Molecular Dynamics Simulations on the Role of Structural Mg2+Ions in Phosphoryl Transfer Catalyzed by GSK-3β
SUN Hao JIANG Yong-Jun YU Qing-Sen GAO Hui
(1Southwest Forestry University,Kunming 650224,P.R.China;2Key Laboratory for Molecular Design and Nutrition Engineering of Ningbo City,Ningbo Institute of Technology,Zhejiang University,Ningbo 315100,Zhejiang Province,P.R.China;3Department of Chemistry,Zhejiang University,Hangzhou 310027,P.R.China)
Molecular Dynamics Simulations on the Role of Structural Mg2+Ions in Phosphoryl Transfer Catalyzed by GSK-3β
SUN Hao1JIANG Yong-Jun2,*YU Qing-Sen3GAO Hui3
(1Southwest Forestry University,Kunming 650224,P.R.China;2Key Laboratory for Molecular Design and Nutrition Engineering of Ningbo City,Ningbo Institute of Technology,Zhejiang University,Ningbo 315100,Zhejiang Province,P.R.China;3Department of Chemistry,Zhejiang University,Hangzhou 310027,P.R.China)
Abstract:Glycogen synthase kinase-3β (GSK-3β)is a kind of serine/threonine protein kinase.It regulates the synthesis of glycogen and plays an important part in several signal pathways.It is believed to be an important target for a number of diseases such as diabetes,cancers,chronic inflammation,and Alzheimer′s disease.Mg2+ions are conserved structural metal ions in GSK-3β and they interact with adenosine-triphosphate(ATP).They are very important in phosphoryl transfer in the kinase.In this paper,the effect of two Mg2+ions(Mg,Mg)on GSK-3β is illustrated.Mg2+can stabilize the conformation of GSK-3β and ATP.Without Mg2+,the stabilization of GSK-3β reduces explicitly and the conformation of ATP changes.Mgis important in the phosphorylation reaction while Mgis essential and Lys183 alone cannot maintain the conformation of ATP without the assistance of Mg.ATP forms intramolecular hydrogen bonds and adopts a folded conformation when both Mgand Mgare absent.
Key Words: GSK-3β kinase;Phosphoryl transfer;Mg2+;Structural metal ion;Molecular dynamics simulation
There are more than 500 protein kinase genes identified,representing about 1.7%of all human genes[1].In the large and very diverse family of protein kinases,glycogen synthase kinase-3(GSK-3)is of particular interest.It was originally identified in 1980 and was initially believed to phosphorylate and inactivate glycogen synthase(GS)which was the rate-limiting enzyme of glycogen biosynthesis[2].It is ubiquitously expressed in eukaryote[3-4].There are two major isoforms of GSK-3 in mammals:GSK-3β and GSK-3α,which are encoded by different genes.The kinase domain sequences of the two isoforms are almost the same and the main differences occur at the N and C termini[5-7].
Many different pathways have been described in which GSK-3β plays an important role.Historically GSK-3β fulfilled a significant role in the insulin/IGF1(insulin-like growth factor 1)and Wnt/Shaggy signaling pathways.However,recently it has become clear that GSK-3β is present in many other pathways such as those involving NGF(nerve growth factor)signaling,estradiol signaling,or reelin pathways[8].
GSK-3β phosphorylates many of its substrates via a primedphosphorylation mechanism,recognizing the canonical phosphorylation motif SXXXpS.This motif contains the phosphoaccepting Ser or Thr that is separated by three residues from a phospho-serine or phospho-threonine.The phosphorylation mechanism is called primed-phosphorylation because a different kinase must first phosphorylate the substrate at the P+4 position before GSK-3β can phosphorylate the P0 residue[9].
Since GSK-3β has more than 40 substrates and the list is still growing[10],it has being considered as one of the most promising drug targets for adult onset type 2 diabetes[11-13],stroke[14-15],neurodegenerative disorders(Alzheimer′s disease)[16-17],bipolar disorder[18],and schizophrenia[19-20],acute inflammatory processes[21],cancer[2223],and so forth.
