热处理温度对锂氧气电池用Co-N/C催化剂催化性能的影响
阳炳检 王 红 李 磊 黄博文 廖小珍 何雨石 马紫峰
(上海交通大学化学化工学院化学工程系电化学与能源技术研究所,上海 200240)
1 Introduction
Metal-air battery has attracted much attention as a possible alternative energy conversion and storage devices because of its extremely high energy density compared to that of other re-chargeable batteries.1-5However,despite some progresses that have been reported on cathode materials and electrolytes,there are several key challenges that limit the practical use of this battery system.One of the most important issues is to develop inexpensive and effective oxygen reduction reaction/oxygen evolution reaction(ORR/OER)catalysts,which play key roles in Li/O2batteries.6-10Until now,noble metals such as Pt,Au and their alloys as ORR/OER catalysts in Li/O2battery have shown the best overall catalytic performance.11-13However,the high price and scarcity of precious metals severely limit their wide spread applications.Therefore,developing alternative,low cost catalysts to reduce or completely replace Pt,Au-based catalysts is necessary.
It has been demonstrated that transition-metal macrocycles such as phthalocyanine,porphyrin,and their derivatives have high catalytic activity towards the oxygen reduction reaction after pyrolysis.Furthermore,cobalt phthalocyanine showed considerable catalytic activity toward electrochemical ORR/OER in Li/O2battery reported by Abraham and Jiang.14Despite insufficient activity and stability compared to Pt-based catalysts,non-noble metal-nitrogen catalysts can be applied as promising catalysts for Li/O2batteries.14-16,20-22Since it has been found that simple nitrogen ligands also have the same ORR catalyst performance as the organic macrocycles,we used a simple and cheap chemical called phenanthroline(phen)as N-containing ligand to prepare the cobalt-phen(Co-phen)complex.The asprepared Co-phen complexes were coated on BP2000 and then heat-treated at different temperatures(600-900°C)in an inert atmosphere to achieve ORR/OER electrocatalysts.The catalysts obtained at different calcination temperatures were characterized by X-ray diffraction(XRD)and X-ray photoelectron spectroscopy(XPS)analyses.The ORR/OER activities of the prepared catalysts were characterized in an alkaline medium and an organic electrolyte,respectively,using rotating disk electrode(RDE).The Li/O2cell performance of the prepared catalysts was investigated.
2 Experimental
The Co-N/C catalysts were prepared using BP2000(American Cabot Co.)as carbon support.The BP2000 carbon was activated by refluxing with 30%H2O2(AR)over night,washed with de-ionized water and then dried in a vacuum oven at 100°C for 4 h.The dried BP2000 powder was milled for 2 h in a planetary mill(Fritsch Pulversette-6)with an agate vessel.
Following is the preparing procedure for the Co-N/C electrocatalysts.First,0.126 g cobalt acetate was dissolved in 25 mL ethanol(AR)and 0.202 g phenanthroline(99%)ligand was added to form Co(phen)2chelate.The obtained solution was mixed with 1.0 g activated BP2000 for 1 h under ultrasonic condition and then kept stirring for 2 h.After drying to remove ethanol,the resulting powder was calcinated at 600,700,800,and 900°C,respectively,for 90 min under an argon atmosphere to obtain final Co-N/C products.
For comparison,the cobalt(II)tetramethoxyphenylporphyrin(CoTMPP/C)electrocatalyst was also prepared as following:0.405 g cobalt tetramethoxyphenylporphyrin(96%,Aldrich)was dispersed in ethanol.The obtained suspension was mixed with 1.0 g activated BP2000 for 1 h under ultrasonic condition and then kept stirring for 2 h.After drying to remove ethanol,the resulting powder was calcinated at 800°C for 90 min under an argon atmosphere to obtain final CoTMPP/C product with 2.61%(w,mass fraction)Co loading.
The prepared Co-N/C catalysts and BP2000 were characterized using X-ray diffraction(D/max-2200/PC,Rigaku Co.Ltd.,Tokyo,Japan)with filtered Cu Kαradiation.The XRD patterns were collected at room temperature by step scanning at the range of 20°≤2θ≤80°.Cobalt content in the samples was determined by inductively coupled plasma mass spectrometer(Agilent 7500cx)and Nitrogen elemental analysis was conducted by Elementar Vario EL-III/Isoprime.XPS experiments were carried out on a KratosTMAxis Ultra DLD surface analysis instrument with Al Kαradiation(hv=1486.6 eV).The binding energy scale was calibrated with the C 1s peak(284.8 eV)of adventitious carbon on the sample surface.
