Self-Assembly of 3-Aminopropyltrimethoxysilane to Improve the Efficiency of Dye-Sensitized Solar Cells
LAO Chun-Feng CHU Zeng-Ze ZOU De-Chun,*
(1Researchand Development Center of Haier Group,Qingdao 266103,Shandong Province,P.R.China;2College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,P.R.China)
Self-Assembly of 3-Aminopropyltrimethoxysilane to Improve the Efficiency of Dye-Sensitized Solar Cells
LAO Chun-Feng1CHU Zeng-Ze2ZOU De-Chun2,*
(1Researchand Development Center of Haier Group,Qingdao 266103,Shandong Province,P.R.China;2College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,P.R.China)
Abstract: A dye-sensitized solar cell(DSSC)based on a 3-aminopropyltrimethoxysilane(APTS)-modified TiO2electrode was fabricated.This cell generated a short current of 18.32 mA·cm-2,an open voltage of 775.9 mV,and its overall photo-to-electricity conversion efficiency was 9.15%under 100 mW·cm-2white light irradiation from a xenon lamp.The three DSSC parameters for the bare TiO2electrode were found to be 18.08 mA·cm-2,749.9 mV,and 7.70%.Compared with the unmodified solar cell,the overall conversion efficiency improved by 18.8%and the fill factor improved from 0.57 to 0.64.This improvement is attributed to the inhibition of the back reaction at the interface between the semiconductor and the electrolyte.The dark current-applied voltage curve shows that the onset voltage shifts from-0.30 to-0.40 V,which indicates a reduction in defects and surface states on the TiO2surface because of the presence of APTS.Furthermore,special experiments were conducted to investigate the interaction among TiO2,APTS,and the cis-Ru(dcpyH2)2(SCN)2dye.In these experiments,APTS and the dye were self-assembled onto a TiO2electrode in layers.The interaction was characterized by X-ray photoelectron spectroscopy(XPS).Qualitative and quantitative results showed that the―OCH2CH3was partially removed and it formed mono-bridge or bi-bridge Si―O―Ti bonds.The cis-Ru(dcpyH2)2(SCN)2dye adsorbed onto APTS through an electrostatic interaction between―COOH and―NH2from the dye.FT-IR spectra further confirmed this inner interaction.
Key Words:Dye-sensitized solar cell;3-Aminopropyltrimethoxysilane;Self-assembly;TiO2;X-ray photoelectron spectroscopy
1 Introduction
Dye-sensitized solar cells(DSSCs)have attracted much attention ever since power conversion efficiency reached the high value of 10%.1,2In a Grätzel solar cell,a nano-crystalline semiconductor plays an important role.Several oxides,such as TiO2,1ZnO,3SnO2,4Fe2O3,5Nb2O5,6ZrO2,7Al2O3,8etc.,have been studied for photoelectrical conversion.Among them,TiO2has shown the best performance when using cis-Ru(dcpyH2)2(SCN)2as the sensitiser.In DSSCs,for the lack of space charge layer,9the charge transport from the excited dye to the back contactbecomesakeyproblemthatinfluencestheoverallphototo-current efficiency(η).The charge lost may come from two aspects:one is the photo-injected electron back reaction with triodide ions in the electrolyte,10and the other is the presence of an electron acceptor,such as oxygen and iodine,which leads to the loss of the photo-generated electron at the interface between the nanocrystal of TiO2and the electrolyte layer during the transport of electrons to the back contact.11
Traditionally,an inorganic semiconductor or insulating metal oxides which act as a blocking layer were employed to modify TiO2.12-14They act as shells to protect the electron from back reaction.The reported organic material that modifies the TiO2electrode is the 4-tert-butylpyridine.2After treatment for 15 min,the electrode showed better light-to-electricity conversion efficiency as the fill factor and open voltage improved.This mechanism proposes that the state of Ti(IV)ions at the surface of TiO2interacts with the pyridine derivative.And also,there are reports that using La3+15and Ho3+16to optimize the electron injection process.