Structural metal ions are important.They can influence the structure of kinases,and the binding of structural metal ions is energetically favored[24].Mg2+is conserved structural metal ion in GSK-3β.Experimental studies[8]on GSK-3β revealed that in GSK-3β,two Mg2+binding sites mainly involve the conserved residues Asn186 and Asp200,like PKA(protein kinase A),CDK(cyclin-dependent kinases)and other protein kinases.
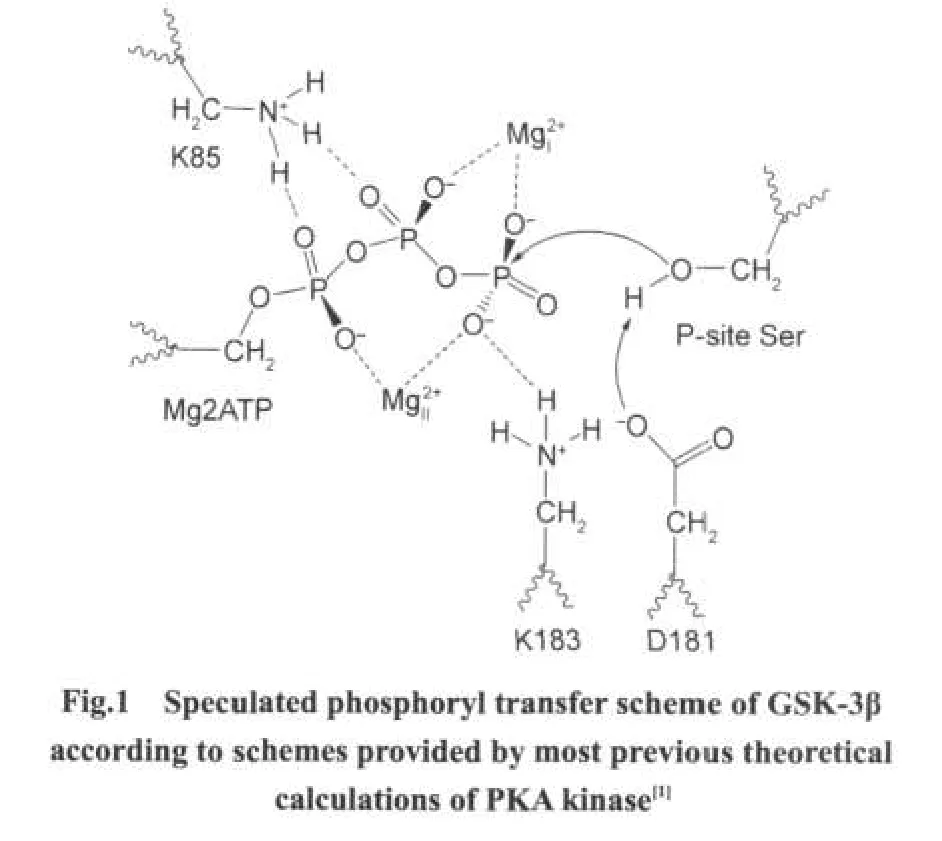
Many studies showed that Mg2+could increase the activity of GSK-3β,but the details of Mg2+function in GSK-3β still were indistinct.However,some useful information can be learned by referring the other kinase:PKA,because of the structural similarity[8].In PKA,Mg(Mg2+binding with β-and γ-phosphates of ATP)is generally identified as a catalytic activator,while Mg(Mg2+binding with α-and γ-phosphates of ATP)as an inhibitor[25-26].Ab initio studies[1]on PKA revealed that the phosphorylation reaction probably proceeded through a mainly dissociative transition state,and the conserved Asp166(corresponding to Asp181 in GSK-3β)served as the catalytic base to accept the late proton transfer,shown in Fig.1.That study also reported that both metal ions contributed greatly to lower the energy barrier through electrostatic interactions,and the catalytic role of Lys168(corresponding to Lys183 in GSK-3β)was demonstrated to keep ATP and substrate peptide in the near-attack reactive conformation[1,27-28].Because of the conservation of kinase domain structures,we can presume the functions of Mg2+in GSK-3β on the foundation of PKAstudies.In order to reveal the functions of Mg2+in GSK-3β,we performed computational studies on GSK-3β using molecular mechanical methods.In this paper,four enzyme complexes were investigated,which respectively contained two Mg2+,Mgonly,Mgonly,and no Mg2+.