The catalytic activity toward ORR was evaluated by RDE measurements using a CHI RRDE-3A system.The catalyst ink was prepared by sonicating 5 mg of material in 30 μL of Nafion(5%(w))and 1 mL of Milli-Q H2O.8 μL of the suspension was deposited onto a polished tip of the RDE of 3 mm diameter(catalyst loading:40 μg)and dry at room temperature.All electrochemical measurements were performed at room temperature in the RDE electrochemical cell using an Ag/AgCl reference electrode,a Pt wire counter electrode and 0.1 mol·L-1aqueous KOH solution as electrolyte.For ORR and OER measurements in 1 mol·L-1lithium bis(trifluoromethanesulfonyl)imide(LiTFSI)/propylene carbonate(PC):diethyl carbonate(DEC)solution,the catalyst ink was deposited onto a polished tip of the RDE and dry at 40°C.Pt wires were used as reference electrode and counter electrode,which was calibrated against the Li metal electrode in 1 mol·L-1LiTFSI/PC:DEC solution.
The oxygen electrodes were prepared by casting a mixture of Co-N/C catalysts and polytetrafluoroethylene(PTFE)binder in a mass ratio of 85:15 onto carbon paper.The electrode disks with a diameter of 20 mm were punch and then dried at 120°C for 8 h.The mass load of catalyst layer was 2 mg·cm-2.The lithium-oxygen cells with Li metal as anode,oxygen electrode as cathode,and polymer electrolyte membrane(polyvinylidenefluoride-hexafluoro propylene(PVDF-HFP))as separator were assembled for electrochemical test.The polymer electrolyte membrane was soaked in the electrolyte(consisting of 1 mol·L-1LiTFSI(99%,Aladdin)dissolved in a mixture of PC and DEC(1:1 in volume)before assembly.All processes of assembling and dismantling the battery cells were carried out in an argon atmosphere in a glove box.
The Li/O2batteries were tested in 105Pa oxygen atmosphere using a battery test system(LAND CT2001 A model,Wuhan Jinnuo Electronic Co.,Ltd.)at 25°C.Discharge/charge curves were recorded galvanostatically with various current densities at the voltage range of 4.5-2.0 V.The specific capacities are normalized with respect to the mass of catalyst in the cathode.All measurements were carried out under oxygen atmosphere.
3 Results and discussion
Powder XRD patterns of the prepared Co-N/C catalysts with different calcination temperatures,and also the carbon support BP2000 are displayed in Fig.1.It was observed that the samples calcinated at 900 and 800°C showed two small sharp peaks at around 44°and 52°,corresponding to the Co(111),and Co(200)planes of a face-centered cubic(fcc)crystalline α-Co phase(PDF No.89-4307),while no obvious peaks at the same place were detected for the other samples calcinated at lower temperature.These results indicate that cobalt crystallized when the heat-treating temperature was up to 800°C.Fig.2 displays the results of element analysis.It can be seen that the cobalt content of the prepared four Co-N/C samples(600,700,800,and 900°C)was in the range of 2.41%-2.64%.In the other hand,the total nitrogen content in the samples decreased obviously with the calcinating temperature due to the pyrolysis of the N containing component.This phenomenon was also reported by other researchers.23
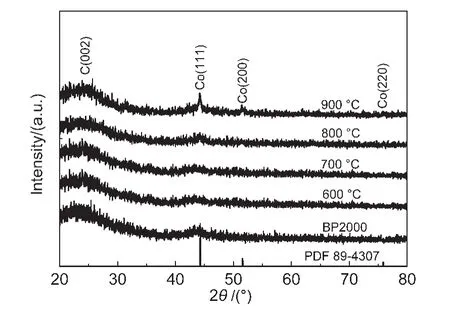
Fig.1 XRD patterns of the carbon supported BP2000 and Co-N/C catalysts with different calcination temperatures
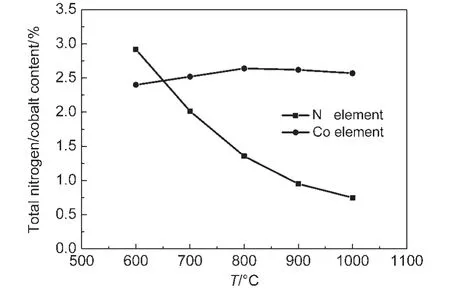
Fig.2 Total cobalt and nitrogen contents(w)of the Co-N/C catalysts prepared from different calcination temperatures