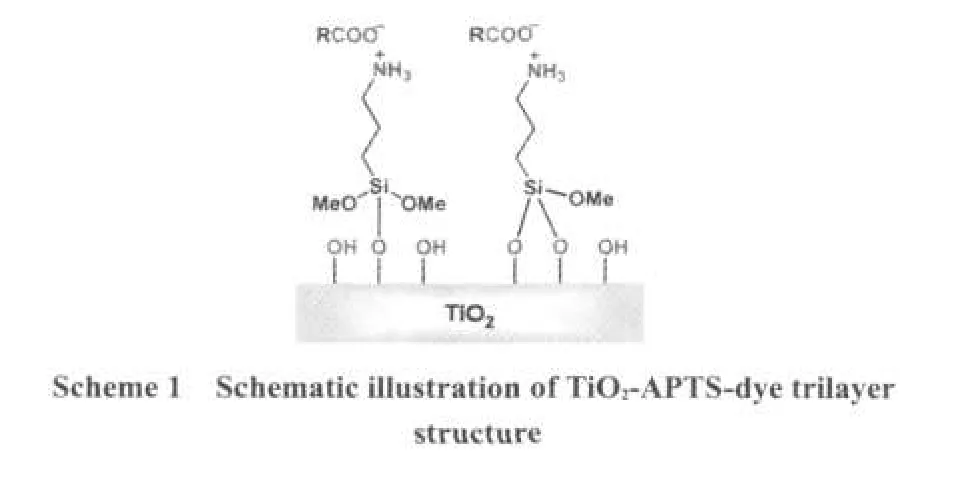
Self-assembly(SA)has been widely used since 1997.17In SA,3-aminopropyltrimethoxysilane(APTS)is often used to functionalize the surface of silica,18zeolite,19Pt,Au particle,etc.In these works,APTS usually serves as the source of the amino group(―NH2),which is the most significant component in electrostatic self-assembly.APTS-modified titania has a good performance in catalysis.20The effect of APTS on DSSCs has already been reported.21In this study,we employed APTS as a blocking layer to modify TiO2photoelectrode and further fabricated the dye-sensitized solar cell.The design idea is not only to separate dye layer and TiO2film using APTS layer to retard electron recombination at interface,but also to improve the surface properties of TiO2film.We also noticed that a similar work was reported very recently.22In contrast to their post-modification using APTS after N3-sensitization of TiO2photoanode,our method is to directly functionalize the surface of TiO2film with APTS,and then coat dye molecules onto APTS-modified TiO2electrode,as illustrated in Scheme 1.
2 Experimental
2.1 Materials
Titanium tetra-isopropoxide(Ti(i-OC3H7)4),4-tert-butylpyridine,propylene carbonate(PC),3-aminopropyltrimethoxysilane(APTS),and poly(ethylene glycol)(PEG,MW=20000)were purchased from Acros.An optically transparent conducting glass(CTO,F-doped SnO2)was obtained from Asahi Glass Co.Ltd.,Japan.cis-Ru(dcpyH2)2(SCN)2(Ru535)was taken from solaronix SA.Other chemicals and solvents used in the experiment were at least reagent grade(crc-bj.com Inc.,China)and were used without further purification.Tetrabutylammonium perchlorate(TBAP),lithium perchlorate,and acetonitrile were dried before using them in electrochemical measurement.
2.2 TiO2and modified TiO2electrode
The TiO2colloid was prepared following the procedure indicated in the literature.2The TiO2electrode was prepared in the same manner as we reported before23but with some modifications this time.The TiO2colloid was cast on the CTO by means of the doctor-blade method.The as-cast films were heated at 450°C for 30 min in air.This procedure was repeated for five times until the thickness reached 10 μm.After washing with 0.1 mol·L-1TiCl4for four times,the films were rinsed with deionised water and were heated at 450°C for another 30 min.
The prepared-TiO2electrode was immersed in the solution of APTS(dissolved in dichloromethane,6.625×10-3mol·L-1)when it was still hot(ca 80°C),and it was kept in this solution for 20 min.Then it was washed with dichloromethane for five times and dried at 80°C in an oven for 10 min.The APTS-modified and unmodified TiO2electrode were immersed in 3×10-4mol·L-1cis-Ru(dcbpyH2)2(SCN)2for 24 h,and then they were used to assemble the DSSCs.The counter electrode,a 2-μm thick Pt on CTO glass,was placed directly on top of the TiO2electrode,and together,they were clipped tightly.The electrolyte,which was composed of 0.5 mol·L-1LiI,0.1 mol·L-14-tert-butylpyridine,and 0.05 mol·L-1I2in acetonitrile/PC(volume ratio 1/1),was attracted into the space by capillary force.
