Hg2+荧光传感器的设计及合成
祖福丽,李 茜,宋 盼,王晨娟,徐括喜
(河南大学 化学化工学院,河南 开封 475004)
Hg2+荧光传感器的设计及合成
祖福丽,李 茜,宋 盼,王晨娟,徐括喜
(河南大学 化学化工学院,河南 开封 475004)
设计合成了一种新颖的基于萘荧光基团的Hg2+荧光传感器. 通过荧光光谱滴定实验研究了其对Hg2+、Li+、 Na+、K+、Zn2+、Co2+、Ni2+、Cu2+、Fe2+、Mn2+、Cr3+和 Fe3+等金属离子的选择性识别能力. 结果表明,该传感器在生理pH = 7.4的H2O-DMSO中对Hg2+表现出较高的选择性,并且形成1∶1的配合物;主客体相互作用荧光猝灭的络合常数为(9.07±0.41)×104.
荧光;化学传感器;Hg2+;设计;合成
Over the last few decades, among the heavy and transition metal (HTM) ions, Hg2+has been consi-dered as the most toxic heavy metal ion and a common pollutant which possesses severe risks for human health and environment[1-3]. Although traditional analytical techniques like atomic absorption spectrometry (AAS), atomic fluorescence spectrometry (AFS) and inductively coupled plasma-mass spectrometry (ICP-MS) have been applied to detect the concentration of Hg2+, the wide utilization of these methods is largely limited due to the expensive equipment and consumed time for the procedures of preparing samples[4-5]. In view of these sophisticated experimental methods, many efforts have been devoted to pursue an innovative and convenient optical chemosensor for the detection of Hg2+which can offer high sensitivity and selectivity[6-9]. In optical sensors, the use of fluorescence as detecting method offers distinct advantages in terms of sensitivity, selectivity and response time. Fluorescent sensors have attracted considerable interest because of their intrinsic sensitivity and selectivity[10-11]. Fluorescent sensors consist in a recognition moiety (ionophore) linked to a transducting moiety (fluorophore). The choice of the fluorophore is of major importance because it governs the recognition event into an optical signal owing to the change of its photophysical characteristics due to the perturbation by the bound cation of various photoinduced processes (electron transfer, energy transfer, charge transfer). The recognition moiety is responsible for the efficiency and selectivity of binding. Comparing with traditional approaches for Hg2+analysis relied on complicated sample preparation or sophisticated instrumentation, fluorogenic chemosensors provide a rapid, facile, and sensitive tool for Hg2+detection.
Although many fluorescent sensors have been designed for Hg2+sensing, these sensons lack the suitability for commercial and practical uses due to multi-step syntheses, high costs of starting materials or high detection limits of Hg2+[12-13]. Besides, they often suffer from cross-sensitivity toward other ions, particularly potential competitors such as copper (Cu2+) and lead (Pb2+) due to their similar chemical behavior to Hg2+[14].
Therefore, for practical applications, it is necessary to develop Hg2+sensors which are easily prepared and possess selective and sensitive signaling in protic solutions. Herein, we design and synthesize a novel fluorescent sensor1a(Fig.1), of which the two naphthyls are used as signal transduction and the two amide groups and pyridine as binding sites. We expect1acan recognize Hg2+by changing its fluorescent intensity in aqueous solution. For comparison,1bbearing benzol moiety should be also designed as a comparative compound.
1 Experimental
1.1 Apparatus and materials
1H NMR spectra were recorded on a Bruker AV-400HZ spectrometer. High resolution mass spectra (HRMS) were measured on a Agilent 1290 LC-6540 Accurate Mass Q-TOF using electrospray ionization (ESI). Fluorescent spectra were obtained with Hitachi F-7000 FL. Melting point was determined with a Mel-TEMP melting-point apparatus. The regents used were purchased from commercial supplies and employed without further purification, and double-distilled water was used throughout the experiments.
