of duodenal villus length to crypt depth ratio in each experimental group
Discussion
Normal structure and function of the small intestine basically ensure full digestion and absorption of nutrients.Especially,villus length and mucosal thickness are two important indicator determining the digestion and absorption function of the small intestine[3].Villus length to crypt depth ratio reflects the atrophy rate of intestinal villi[4].According to the experimental results,under heat stress,duodenal mucosal thickness,villus length and villus length to crypt depth ratio (V/C) were reduced gradually,and the villi were scarce and thin.Intestine is the central organ in response to stress.Acute heat stress can cause pathological damages to organs and tissues of experimental chickens.Stress injuries are extremely serious at the early stage (6-10 d) of heat stress[5],which is consistent with the experimental results of this study.The traditional Chinese herbal compound agent used in the present study was prepared with gypsum,patchouli,mint,dried rehamnnia root,Rhizoma Atractylodis and Rhodiola rosea L.Modern medical researches confirm that β-Eudesmol,as an active ingredient of Rhizoma Atractylodis,can significantly promote the gastrointestinal motility;in addition,Rhizoma Atractylodis also exhibits various functions such as anti-inflammation and anti diarrhea[6].Patchouli essential oil can promote the secretion of gastric juice,improve digestion and relieve gastrointestinal spasm.Golden cypress has functions of anti-inflammation,anti-bacteria and anti-oxidative damage[7-8].Gypsum can inhibit thermotaxic centre excitement under heat stress.Rhizoma Anemarrhenae can clear heat-fire and promote the secretion of saliva or body fluid,which is commonly used to treat heat diseases,high fever and polydipsia,cough due to lung dryness,osteopyrexia and fever,heat diabetes and constipation due to intestinal dryness[9].
Conclusion
In the present study,various experimental indicators of duodenal mucosa of chickens under heat stress were reduced gradually.In addition,duodenal villi became scarce and thin with shedding and breakage.Under high temperature conditions,high-,moderate-and low-doses of traditional Chinese herbal medicine reduced pathological changes of duodenal mucosa and promoted villus growth.High-dose administration group showed significantly better results than other administration groups and Vc control group,exhibiting significant protective effects on duodenal mucosa of chickens under heat stress.
[1]LIU YX(刘永学),GAO Y(高月).Recent advance in stress research(应激的研究进展)[J].Chinese Journal of Pathophysiology(中国病理生理杂志),2002,18(2):218.
[2]TAN X (谭勋).Compensatory reaction against heat stress and its consequences in chickens(热应激的代偿反应及后果)[J].Shangdong Journal of Animal Science and Veterinary Medicine(山东畜牧兽医),1999,1:32-33.
[3]WANG XL(王小龙),GAO DY(高得仪),CHEN WF (陈万芳),et al.Veterinary Clinical Pathology (兽医临床病理学)[M].Beijing:China Agriculture Press(北京:中国农业出版社),1995.114-148.
[4]YU X (于显),YUAN X (袁续).The research progress of avian heat stress mechanism and influence factors (家禽热应激机理及其影响因素的研究进展)[J].Seed Landscape (饲料广角),2003(8):28-30.
[5]LI SJ (李守军).Acute heat stress damage and its pathogenesis in heatstressed broilers (肉鸡急性热应激损伤及其机理的研究)[D].Beijing:China Agricultural University (北京:中国农业大学),2004.
[6]ZHENG S(郑枢),AN LL(安立龙),FENG Y (冯业),et al.Study on effect of Chinese herbal feed additive on anti-heat stress for broilers(中草药饲料添加剂抗肉鸡热应激研究进展)[J].Acta Ecologiae Animalis Domastici(家畜生态学报),2007,28(2):28-30.
[7]WANG HQ (王衡奇),QIN MJ (秦民坚),YU GD(余国奠).Advances in the study on chemical constituents and pharmacology of Phellodendron amurense (黄柏的化学成分及药理学研究进展)[J].Chinese Wild Plant Resources (中国野生植物资源),2001,20(4):6-8.
[8]KONG LD(孔令东),YANG C(杨澄),QIU X (仇熙),et al.Effects of different processing products of Cortex Phellodendri on scavenging oxygen free radicals and anti-lipidperoxidation (黄柏炮制品清除氧自由基和抗脂质过氧化作用)[J].China Journal of Chinese Materia Medica(中国中药杂志),2001,26(4):247.
[9]LIU ZJ(刘忠杰),XU JQ(许剑琴).Traditional Chinese Veterinary Medicine (中兽医学)[M].Beijing:China Agriculture Press (北京:中国农业出版社),Third Edition,2006.166.
 Agricultural Science & Technology2015年1期
Agricultural Science & Technology2015年1期
- Agricultural Science & Technology的其它文章
- Research Status and Application Prospects of Monascus sp.
- A Study on the Relationships of Ecological Civilization Construction with Natural Conservation and Sustainable Resources Use
- Inquiry of Modernization Managementof Rural Community Self-organizing on the Perspective of SocialWork
- Research Advances in Spatio-Temporal Coupling of Water and Fertilizer in Sugarcane
- Instrucions for Authors
- About Agricultural Science Technology