To characterize the nitrogen species on the surface of the catalyst samples,XPS analyses of N 1s were recorded as shown in Fig.3.It can be seen that there were three types of nitrogen on the catalyst surface with binding energies of 398.5-398.6 eV,400.4-401.1 eV,and 402.2-403.7 eV,which may be assigned to pyridinic-type N,pyrrolic-type N,and highly coordinated N atoms(N atoms bound to three carbon atoms within a graphene layer),respectively.With the calcination temperature increased from 600 to 900°C,the proportion of pyrrolic-N decreased significantly.Part of pyrrolic-N was gradually converted to pyridinic-N and highly coordinated nitrogen with the increased temperature.The pyridinic-N and highly coordinated N as the active sites play an important role in oxygen reduce reaction.It is known that O2molecules prefer to adsorb at carbon sites on graphene-like zigzag edges where highly coordinated N is located nearby.24According to density functional theory(DFT)calculation,the presence of pyridine-type N species in graphene structure can activate oxygen reduction.24The experimental results in literature25,26showed that the catalysts with a higher content of highly coordinated N had higher ORR activity.
The effect of calcination temperature on the ORR catalytic activity of Co-N/C catalysts was investigated in aqueous 0.1 mol·L-1KOH solution,as shown in Fig.4a.The ORR performance of the CoTMPP/C catalyst and bare BP2000 were also compared with the Co-N/C catalysts.It can be seen that the Co-N/C catalysts exhibited obviously superior electrochemical performance to the bare BP2000,and closed to the CoTMPP/C catalyst.Furthermore,the catalyst Co-N/C(800°C)showed slightly higher limiting current density and more positive ORR half-wave potential than the other Co-N/C samples.The catalytic performance of Co-N/C(800°C)is comparable to CoTMPP/C catalyst.It seems that 800°C is a proper calcination temperature for preparing high performance Co-N/C catalyst.It is known that nitrogen plays an importance role in the active site of carbon materials for ORR catalysts.24-26From Fig.3 it can be seen that the Co-N/C(800°C)sample showed higher proportion of the active pyridinic-type N and highly coordinated N.In the other hand,when calcination temperature increased to 900°C,the total N content decreased to a very low value,which was disadvantage to the catalytic activity of the catalyst.The number of cobalt active sites is another important factor that closely related to the behavior of the oxygen cathode.XRD patterns in Fig.1 show that Co-N/C(900°C)sample exhibited a sharp XRD peak at 44.2°of metallic Co(111),while only a very small peak at the same place was observed for Co-N/C(800°C).This result may indicate that cobalt nanoparticles agglomerated when the sample was calcinated at 900°C,which decreased the number of cobalt active sites.

Fig.3 XPS spectra of N 1s in the Co-N/C catalysts at different calcination temperatures
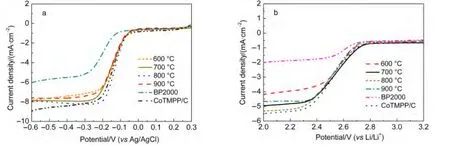
Fig.4 Comparison of the voltammetry curves for the Co-N/C,CoTMPP/C,and BP2000 at the rotation rate of 3600 r·min-1 in oxygen-saturated solution
We further characterized the ORR catalytic activity of the Co-N/C catalysts in a non-aqueous electrolyte of O2-saturated 1 mol·L-1LiTFSI/PC:DEC as shown in Fig.4b.It is clear that the Co-N/C(700,800°C)samples showed similar electrochemical activity as CoTMPP/C electrode,and superior to the other samples.The half-wave potentials of CoTMPP/C,Co-N/C(700 °C),and Co-N/C(800 °C)electrodes were 2.53,2.54,and 2.52 V(vs Li/Li+),respectively.According to the results in Fig.4a and Fig.4b,the proper heat treating temperature for Co-N/C catalyst is 700-800°C.