2.3 Equipments and methods
The UV absorption spectra were measured by means of a UV-Vis spectrophotometer V-550(JASCO,Japan).The prepared TiO2nano-particle was analyzed by X-ray diffraction(XRD)using CuKαradiation at 40 kV and 100 mA,and a graphite monochromator.In addition,it was scanned at a 2θof 2(°)·min-1with a diffractometer(Model Dmax-2000,Rigaku Co.,Tokyo,Japan)to determine the phase of the crystalline products.The morphology of the products was observed using transmission electron microscopy(TEM,Model JEM-200CX,JEOL,Ltd.,Tokyo,Japan).The thickness of films was determined with a DEKTAK 3 profilometer(Vecco,USA).The APTS-modified TiO2electrodes and the self-assembly of Ru535 on them were characterized with X-ray photoelecton spectrameter(XPS).The XPS data were taken on an AXISUltra instrument from Kratos Analytical(England)using monochromatic AlKαradiation(225 W,15 mA,15 kV)and lowenergy electron flooding for charge compensation.To compensate for surface charges effects,binding energies were calibrated using C 1shydrocarbon peak at 284.80 eV.
The chemical structures of the dye and the dye on APTS were measured on a FT-IR microscope(Nicolet Magna-IR 750,Nicolet NicPlan IR Microscope,UK)with the detector of MCT/A.
AM1.5 solar light was simulated by a 500-W Xe lamp,with the L-42(Toshiba,Japan)filter to cut off the light with a wavelength of less than 420 nm,while IRA-25S(Toshiba,Japan)removed the infrared radiation.The intensity of the light was determined by the Multi function optical Meter Mode 1835-C(Newport,USA).
The photocurrent density-voltage curve was measured by Multimeter 2000(Keithley,USA)which was controlled by a computer system.The effect of the applied voltage on the dark current-voltage was measured in a three-electrode system.TiO2or APTS-modified TiO2,platinum wire,and Ag/AgCl functioned as the working electrode,counter electrode,and reference electrode,respectively.The electrolyte solution was 0.2 mol·L-1TBAP in CH3CN containing 0.1 mol·L-1LiClO4.Potential control was carried out on a model 600 voltammetric analyser(CH Instruments,USA).The amount of adsorbed dye was determined by means of desorption in a 0.01 mol·L-1NaOH solution with methanol as the solvent.
3 Results and discussion
3.1 Current-voltage characteristics
Fig.1 shows the photocurrent density-voltage curve.After treatment with APTS,the fill factor(FF)greatly improved from 0.57 to 0.64.Also,the open-circuit voltage(Voc)increased from 749.9 to 775.9 mV,while there was a little shift from 18.08 to 18.32 mA·cm-2in short current density(Isc).As a result,the photo-to-electricity conversion efficiency(η)improved by 18.8%from 7.70%to 9.15%,as shown in Table 1.
The fill factor(FF)is defined as


where,VoptandIoptcorrespond to the voltage and current density when the output power efficiency is in the maximum.
The photo-to-electricity conversion efficiency is defined as

where,Pinis the light intensity.
It shoud be pointed out that all the data related to fill factor and conversion efficiency were not corrected by the absorption and reflection of the CTO(F-doped SnO2)substrate glass to the light in the range of 420-800 nm.
In Fig.1,the inset shows the light transmittance(TL)-wavelength(λ)curve of the CTO glass in the range of 420-800 nm.The CTO glass used here was more opaque than others(usually>90%in the visible region).Photo flux(P(W·m-2))was obtained through the following expression:

where,F(λ)is the incident photo flux density at wavelengthλ,andTL(λ)is the transmittance of the CTO glass at wavelengthλ.According to equation(3),at the irradiation of 100 mW·cm-2,the intensity of light that reaches the interface of the TiO2and the CTO glass is only 71.4 mW·cm-2.Because short-circuit current density is almost linear to light intensity,2,12if the transmittance could reach the value of 90%,the short-circuit current density will reach the value of 23.08 and 22.78 mA·cm-2for the APTS-modified and unmodified TiO2electrode,respectively.If there is not muches change in the open voltageand fill factor,the conversion efficiency of the solar cells made from the TiO2electrode will reach as high as 9.7%,and the ATPS-modified solar cells will reach an efficiency as high as 11.5%.