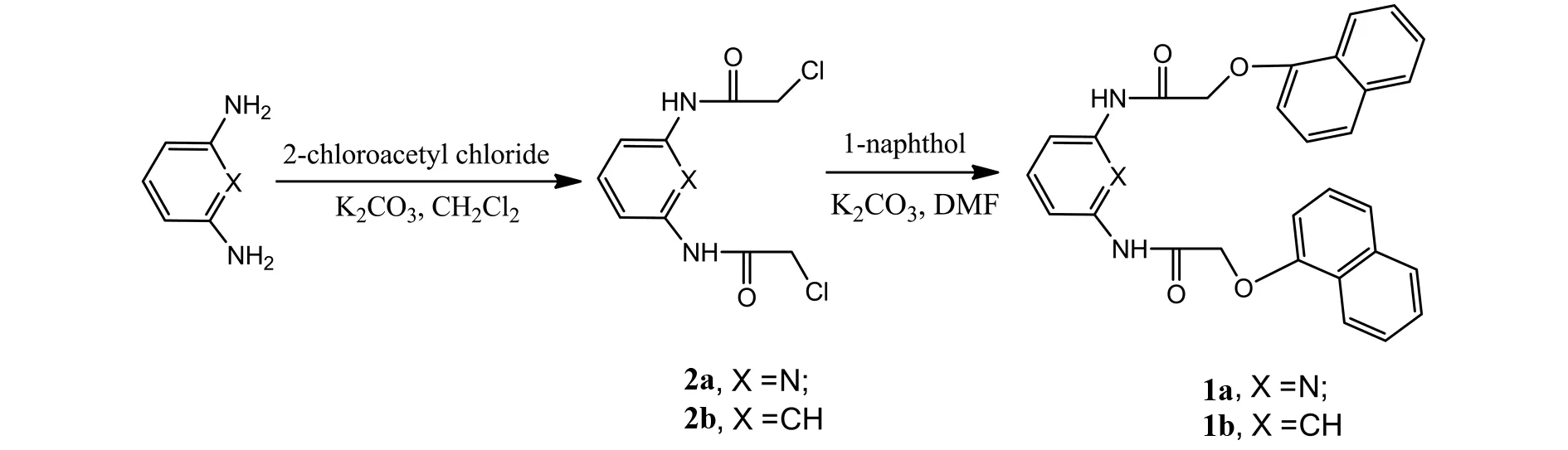
Fig.1 Synthesis of the compounds 1a, 1b
1.2 Synthesis of compounds 2a and 2b
2,6-Pyridinediamine orm-phenylenediamine (1.0 mmol) was dissolved in 30 mL of dried chloroform and cooled in the ice bath. 0.35 g (2.5 mmol) anhydrous K2CO3was placed in the solution as the base. 2-chloroacetyl chloride (0.27 g, 2.4 mmol) was added to the mixture drop-by-drop within 30 min. The mixture was then stirred at room temperature for 3 h. After filtration, the filtrate was collected and washed with 20 mL 10% HCl, saturated NaHCO3and water respectively. The organic layer was dried over anhydrous Na2SO4and the solvent was evaporated under reduced pressure. The residue was then purified by column chromatography on silica gel using CHCl3/MeOH (50∶1,V/V) as eluent to obtain pure product2a(86%).1H NMR (CDCl3, 400 MHz)δ: 8.64 (br, 2H), 7.96 (d,J= 8.0 Hz, 2H), 7.78 (t,J= 8.0 Hz, 1H), 4.20 (s, 4H). Elemental analysis calcd (%) for C9H9Cl2N3O2: C 41.24, H 3.46, N 16.03, Found: C 41.12, H 3.51, N 15.96.2b(92%).1H NMR (CDCl3, 400 MHz)δ: 8.44 (br, 2H), 8.02 (s, 1H), 7.87 (d,J= 7.6 Hz, 2H), 7.47 (t,J= 7.6 Hz, 1H), 4.23 (s, 4H). Elemental analysis calcd (%) for C10H10Cl2N2O2: C 46.00, H 3.86, N 10.73, Found: C 45.94, H 3.88, N 10.68.
1.3 Synthesis of compound 1a and 1b
The synthesis procedure of compound(1aor1b) would be explained as follow. 0.5 mmol compound2awas dissolved in 10 mL dried chloroform (containing 2 mL dried DMF), 0.28 g (2 mmol) anhydrous K2CO3, then a solution of 0.15 g (1.1 mmol) 1-naphthol dissolved in 10 mL dry chloroform (containing 2 mL dried DMF) was added to the mixture drop-by-drolp. After that, the solution was stirred at 60 ℃ for 8 h, monitoring with TLC. When completed, the mixture was poured into ice-water, adjusted to weak acid by using 10% dilute HCl, and extracted by CH2Cl2. The organic phase was washed with saturated NaHCO3(3×50 mL), water (3×50 mL), and then dried with anhydrous Na2SO4. After removing the solvent by rotary evaporation, the crude product was purified by silica column chromatography by using CH2Cl2/MeOH (50∶1,V/V) as eluent to get white product.