We further study the ORR and OER catalytic activity of Co-N/C(800°C)sample in non-aqueous electrolyte,the cyclic voltammograms were measured in O2.As shown in Fig.5,a pair of redox peaks was detected with anodic peak at 2.5 V and cathodic peak at 3.4 V at a scan rate of 10 mV·s-1under O2atmosphere,which indicates excellent ORR and OER activity of the Co-N/C(800°C)catalysts.Furthermore,it is interesting to find that the oxygen reduction overpotential of CoTMPP/C catalyst was larger than Co-N/C catalyst,and the oxidic peak(OER)of CoTMPP/C catalyst was obviously smaller than that of Co-N/C catalyst.These results suggested that the Co-N/C(800°C)catalyst could be an effective ORR and OER catalyst for Li/O2cells.
The number of electrons involved in the ORR is an important parameter for evaluating the catalyst performance.Rotating disk electrode(RDE)voltammetry measurements for Co-N/C catalysts in both aqueous 0.1 mol·L-1KOH and non-aqueous 1 mol·L-1LiTFSI/PC:DEC electrolytes were conducted at various rotation rates(Fig.6A and Fig.7A,respectively).The Koutecky-Levich(K-L)equation is used to analyze the RDE data.27,28
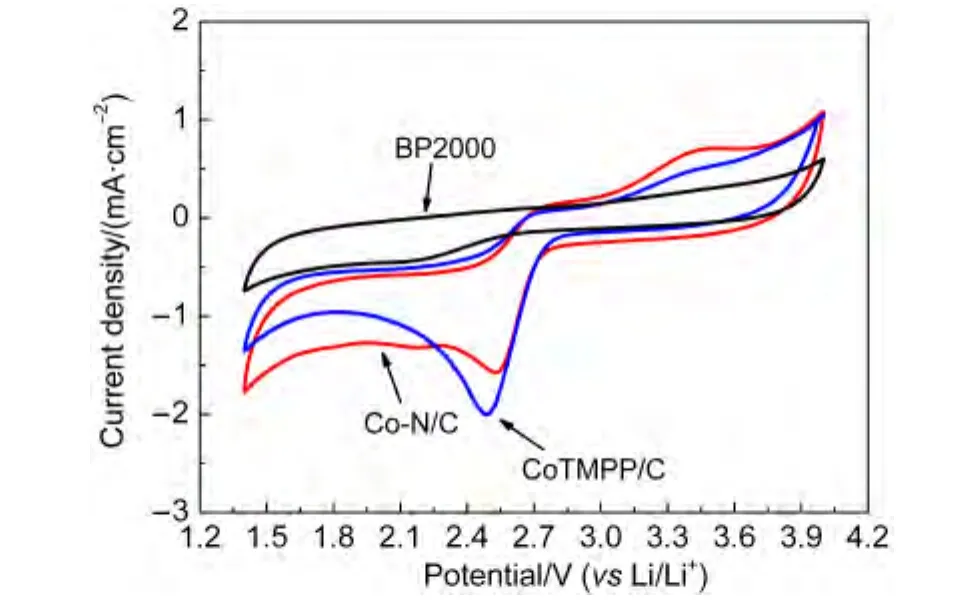
Fig.5 CVs of the Co-N/C,CoTMPP/C,and BP2000 catalysts in oxygen-saturated 1 mol·L-1 LiTFSI-PC:DEC solution
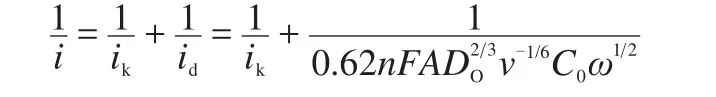
In the above equations,i is the current at different potentials,ikand idare the kinetic and diffusion limited currents respectively,F is Faraday constant,A is the electrode area,ω is angular velocity,C0is bulk concentration of O2in the electrolyte solution,DOis the diffusion coefficient of O2,and v is the kinematic viscosity of the solution.27Fig.6b and Fig.7b show typical K-L plots for oxygen reduction in 0.1 mol·L-1NaOH and non-aqueous 1 mol·L-1LiTFSI/PC:DEC electrolytes,respectively.The numbers of electron transferred per O2molecule in ORR is calculated from the slopes of K-L plots,which were~3.7 in aqueous 0.1 mol·L-1KOH electrolyte and~1.7 in LiTFSI/PC:DEC electrolyte.It is clear that the catalytic activity of Co-N/C is similar to the typical transition-metal macrocycle electrocatalyst CoTMPP/C.17-19We speculate that the product of reduction should be Li2O2or Li2O in non-aqueous Li/O2cell.