Table 1 Performance parameters of the cells based on the TiO2electrode and theAPTS-modified TiO2electrode
The high short-circuit current densities could be partly attributed to the spectrum of the Xe lamp we used.Fig.2 illustrates the spectral comparison of Xe lamp and AM1.5 solar emission.The wavelength range of Xe lamp is mainly from 450 to 700 nm,and this region matches properly with the spectral region in which our solar cells can harvest sun flux more efficiently and perform better,which can be seen from monochromatic incident photon-to-electron conversion efficiency(IPCE)-wavelength curve.This probably contributes to the high short-circuit current densities.

3.2 Self-assembly ofAPTStosuppress back reaction
A little decrease in dye adsorption of the ATPS-modified TiO2film(1.013×10-7mol·cm-2)was found when it was compared to the unmodified TiO2film(1.368×10-7mol·cm-2).However,there is a little shift in short-circuit photocurrent,fill factor,open-circuit voltage,and overall conversion efficiency.This suggests that the decrease in dye adsorption is compensated by the fact that APTS makes the TiO2film favourable in the electron transport,12and restrains back reaction at the semiconductor electrolyte junction.The back reaction mainly arises from the reduction of triiodide by the conduction band electron despite the existence of the monolayer of Ru535.

This occurs when the small-size triiodide penetrates the nanosized pores that the dye cannot cover.As can be seen in Fig.3,after being modified with APTS,the onset of dark current was shifted by-0.10 V from-0.30 to-0.40 V,which means that the dark current from the combination of the electron in the conduction band of TiO2with triiodide or other surface state has been suppressed.APTS is smaller in size as compared to Ru535,so it can be easily filled into the pores.As a result,the back reaction caused by triiodide and the conduction band electron could be partly avoided.For general regenerative photoelectrochemical systems,the following relation holds:24,25


where,Iinjis the flux of charge resulting from sensitized injection,ncbis the concentration of electrons at the TiO2surface,[I-3]represents the concentration of the I-3ions and ketis the rate constant for triiodide reduction.After being covered by APTS,the product of ncb,ket,and[I-3]was reduced,which led to the increase of the open-circuit photovoltage according to Eq.(5).The surface states of Ti(IV)are more active in charge transfer.2,26After being partly covered by APTS,these surface states were mostly blocked.The fill factor and short-circuit photocurrent density were consequently improved for the suppression of back reaction.Just as Kay et al.reported,13in metal oxide functionalised mesoporous SnO2solar cells,the insulating layer has an optimum thickness of only a few angstroms.If the concentration of APTS in dichloromethane is too dense,or if the TiO2film is treated for an excessively long time,the short-circuit photocurrent decreases greatly.Therefore,the concentration and treatment time in our experiment were optimised.This can be contributed to the much higher conducting band in APTS.If the APTS layer is too thick,the electron will not be able to penetrate this layer and be injected into the conduction band of TiO2.Also,too much APTS will block the adsorption of the dye,which is the most important for a higher photocurrent.
3.3 Characteristics of a dye-sensitized APTS-modified TiO2electrode
In APTS-modified DSSCs,the interaction among the dye,APTS,and TiO2film is the most important.In unmodified DSSCs,however,the dye coordinates with the TiO2surface through the carboxylate group in bridging the badentate mode in F type,27-29and it uses two of its four carboxylic acid groups.In previous study,the interaction between APTS and TiO2was thoroughly investigated.20,30After slightly being heated,APTS was inclined to lose methoxy and interacted with TiO2to form the covalent bond of Si―O―Ti.In water solution,APTS acted with carboxylic acid in electrostatic force,17,31,32as also provenin this system.After immersing in APTS,the interaction among the dye,APTS,and TiO2surface is demonstrated,as shown in Scheme2.