1a: Yield, 76.4%, m.p. 219-220 ℃.1H NMR (DMSO-d6, 400 MHz)δ: 10.52 (s, 2H), 8.27 (d,J= 4.8 Hz, 2H), 7.89 (d,J= 4.6 Hz, 2H), 7.82 (t,J= 7.8 Hz, 2H), 7.76-7.72 (m, 3H), 7.56-7.51 (m, 4H), 7.41 (d,J= 8.0 Hz, 2H), 6.91 (d,J= 7.6 Hz, 2H), 5.00 (s, 4H);13C NMR: 166.90, 152.80, 148.76, 141.09, 134.72, 127.91, 126.90, 125.74, 122.36, 121.42, 110.61, 110.07, 106.06, 68.11; HRMSm/z: calculated for C29H23N3O4, [M+Na]+requires 500.158 1, found 500.158 2, [M+H]+requires 478.176 1, found 478.176 2 . IR: 3 401, 1 680, 1 587, 1 243 cm-1,
1b: Yield, 83.7%, m.p. 213-214 ℃.1H NMR (DMSO-d6, 400 MHz)δ: 10.30 (s, 2H), 8.31 (d,J= 4.8 Hz, 2H), 8.03 (s, 1H), 7.89 (d,J= 4.6 Hz, 2H), 7.55-7.50 (m, 5H), 7.43-7.37 (m, 4H), 7.28 (t,J= 8.0 Hz, 2H), 6.92 (d,J= 7.6 Hz, 2H), 4.91 (s, 4H);13C NMR: 166.58, 152.78, 137.54, 134.69, 129.84, 127.95, 126.88, 125.76, 125.19, 122.33, 121.22, 116.67, 111.90, 106.25, 68.23; HRMSm/z: calculated for C30H24N2O4, [M+H]+requires 477.180 9, found 477.180 8, [M+Na]+requires 499.162 8, found 499.162 7, IR: 3 409, 1 694, 1 609, 1 238 cm-1.
1.4 Binding experiments
General procedure of the fluorescent measurement: Metal ions were prepared in H2O-DMSO (9∶1,V/V, 0.01 mol/L HEPES-HCl buffer, pH=7.4). Compound1aor1bwas dissolved in DMSO, and then diluted to 0.1 mol/L with buffered H2O (0.01 mol/L HEPES-HCl, pH=7.4). The test solutions were prepared by adding different volumes of metal ions solution to a series of test tubes, and then the same amount of stock solution of compound1aor1bwas added to each of the test tubes and diluted to 3.0 mL with H2O (0.01 mol/L HEPES-HCl buffer, pH=7.4). After shaken for several minutes, the test solutions were analyzed immediately.
2 Results and discussion
2.1 Synthesis
As shown in Fig.1, intermediates2aand2bwere prepared at high yield by reacting 2,6-pyridinediamine orm-phenylenediamine with 2-chloroacetyl chloride in double molar ratio in dried chloroform. The structure was confirmed via1H NMR,13C NMR and mass spectroscopy.
2.2 Fluorescent titration studies
The fluorescent spectra of sensor1aand1bwere studied by using a solution (3.0×10-5mol/L) of H2O-DMSO (9∶1,V/V, 0.01mol/L HEPES-HCl buffer, pH=7.4) in the absence and presence of various chloride salts of Li+, Na+, K+, Hg2+, Zn2+, Co2+, Ni2+, Cu2+, Fe2+, Mn2+, Fe3+and Cr3+. Sensor1aexhibited a fluorescent emission at about 416 nm (λex=360 nm) in the buffered H2O. As shown in Fig.2, when Hg2+was added, the fluorescence of1awas significantly decreased. A good sensor system is important to attain a high selectivity. Thus, we tested our sensor with possible interferences of other metal ions in the same conditions. All metal ions caused negligible changes in the fluorescent change of sensor1a(Fig.2). Sensor1a, therefore, can act as a “turn-off” sensor for Hg2+and has high selectivity toward Hg2+detection. In particular,1aillustrated the high selectivity for Hg2+over Cu2+and Pb2+which are potential competitors and revealed a greater affinity over several previously reported Hg2+sensors[15].