1 Computational methods
1.1 Preparation of the systems
The structure of GSK-3β in a complex with ATP mimic AMP-PNP(PDB code:1PYX)was chosen as the initial structure.Absent residues on disordered loop of the crystal structure were added and the conformations of the residues were modeled using Loop Search module of Sybyl 6.8(Tripos Inc.).The structure of AMP-PNP was changed to that of ATP by replacing the nitrogen atom N3B in 1PYX with an oxygen atom.Four systems were prepared.System 1,complex-2Mg featured GSK-3β with ATP and two Mg2+ions.System 2,complex-MgI,featured GSK-3β with ATP and Mg,whileMgwas removed.System 3,complex-MgII featured GSK-3β with ATP and Mgwhile Mgwas removed.System 4,complex-noMg,featured GSK-3β withATP,while both Mgand Mgwere removed.
1.2 Molecular dynamics simulations
Molecular dynamics simulations were carried out on the four systems respectively,using the SANDER module of AMBER 9.0 with the Amber FF03[29-30]and GAFF force field[31].The parameters of ATP were provided by Amber web site[32].All simulations were carried out at neutral pH.Lys and Arg residues were positively charged,and Asp and Glu residues were negatively charged.Default His protonation state in AMBER9 was adopted.To maintain the electroneutrality of the systems,seven counterions(Cl-)were added into complex-2Mg;fivecounterions(Cl-)were added into complex-MgI and complex-MgII;and three counterions(Cl-)were added into complex-noMg.Every system was immersed in a 1 nm truncated octahedron periodic water box,and the structure water molecules were maintained.The box of water molecules in all systems contained around 13635 TIP3P[33]water molecules.A 2 fs time step was used in all simulations,and long-range electrostatic interactions were treated with the particle mesh Ewald(PME)procedure[34]with a 1 nm non-bonded cutoff.Bond lengths involving hydrogen atoms were constrained using the SHAKE algorithm[35].All systems were minimized prior to the production run.The minimization employed SANDER module under constant volume condition.The solvent molecules were firstly relaxed,while all heavy atoms in both protein and ATP were restrained with forces of 2.0×105kJ·mol-1·nm-2.Then,the systems were continually relaxed.All heavy atoms of the system were restrained with forces of 2.0×105kJ·mol-1·nm-2,except the atoms of the residues modeled by Loop Search module of Sybyl 6.8.Finally,all restraints were lifted and whole system was relaxed.The 3 steps above all featured 1000 cycles of steepest descent followed by 1000 cycles of conjugate gradient minimization.After the relaxation,300 ps of MD simulations were carried out at constant volume,with 4.0×103kJ·mol-1·nm-2restraint on solute.Then 2 ns of equilibration MD followed by 3 ns of production MD were respectively carried out on all systems at constant pressure(101325 Pa).All simulations were performed at 300 K.
2 Results and discussion
The root-mean-squared deviations(RMSD)value curves of backbone atoms during the MD simulation have been obtained.The curves in Fig.2 show that corresponding to the relaxation of the systems,the RMSD values of backbone atoms of the complex increase slowly before 1000 ps.And after 1000 ps,the RMSD values are fairly stable around 0.20 nm.The total potential energy fluctuates around a constant mean value after 2 ns.This indicates that the systems attain equilibrium.