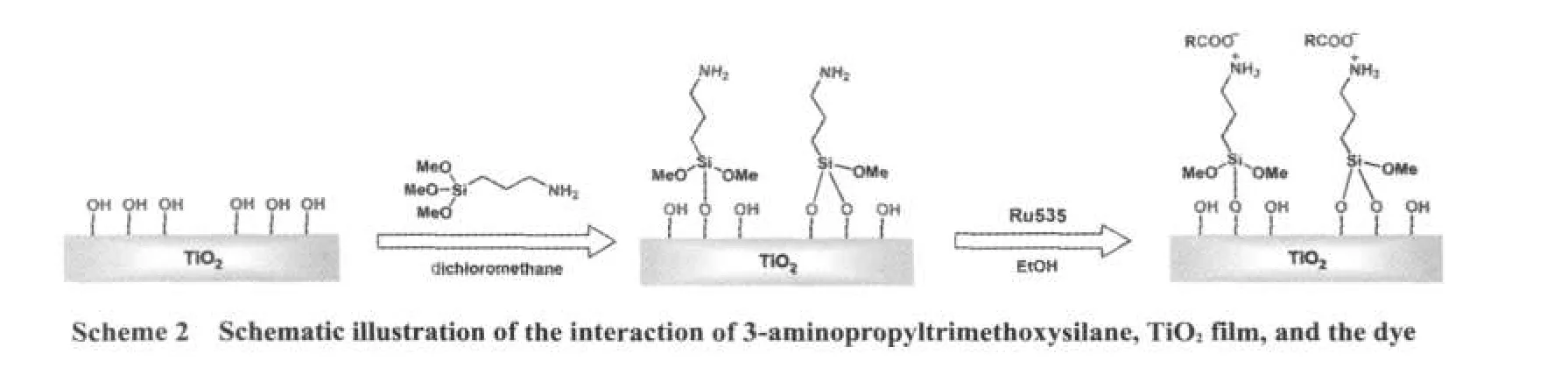

XPS proved to be an efficient method to characterize APTS-modified multi-layer films.33,34Under anhydrous condition,APTS can act with inorganic oxide in three possible modes,35such as mono-bridge,bi-bridge,and tri-bridge modes.Because APTS and the dye have a monolayer self-assembly,the signals are not big enough to be distinguished from each other in a dye-sensitized TiO2electrode.It is even difficult to analyze the element with ICP.In order to investigate the interaction by XPS,new experiments were conducted.Thin films of TiO2(10 nm)were prepared by means of spin-coating on CTO substrate,36and then immersing them in the solution of APTS in dichloromethane with the concentration of 6.625×10-3mol·L-1for 5 min.After which,the films were dried at 80°C in an oven for 10 min.Dye sensitising for 20 min was carried out in the same concentration just as described above.
The XPS spectra of the APTS-modified TiO2film and dyesensitized APTS-modified TiO2film are shown in Fig.4.In the ATS-modified TiO2film,the signals of Si 2p,N 1s,and C 1sappeared.The atomic concentration ratio(in Table 2)of C,O to Si(or N)revealed that the―OCH2CH3was partially removed,and APTS was adsorbed onto the TiO2surface by the Si―O―Ti bond.The bottom part shows the XPS spectrum of the dye-sensitized APTS-modified TiO2film,with peaks of 280.71,284.81,and 162.35 eV which correspond to the binding energy of Ru 3d5,Ru 3d3,and S 2p,respectively.Also,the atomic concentration in Table 1 reveals that the dye was adsorbed ontoAPTS by part of its―COOH.