A rechargeable Li/O2cell using Co-N/C(800°C)as cathode catalyst was fabricated and characterized.The cell performance of the CoTMPP/C catalyst and bare BP2000 were also compared with the Co-N/C catalyst.Fig.8 shows the discharge and charge behavior of these Li/O2cells at 0.1 mA·cm-2.It can be seen that the Co-N/C cell exhibited very similar performance with Li/O2(CoTMPP/C)cell,which was obvious superior to BP2000 cell.The Co-N/C cathode showed a constant discharge potential plateau at about 2.9-2.7 V(vs Li/Li+).The first discharge capacities of the Li/O2(Co-N/C)cell was 3221 mAh·g-1,which is slightly higher than the Li/O2(CoTMPP/C)cell(3195 mAh·g-1,with potential plateau at around 2.9-2.7 V(vs Li/Li+)).The Li/O2cell performance of the catalysts was consistent with the results of RDE measurement.Fig.9 further compares the rate performance of Li/O2(Co-N/C)cell and Li/O2(CoTMPP/C)cell.It is clear that the Co-N/C catalyst show comparable performance to CoTMPP/C at all the three test current densities.The discharge capacities of the Li/O2(Co-N/C)cell were 2502 mAh·g-1(0.15 mA·cm-2)and 2101 mAh·g-1(0.2 mA·cm-2),respectively.The good performance of Co-N/C cathode was attributed to the cobalt-nitrogen catalyst which played a key role to promote the ORR and OER reversibility.
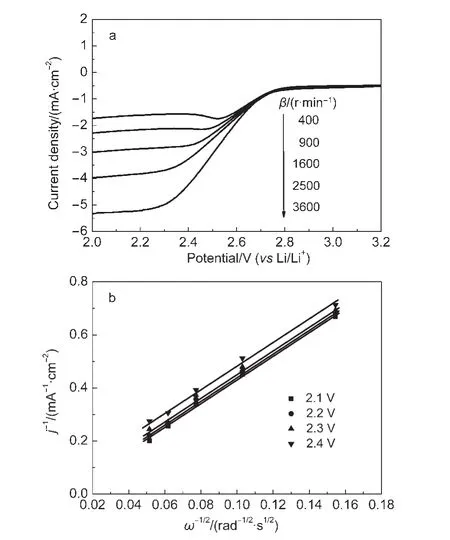
Fig.7 (a)RDE voltammograms of the Co-N/C catalysts in oxygen-saturated 1 mol·L-1 LiTFSI/PC:DEC solution at a scan rate of 10 mV·s-1;(b)Koutecky-Levich plots at different potentials
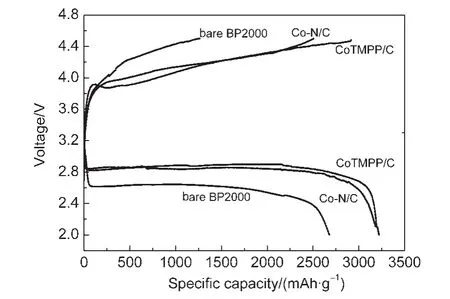
Fig.8 Discharge and charge curves corresponding to the first cycle for the Co-N/C,CoTMPP/C,and BP2000 cathodes at 0.1 mA·cm-2

Fig.9 Discharge and charge curves at different current densities corresponding to the first cycle for the Co-N/C and CoTMPP/C cathodes
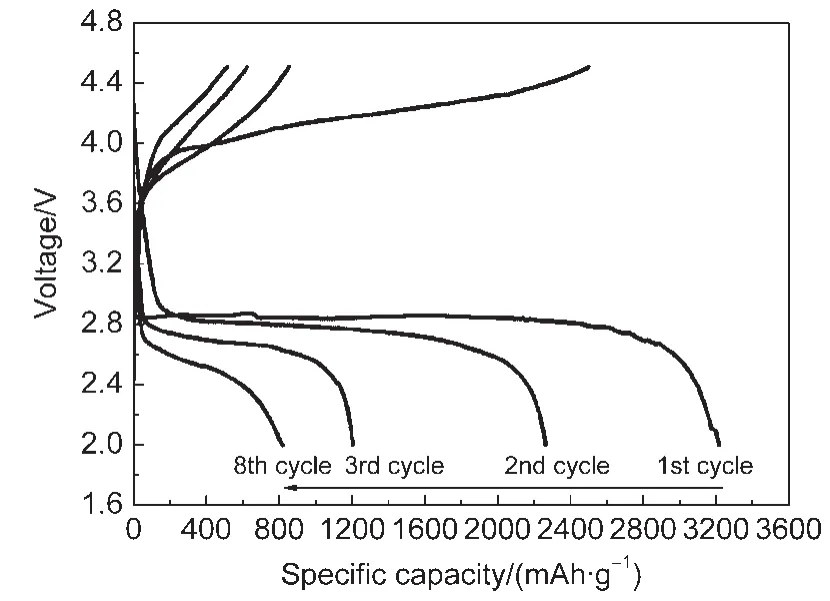
Fig.10 Cycling performance of the Li/O2using Co-N/C(800 °C)as cathode at 0.1 mA·cm-2