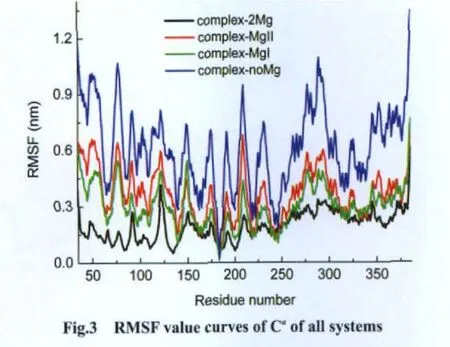
Mg2+can stabilize the structure of GSK-3β.RMS fluctuation(RMSF)values from structure provide an approach to evaluate the convergence of the dynamical properties of the system.As shown in Fig.3,the fluctuation values of complex-2Mg(black curve)are the lowest,while the values of complex-noMg(blue curve)are the highest.The fluctuation values of systems containing only one Mg2+are moderate,and the values of complex-MgI(green curve)are lower than that of complex-MgII(red curve).These indicate that Mg2+can stabilize GSK-3β,just like Mg2+in other kinases[26,36].Furthermore,we can conclude that Mgis more powerful than Mgin stabilizing GSK-3β,because of the lower fluctuation values of complex-MgI.

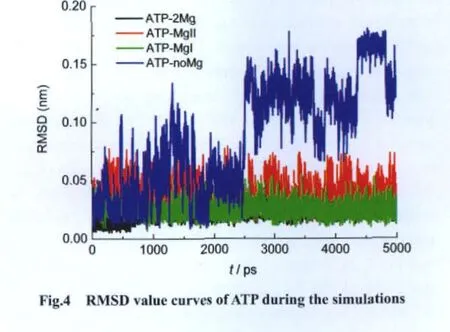
Mg2+can influence the conformation of ATP.The stability of conformation of ATP is essential to catalytic reaction[37-38].RMSD values of ATP in different systems during simulations confirm the importance of Mg2+and the necessity of Mg.As shown in Fig.4,the RMSD values of ATP of complex-2Mg(black curve)and complex-MgI(green curve)are stable around 0.025 nm,while the values of complex-MgII are stable around 0.050 nm(red curve).The RMSD values of ATP of complex-noMg(blue curve)increase continuously,corresponding to remarkable conformation change of ATP,which is adverse to phosphoryl transfer.
To facilitate phosphoryl transfer,ATP and substrates must keep the near-attack reactive conformations(in-line phosphoryl transfer mechanism)[38].The right conformation of ATP is guaranteed by the H-bond between γ-phosphate of ATP and conserved Lys183[1,39-40].As shown in Fig.5(a),in complex-2Mg,the oxygen atom on γ-phosphate of ATP can form H-bond with Lys183,and ATP can adopt right conformation.As shown in Fig.5(d),in complex-noMg,ATP moves away from phosphate transfer region and forms H-bond with Asn64 and Ser66,but without Lys183.As shown in Fig.5(b),in complex-MgII,ATP can form H-bond with Lys183,while as shown in Fig.5(c),in complex-MgI,ATP does not form H-bond with Lys183,but forms H-bond with Ser66.These indicate that Mgplays an important role in keeping the right position of γ-phosphate ofATP.