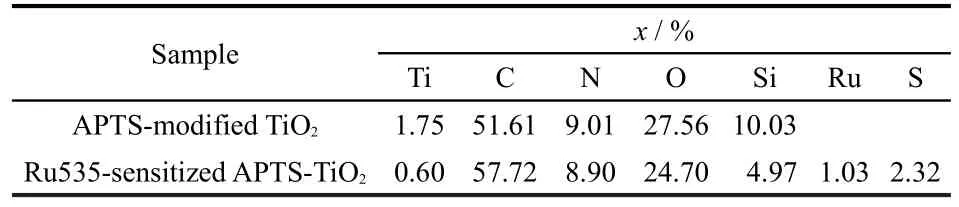
Table 2 XPS atomic percentages(x)forAPTS-modified TiO2and dye-sensitizedAPTS-TiO2
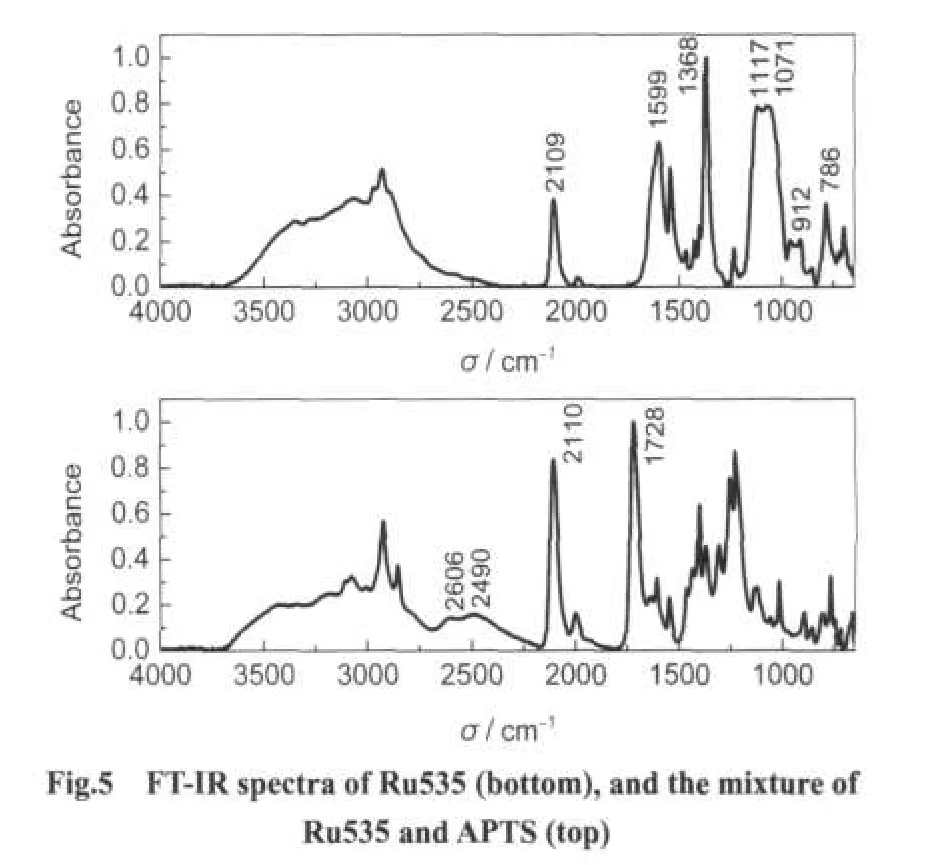
As illuminated in Scheme 2,the dye acted with APTS by means of electrostatic force.Fig.5 shows the FT-IR spectra of Ru535,and the mixture of Ru535 and APTS.A band with intense peak centered at 1728 cm-1corresponded toνC=Oof carboxylic acid in Ru535.After acting with APTS,the peak moved to 1599 cm-1,which corresponded to the asymmetric―COO-stretch.Moreover,the broad band with the peaks of 2490 and 2606 cm-1almost disappeared after acting with APTS,which is another characteristic of carboxylic acid.APTS can also be identified(by order of descending energy)as Si―O―C stretch(intense peaks centred at 1117 and 1071 cm-1),Ti―O―Si stretch(centred at 912 cm-1),and N―H bend(786 cm-1).
4 Conclusions
In summary,it was found that APTS acted with the TiO2film in the form of covalence,and with the dye of Ru535 by means of electrostatic force.This self-assembly APTS layer reduced the defects and surface states of TiO2.It also inhibited the back reaction at the semiconductor electrolyte junction.As a result,modifying the TiO2electrode with APTS increased the short-circuit photocurrent,the open-circuit photovoltage,and the fill factor.All of these caused the overall power conversionefficiency to increase by 18.8%.
APTS acts as insulating layer in DSSCs,it may improve cell stability under UV irradiation.The band gap of anatase TiO2is 3.2 eV,and it can absorb light below 388 nm,which leads to the formation of strongly oxidised valence band holes that can oxidise the solvent irreversibly and result in an unrecoverable loss of.The APTS-modified TiO2film reduces the chance of valence band holes′reaction with I-ions and may be used to fabricate UV-resisted DSSCs.