Fig.10 shows the cycling performance of the Li/O2using Co-N/C(800°C)as cathode catalyst.The discharge-charge current density was 0.1 mA·cm-2.The initial discharge capacity of the Co(phen)2/C cathode was 3221 mAh·g-1,which dropped to 2267 mAh·g-1at the second cycle and retained a value of 819 mAh·g-1after 8 cycles.Further work on optimizing air electrodes,developing good performance electrolytes is necessary to improve the Li/O2cell cycling performance.
4 Conclusions
The Co-N/C non-noble metal electrocatalysts were synthesized by calcinating cobalt phenanthroline(phen)chelate,which was coated on a carbon support BP2000.The influences of calcination temperature on the catalytic activities of the obtained catalysts have been investigated using cyclic voltammogram and rotating-disk electrode in oxygen saturated in aqueous KOH and non-aqueous PC/DEC electrolyte.The Co-N/C samples calcinated at 700 and 800°C showed higher activity than those calcinated at 600 and 900°C.The Li/O2cell using Co-N/C(800°C)catalyst shows comparable performance with the cell using typical CoTMPP/C catalyst.The cheap Co-N/C catalyst may be a promising candidate for practical application in rechargeable Li/O2cells.
(2)Lee,J.S.;Kim,S.T.;Cao,R.;Choi,N.S.;Liu,M.;Lee,K.T.;Cho,J.Adv.Energy Mater.2011,1,34.doi:10.1002/aenm.201000010
(3)Peng,Z.;Freunberger,S.A.;Chen,Y.;Bruce,P.G.Science 2012,337,563.doi:10.1126/science.1223985
(4)Wang,H.;Liao,X.Z.;Li,L.;Chen,H.;Jiang,Q.Z.;He,Y.;Ma,Z.F.J.Electrochem.Soc.2012,159,A1874.
(5)Lu,J.;Qin,Y.;Du,P.;Luo,X.;Wu,T.;Ren,Y.;Wen,J.;Miller,D.J.;Millera,J.T.;Amine,K.RSC Adv.2013,3,8276.doi:10.1039/c3ra40451j
(6)Christensen,J.;Albertus,P.;Sanchez-Carrera,R.S.J.Electrochem.Soc.2012,159,R1.
(7)Huang,B.W.;Liao,X.Z.;Wang,H.;Wang,C.N.;He,Y.S.;Ma,Z.F.Journal of Electrochemical Society 2013,160,A1112.
(8)Kraytsberg,A.;Ein-Eli,Y.J.Power Sources 2011,196,886.doi:10.1016/j.jpowsour.2010.09.031
(9)Wang,H.;Liao,X.Z.;Jiang,Q.Z.;Yang,X.W.;He,Y.S.;Ma,Z.F.Chin.Sci.Bull.2012,57,1959.doi:10.1007/s11434-011-4944-7
(10)Débart,A.;Bao,J.;Armstrong,G.;Bruce,P.G.Angew.Chem.Int.Edit.2008,47,4521.