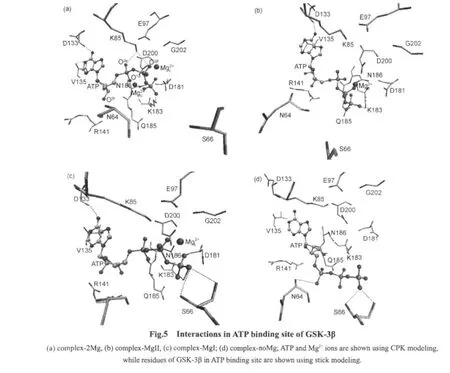
Mg2+can influence the interactions between ATP and Lys85.Lys85 is a conserved catalytic residue which anchors α-and βphosphate of ATP by H-bond.Experimental studies illustrated that if Lys85 was mutated to Arg,GSK-3β would lose its activity[37,41].Calculation studies showed that in kinase,PKA for example,this conserved Lys could strongly stabilize the transition state through electrostatic interactions during phosphoryl transfer[1].To investigate the effect of Mg2+on the interactions between ATP and Lys85,the distances between atoms were monitored:the distances between the oxygen atom of ATP,O1α,and the nitrogen atom of Lys85,NZ,are shown in Fig.6;the distances between the oxygen atom of ATP,O2β,and the nitrogen atom of Lys85,NZ,are shown in Fig.7.As shown in Fig.6 and Fig.7,in complex-2Mg(black curve)and complex-MgI(green curve),the distances between O1αand NZ,and between O2βand NZ are around 0.30 nm,which indicates that stable H-bond between ATP and Lys85 can form,referring to Fig.5(a)and Fig.5(c).In complex-MgII(red curve),the distances are more than 0.35 nm,which indicates that stable H-bonds between ATP and Lys85 can not form,referring to Fig.5(b).In complex-noMg(blue curve),the distances increase continuously,which indicates that ATP is moving away from Lys85,referring to Fig.5(d).The interactions between ATP and Lys85 are important to phosphoryl transfer.The important roles of Mgare evident,because the interactions are demolished when Mgions are absent.
Mg2+can influence the formation of the conserved H-bonds between adenine moiety of ATP and GSK-3β.The H-bonds between adenine moiety of ATP and Asp133,Val135 are conserved in kinase ATP binding sites[6].These H-bonds can strengthen the binding of ATP to kinases.As shown in Figs.5(a),5(b),and 5(c),in complex-2Mg,complex-MgII,and complex-MgI,the conserved H-bonds can form.As shown in Fig.5(d),in complex-noMg,the conserved H-bonds can not form,suggesting the drifting of ATP in binding site and the weakening ofATP binding.