The electrostatic force between Ru535 and APTS is not so stable as the covalent interaction of Ru535 and TiO2film in liquid-state DSSCs.The experiments show that the APTS-modified solar cell is not so stable as the unmodified solar cell.However,further research work may be done to change the weak leakage to the covalence bond.Also,when used in solidstate dye-sensitized solar cells,the APTS-modified TiO2electrode may show a more outstanding performance.
(1) O′Regan,B.;Grätzel,M.Nature 1991,353,737.
(2) Nazeeruddin,M.K.;Rodicio,I.;Humphry-Baker,R.;Muller,E.;Liska,P.;Vlachopoulos,N.;Grätzel,M.J.Am.Chem.Soc.1993,115,6382.
(3)Keis,K.;Bauer,C.;Boschloo,G.;Hagfeldt,A.;Westermark,K.;Rensmo,H.;Siegbahn,H.J.Photochem.Photobio.A 2002,148,57.
(4) Stergiopoulos,T.;Arabatzis,I.M.;Cachet,H.;Falaras,P.J.Photochem.Photobio.A 2003,155,163.
(5) Fitzmaurice,D.J.;Frei,H.Langmuir 1991,7,1129.
(6) Hara,K.;Horiguchi,T.;Kinoshita,T.;Sayama,K.;Sugihara,H.;Arakawa,H.Sol.Energy Mater.Sol.Cells 2000,64,115.
(7) Heimer,T.A.;D′Arcangelis,S.T.;Farzad,F.;Stipkala,J.M.;Meyer,G.J.Inorg.Chem.1996,35,5319.
(8) Nüesch,F.;Moser,J.E.;Shklover,V.;Grätzel,M.J.Am.Chem.Soc.1996,118,5420.
(9)Hagfeldt,A.;Grätzel,M.Chem.Rev.1995,95,49.
(10)Peter,L.M.;Wijayantha,K.G.U.Electrochem.Commun.1999,1,576.
(11) Rensmo,H.;Lindstrom,H.;Sodergren,S.;Willstedt,A.K.;Solbrand,A.;Hagfeldt,A.;Lindquist,S.E.J.Electrochem.Soc.1996,143,3173.
(12)Wang,Z.S.;Huang,C.H.;Huang,Y.Y.;Hou,Y.J.;Xie,P.H.;Zhang,B.W.;Cheng,H.M.Chem.Mater.2001,13,678.
(13)Kay,A.;Grätzel,M.Chem.Mater.2002,14,2930.
(14) Palomares,E.;Clifford,J.N.;Haque,S.A.;Lutz,T.;Durrant,J.R.J.Am.Chem.Soc.2003,125,475.
(15) Zhang,L.;Ren,Y.J.;Cai,S.M.Electrochemistry 2002,8,27.[张 莉,任焱杰,蔡生民.电化学,2002,8,27.]
(16)Yang,S.M.;Kou,H.Z.;Wang,L.;Wang,H.J.;Fu,W.H.Acta Phys.-Chim.Sin.2009,25,1219.[杨术明,寇慧芝,汪 玲,王红军,付文红.物理化学学报,2009,25,1219.]
(17) Decher,G.Science 1997,277,1232.
(18) Lee,C.H.;Lin,T.S.;Mou,C.Y.J.Phys.Chem.B 2003,107,2543.
(19) Mukhopadhyay,K.;Phadtare,S.;Vinod,V.P.;Kumar,A.;Rao,M.;Chaudhari,R.V.;Sastry,M.Langmuir 2003,19,3858.
(20)Kominami,H.;Itonaga,M.;Shinonaga,A.;Kagawa,S.;Konishi,S.;Kera,Y.Stu.Sur.Sci.Cat.2002,143,1089.
(21) Lao.C.F.Researches on the Efficiency of Dye-Sensitized Solar Cells.Ph.D.Dissertation,Peking University,Beijing,2006. [劳春峰.染料敏化太阳能电池效率问题的研究[D].北京:北京大学,2006.]
(22) Zhang,J.;Yang,G.T.;Sun,Q.;Zheng J.;Wang,P.Q.;Zhu,Y.J.;Zhao,X.Z.J.Ren.Sust.Energy 2010,013104.