(11)Lu,Y.C.;Xu,Z.;Gasteiger,H.A.;Chen,S.;Kimberly,H.;Yang,S.H.J.Am.Chem.Soc.2010,132,12170.doi:10.1021/ja1036572
(12)Lu,Y.C.;Gasteiger,H.A.;Parent,M.C.;Vazrik,C.;Yang,S.H.Electrochem.Solid-State Lett.2010,13,A69.
(13)Thapa,A.K.;Saimen,K.;Ishihara,T.Electrochem.Solid-State Lett.2010,13,A165.
(14)Abraham,K.M.;Jiang,Z.J.Electrochem.Soc.1996,143,1.doi:10.1149/1.1836378
(15)Wu,J.;Park,H.W.;Yu,A.;Higgins,D.;Chen,Z.J.Phys.Chem.C 2012,116,9427.doi:10.1021/jp301644e
(16)He,P.;Wang,Y.G.;Zhou,H.S.Chem.Commun.2011,47,10701.doi:10.1039/c1cc14144a
(17)Xu,L.;Qiao,J.L.;Ding,L.;Hu,L.Y.;Liu,L.L.;Wang,H.J.Acta Phys.-Chim.Sin.2011,27,2251.[徐 莉,乔锦丽,丁 蕾,胡隆宇,刘玲玲,王海江.物理化学学报,2011,27,2251.]doi:10.3866/PKU.WHXB20111015
(18)Dai,X.F.;Zheng,M.F.;Xu,P.;Shi,J.J.;Ma,C.Y.;Qiao,J.L.Acta Phys.-Chim.Sin.2013,29,1753.[戴先逢,郑明富,徐 攀,石晶晶,马承禺,乔锦丽.物理化学学报,2013,29,1753.]doi:10.3866/PKU.WHXB201306141
(19)Cao,C.H.;Lin,R.;Zhao,T.T.;Huang,Z.;Ma,J.X.Acta Phys.-Chim.Sin.2013,29,95.[曹春晖,林 瑞,赵天天,黄 真,马建新.物理化学学报,2013,29,95.]doi:10.3866/PKU.WHXB201209272
(20)Wang,H.;Liao,X.Z.;Jiang,Q.Z.;Yang,X.W.;He,Y.;Ma,Z.F.Chin.Sci.Bull.2012,57,1.doi:10.1007/s11434-011-9935-1
(21)Yoo,E.;Nakamurab,J.J.;Zhou,H.S.Energy Environ.Sci.2012,5,6928.doi:10.1039/c2ee02830a
(22)Li,Y.L.;Wang,J.J.;Li,X.F.;Geng,D.S.;Banis,M.N.;Li,R.Y.;Sun,X.L.Electrochem.Commun.2012,18,12.doi:10.1016/j.elecom.2012.01.023
(23)Taigo,O.;Takaaki,M.;Shuichi,S.;Jun,K.;Kenji,Y.;Takao,Y.Catalysis Communications 2014,43,66.doi:10.1016/j.catcom.2013.09.011
(24)Ikeda,T.;Boero,M.;Huang,S.F.;Terakura,K.;Oshima,M.;Ozaki,J.I.J.Phys.Chem.C 2008,112,14706.doi:10.1021/jp806084d
(25)Niwa,H.;Horiba,K.;Harada,Y.;Oshima,M.;Ikeda,T.;Terakura,K.;Ozaki,J.;Miyata,S.J.Power Sources 2009,187,93.doi:10.1016/j.jpowsour.2008.10.064
(26)Liu,G.;Li,X.;Lee,J.W.;Popov,B.N.Catal.Sci.Technol.2011,1,207.doi:10.1039/c0cy00053a
(27)Bard,A.J.;Faulkner,L.R.Electrochemical Methods,2nd ed.;John Wiley&Sons:New York,2004.
(28)Dilimon,V.S.;Venkata Narayanan,N.S.;Sampath,S.Electrochimica Acta 2010,55,5930 doi:10.1016/j.electacta.2010.05.047