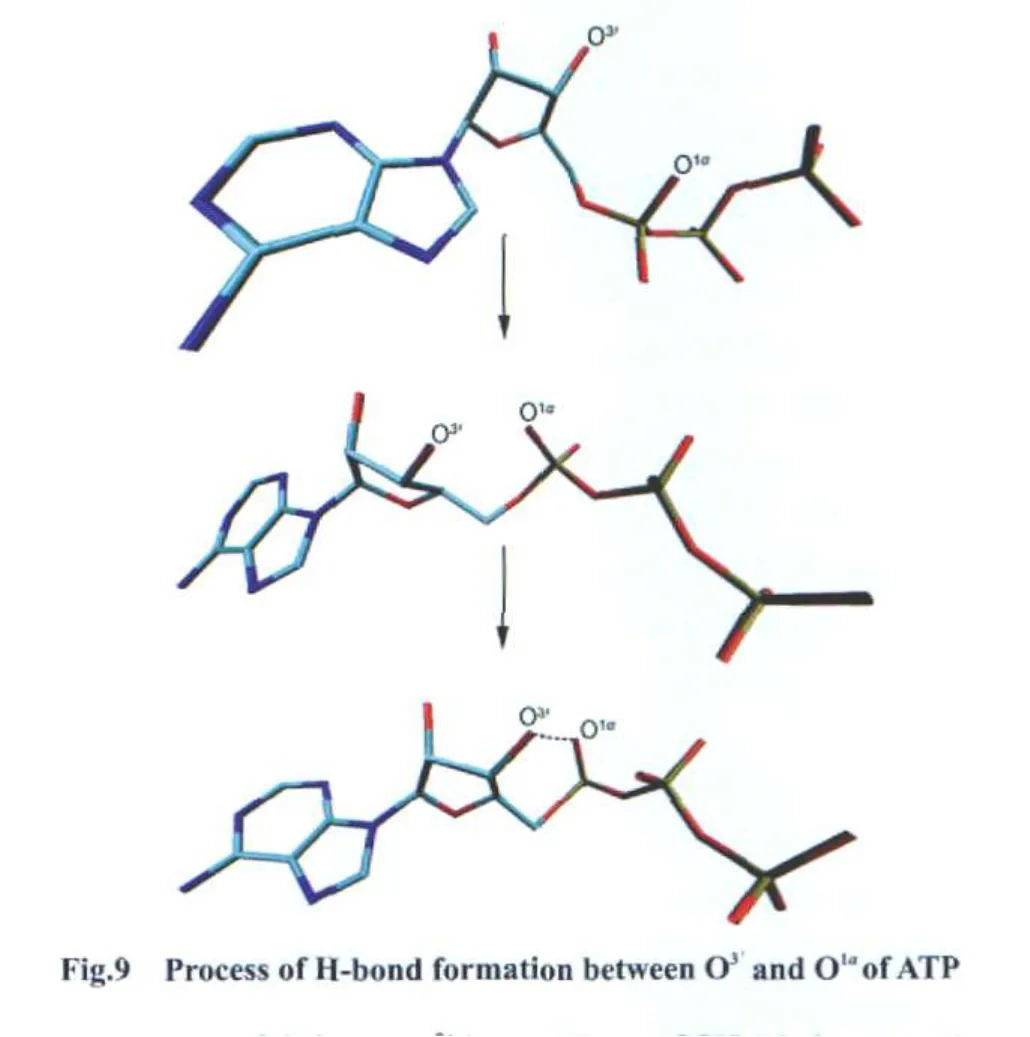
We found interesting phenomena during the simulations.Without Mg2+,ATP can form an intramolecular H-bond intermittently,like ATP in CheA histidine kinase[42].The distances between O3′and O1αwere monitored.As shown in Fig.8,in complex-2Mg(black curve),complex-MgII(red curve),and complex-MgI(green curve),the distances between O3′and O1αareabout 0.60 nm.H-bond can not form obviously.In complexnoMg(blue curve),the distances swing between 0.25 and 0.40 nm,indicating the intermittent forming of H-bond between O3′and O1α,Fig.9 shows the process of the H-bond formation.When this H-bond forms,ATP will adopt folded conformation,which is adverse to phosphoryl transfer.

3 Conclusions
Mg2+ions stabilize the structure of GSK-3β.Complex containing two Mg2+ions has the lowest RMSF values,while complex containing no Mg2+ion has the highest RMSF values.Mg2+Iis more powerful than Mgin stabilizing GSK-3β,because the RMSF values of complex-MgI are lower than those of complex-MgII.Mg2+can also stabilize the conformation of ATP.Without Mg2+,conformation of ATP will change remarkably and the in-line phosphoryl transfer mechanism will be demolished.Mgguarantees the interactions between ATP and Lys85,while Mgguarantees the right position of γ-phosphate of ATP.Without Mg2+,the conserved H-bonds between adenine moiety ofATP and GSK-3β can not form,and the binding of ATP will weaken.Without Mg2+,an intramolecular H-bond of ATP will form intermittently,which disturbs the catalytic reaction.Mg2+ions take an important role in GSK-3β.Mgseems more important than Mg,while Mgis not dispensable.