(23) Lao,C.F.;Chuai,Y.T.;Su,L.;Liu,X.;Huang,L.;Cheng,H.M.;Zou,D.C.Sol.Energy Mater.Sol.Cells 2004,85,457.
(24) Rosenblut,M.L.;Lewis,N.S.J.Phys.Chem.1989,93,3735.
(25)Kumer,A.;Santangelo,P.G.;Lewis,N.S.J.Phys.Chem.1992,96,835.
(26) Moser,J.;Punchihewa,S.;Infelta,P.P.;Grätzel,M.Langmuir 1991,7,3012.
(27) Nazeruddin,M.K.;Humphry-Baker,R.;Liska,P.;Grätzel,M.J.Phys.Chem.B 2003,107,8981.
(28)Rensmo,H.;Westermark,K.;Södergren,S.;Kohle,O.;Persson,P.;Lunell,S.;Siegbahn,H.J.Chem.Phys.1999,111,2744.
(29)Westermark,K.;Rensmo,H.;Lees,A.C.;Vos,J.G.;Siegbahn,H.J.Phys.Chem.B 2002,106,10108.
(30)Chang,C.C.;Chen,W.C.J.Polym.Sci.A:Polym.Chem.2001,39,3419.
(31) Bertrand,P.T.;Jonas,A.;Laschewsky,A.;Legras,R.Macromol.Rapid.Commun.2000,21,319.
(32) Kumar,A.;Mandale,A.B.;Sastry,M.Langmuir 2000,16,6921.
(33) Jarrais,B.;Silva,A.R.;Freire,C.Eur.J.Inorg.Chem.2005,4582.
(34) Noh,J.;Ito,E.;Nakajima,K.;Kim,J.;Lee,H.;Hara,M.J.Phys.Chem.B 2002,106,7139.
(35) Lin,J.;Siddiqui,J.A.;Ottenbrite,R.M.Polym.Adv.Technol.2001,12,285.
(36)Arago,A.C.;Johnson,L.R.;Bliznyuk,V.N.;Schlesinger,Z.;Carter,S.A.;Hörhold,H.H.Adv.Mater.2000,12,1689.
3-氨基丙基三甲氧基硅烷自组装提高染料敏化太阳能电池的效率
劳春峰1初增泽2邹德春2,*
(1海尔集团技术研发中心,山东青岛266103;2北京大学化学与分子工程学院,北京100871)
以3-氨基丙基三甲氧基硅烷(APTS)修饰的二氧化钛为负极制备的染料敏化太阳能电池在100 mW·cm-2的模拟太阳光照下的短路电流、开路电压、光电转换效率分别为18.32 mA·cm-2、775.9 mV、9.15%.而没有经过ATPS修饰的电池三项性能参数分别为18.08 mA·cm-2、749.9 mV、7.70%,修饰后电池的光电转换效率提高了18.8%,同时填充因子由0.57提高为0.64.暗电流-电压曲线显示起始电压从-0.30 V变化到-0.40 V,表明二氧化钛电极和电解液之间的暗反应得到了有效抑制,APTS作为阻挡层减少了二氧化钛电极表面的缺陷与表面态.另外,通过实验设计,将APTS与染料层-层自组装于二氧化钛电极上,通过X射线光电子能谱(XPS)研究了二氧化钛层、APTS、染料的作用形式.定性与定量结果表明:APTS中的乙氧基部分脱除后形成了Si―O―Ti单桥或者双桥键,钌染料cis-Ru(dcpyH2)2(SCN)2通过分子中的部分―COOH与APTS中的―NH2形成的静电作用力吸附在TiO2电极上.傅里叶变换红外(FT-IR)光谱的结果进一步证明了这种分子间作用.
染料敏化太阳能电池;3-氨基丙基三甲氧基硅烷; 自组装; 二氧化钛;X射线光电子能谱
O649
Received:September 13,2010;Revised:November 24,2010;Published on Web:December 22,2010.
∗Corresponding author.Email:dczou@pku.edu.cn;Tel:+86-10-62759799.
The project was supported by the National Natural Science Foundation of China(50125310,90401028)and National Key Basic Research Program of China(973)(2002CB613405).
国家自然科学基金(50125310,90401028)及国家重点基础研究发展规划项目(973)(2002CB613405)资助