1 Cheng,Y.H.;Zhang,Y.K.;McCammon,J.A.J.Am.Chem.Soc.,2005,127:1553
2 Embi,N.;Rylatt,D.B.;Cohen,P.Eur.J.Biochem.,1980,107:519
3 Cross,D.A.;Alessi,D.R.;Cohen,P.;Andelkovich,M.;Hemmings,B.A.Nature,1995,378:785
4 Hoeflich,K.P.;Luo,J.;Rubie,E.A.;Tsao,M.S.;Jin,O.;Woodgett,J.R.Nature,2000,406:86
5 Sun,H.;Jiang,Y.J.;Yu,Q.S.;Zou,J.W.Acta Phys.-Chim.Sin.,2009,25:635 [孙 浩,蒋勇军,俞庆森,邹建卫.物理化学学报,2009,25:635]
6 Zhang,N.;Jiang,Y.J.;Zou,J.W.;Zhuang,S.L.;Jin,H.X.;Yu,Q.S.Proteins,2007,67:941
7 Zhang,N.;Jiang,Y.J.;Zou,J.W.;Zhang,B.;Wang,Y.H.;Yu,Q.S.Eur.J.Med.Chem.,2006,41:373
8 Martinez,A.;Castro,A.;Medina,M.Glycogen synthase kinase 3(gsk-3)and its inhibitors.New Jersey:Wiley,2006:51-54
9 Fiol,C.J.;Wang,A.;Roeske,R.W.;Roach,P.J.J.Biol.Chem.,1990,265:6061
10 Jope,R.S.;Johnson,G.V.Trends Biochem.Sci.,2004,29:95
11 Summers,S.A.;Kao,A.W.;Kohn,A.D.;Backus,G.S.;Roth,R.A.;Pessin,J.E.;Birnbaum,M.J.J.Biol.Chem.,1999,274:17934
12 Ross,S.E.;Erickson,R.L.;Hemati,N.;MacDougald,O.A.Mol.Cell.Biol.,1999,19:8433
13 Wagman,A.S.;Johnson,K.W.;Bussiere,D.E.;Curr.Pharm.Des.,2004,10:1105
14 Martinez,A.;Castro,A.;Dorronsoro,I.;Alonso,M.Med.Res.Rev.,2002,22:373
15 Schafer,M.;Goodenough,S.;Moosmann,B.;Behl,C.Brain Res.,2004,1005:84
16 Phiel,C.J.;Wilson,C.A.;Lee,V.M.;Klein,P.S.Nature,2003,423:435
17 Hernandez,F.;Perez,M.;Lucas,J.J.;Mata,A.M.;Bhat,R.;Avila,J.J.Biol.Chem.,2004,279:3801
18 Gould,T.D.;Zarate,C.A.;Manji,H.K.J.Clin.Psych.,2004,65:10
19 Emamian,E.S.;Hall,D.;Birnbaum,M.J.;Karayiorgou,M.;Gogos,J.A.Nat.Genet.,2004,36:131
20 Bhat,R.V.;Budd,H.S.L.;Avila,J.J.Neurochem.,2004,89:1313
21 Ghosh,S.;Karin,M.Cell,2002,109:81
22 Peifer,M.;Polakis,P.Science,2000,287:1606
23 Pap,M.;Cooper,G.M.J.Biol.Chem.,1998,273:19929
24 Diaz,N.;Suarez,D.Biochemistry,2007,46:8943
25 Ryves,W.J.;Dajani,R.;Pearl,L.;Harwood,A.J.Biochem.Biophys.Res.Commun.,2002,290:967
26 Herberg,F.W.;Doyle,M.L.;Cox,S.;Taylor,S.S.Biochemistry,1999,38:6352
27 Valiev,M.;Kawai,R.;Adams,J.A.;Weare,J.H.J.Am.Chem.Soc.,2003,125:9926
28 Diaz,N.;Field,M.J.J.Am.Chem.Soc.,2004,126:529
29 Duan,Y.;Wu,C.;Chowdhury,S.;Lee,M.C.;Xiong,G.;Zhang,W.;Yang,R.;Cieplak,P.;Luo,R.;Lee,T.J.Comput.Chem.,2003,24:1999
30 Lee,M.C.;Duan,Y.Proteins,2004,55:620
31 Wang,J.;Wolf,R.M.;Caldwell,J.W.;Kollamn,P.A.;Case,D.A.J.Comput.Chem.,2004,25:1157
32 Meagher,K.L.;Redman,L.T.;Carlson,H.A.J.Comput.Chem.,2003,24:1016
33 Jorgensen,W.L.;Chandrasekhar,J.;Madura,J.;Klein,M.L.J.Chem.Phys.,1983,79:926
34 Darden,T.;York,D.;Pedersen,L.J.Chem.Phys.,1993,98:10089
35 Ryckaert,J.P.;Ciccotti,G.;Berendsen,H.J.C.J.Comput.Phys.,1977,23:327
36 Adams,J.A.;Taylor,S.S.Biochemistry,1992,31:8516
37 Hao,S.;Jiang,Y.J.;Yu,Q.S.;Luo,C.C.;Zou,J.W.Biochem.Biophys.Res.Commun.,2008,377:962
38 http://dx.doi.org/10.1007/s00894-010-0738-0
39 Hanks,S.K.;Quinn,A.M.Methods Enzymol.,1991,200:38
40 Knighton,D.R.;Cadena,D.L.;Zheng,J.H.;Teneyck,L.F.;Taylor,S.S.;Sowadski,J.M.;Gill,G.N.Proc.Natl.Acad.Sci.U.S.A.,1993,90:5001
41 Gómez-Sintes,R.;Hernandez,F.;Avila,J.;Gotteland,J.P.;Zaratin,P.;Lucas,J.J.SENC.Rev.Neurol.,2005,41:71
42 Zhang,J.;Xu,Y.H.;Shen,J.H.;Luo,X.M.;Chen,J.G.;Chen,K.X.;Zhu,W.L.;Jiang,H.L.J.Am.Chem.Soc.,2005,127:11709
分子动力学模拟研究结构金属镁离子在GSK-3β激酶磷酸化中的作用
孙 浩1蒋勇军2,*俞庆森3高 慧3
(1西南林业大学,昆明650224;2浙江大学宁波理工学院分子设计与营养工程市重点实验室,浙江宁波315100;3浙江大学化学系,杭州310027)
糖原合成酶激酶-3β(GSK-3β)是一种丝氨酸/苏氨酸蛋白激酶,调节糖原合成酶的活性,并在生物体内的多条信号通路中发挥作用.GSK-3β是糖尿病,肿瘤,急性炎症,早老性痴呆等多种复杂疾病的药物作用靶标.Mg2+是GSK-3β激酶的保守结构金属离子,与三磷酸腺苷(ATP)分子作用,在激酶的磷酸化中扮演重要的角色,本文阐明了两个Mg2+离子(Mg,Mg)在激酶磷酸化中的作用:Mg2+稳定GSK-3β与ATP的构象.缺乏Mg2+离子,GSK-3β结构的柔性增强,同时ATP的构象发生改变,相对Mg离子而言,Mg离子在磷酸化反应中的作用更突出,但Mg离子也是必不可少的,如果没有Mg离子,Lys183无法独立稳定ATP的合适构象.当两个Mg2+离子都不存在时,ATP形成分子内的氢键,成为一种折叠的构象.
GSK-3β激酶; 磷酸化;Mg2+; 结构金属离子; 分子动力学模拟
O641
Received:September 1,2010;Revised:October 15,2010;Published on Web:November 30,2010.
∗Corresponding author.Email:yjjiang@nit.zju.edu.cn;Tel:+86-574-88229517.
The project was supported by the National High Technology Research and Development Program of China(863)(2007AA02Z301),National Natural Science Foundation of China(20803063),Natural Science Foundation of Ningbo,China(2010A610024),and Key Scientific Research Foundation of Southwest Forestry University,China(110932).
国家高技术研究发展计划(863)(2007AA02Z301),国家自然科学基金(20803063),宁波市自然科学基金(2010A610024)及西南林业大学重点科研基金(110932)资助项目