Fig.2 Fluorescent spectra changes of sensor 1a (3.0×10-5 mol/L) measured in H2O-DMSO system (9∶1, V/V, 0.01mol/L HEPES-HCl buffer, pH=7.4) upon the addition of 5.0 equivalent of various metal ions (λex = 360 nm, λem = 416 nm)
Titration of sensor1a(3.0×10-5mol/L) with Hg2+in H2O-DMSO system (9∶1,V/V, 0.01mol/L HEPES-HCl buffer, pH=7.4) by using 0-5.0 equiv. Hg2+was explored. To determine the quantitative sensitivity range and get more information on binding mechanism of1atoward Hg2+, spectrometric titration experiments were performed in the presence of Hg2+. Fig.3a showed the gradual changes in fluorescent intensity of1aupon progressive addition of Hg2+in various concentration ranges. The addition of 4 equiv. of Hg2+showed quenching, which was close to 100% (Fig.3a). The fluorescent behavior of1aclearly demonstrated the ON-OFF switching mechanism which occurred in response to Hg2+ion complexation. In the absence of Hg2+ions, the fluorescence response was at a maximum and fluorescence ‘turn-off’ took place as the increasing of Hg2+concentration. The quenching effect of1atoward Hg2+may be ascribed to a photo-induced electron transfer process from the excited state of naphthyl moieties in1ato the paramagnetic heavy metal ion Hg2+, which was widely reported in some fluorescence quenching chemosensors for Hg2+[16-17]as shown in Fig.4. The excitation of1a·Hg2+complex corresponded to charge transfer from the excited fluorophore to the Hg2+centers and thus provided a pathway for nonradiative deactivation of the excited state.


Fig.3 (a) Fluorescent spectra of sensor 1a (3.0×10-5mol/L) with Hg2+ in H2O-DMSO (0.01 mol/L HEPES at pH=7.4), equiv. of ion: 0→5.0, λex= 360 nm. (b) Changes in the fluorescent intensity of sensor 1a at 416 nm upon the addition of Hg2+. The line shown is a line-fitted curve. The non-linear curve fitting association constant (Kass) is (9.07±0.41)×104 L/mol and the correlation coefficient (R) is 0.993 4
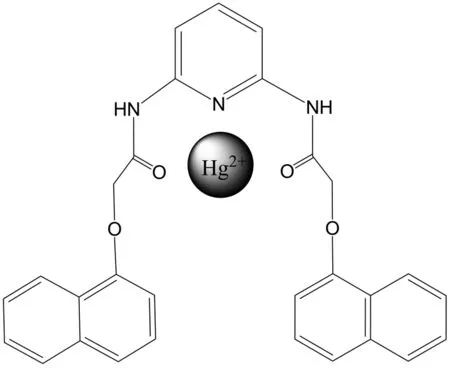
Fig.4 Proposed binding model of 1a with Hg2+
The binding constant (K) of complexes of aforementioned fluorescent sensor with Hg2+was determined by means of titration fluorimetry. With the assumption of a 1∶1 stoichiometry, the complexation of Hg2+(G) with sensor (H) was expressed by Equation (1):


Fig.5 The determination 1a of the detection limit (LOD) for Hg2+ in HEPES buffer
Under the conditions employed,the association constant (Kass) can be calculated by using Equation (2) from the Origin 7.5 software package[18]. The satisfactory non-linear curve fitting (the correlation coefficient was over 0.99, see inset of Fig.3) indicated that sensor10and Hg2+ions formed a 1∶1 complex. WhereIwas the fluorescence intensity,cHandcGwere concentrations of sensor1aand of the Hg2+ion, andc0was the initial concentration of sensor1a. The result of the non-linear curve fitting indicated that sensor1aand Hg2+formed a 1∶1 complex. Thus, the sensor1acould be used as fluorescent sensors to determine readily Hg2+between various metal ions. It was also found that1ashowed 1.24×10-5mol/L of detection limit able to sufficiently sense the Hg2+concentration in H2O-DMSO system(9∶1,V/V, 0.01 mol/L HEPES-HCl buffer, pH=7.4, Fig.5).
To get another fluorescent sensor, we usedm-phenylenediamine as the starting material to synthesize compound1b. The sensing of compound1btowards Hg2+was compared with that of sensor1atowards Hg2+. When compound1bwas treated with Hg2+under the same conditions, the emission change was negligible.
2.31H NMR studies
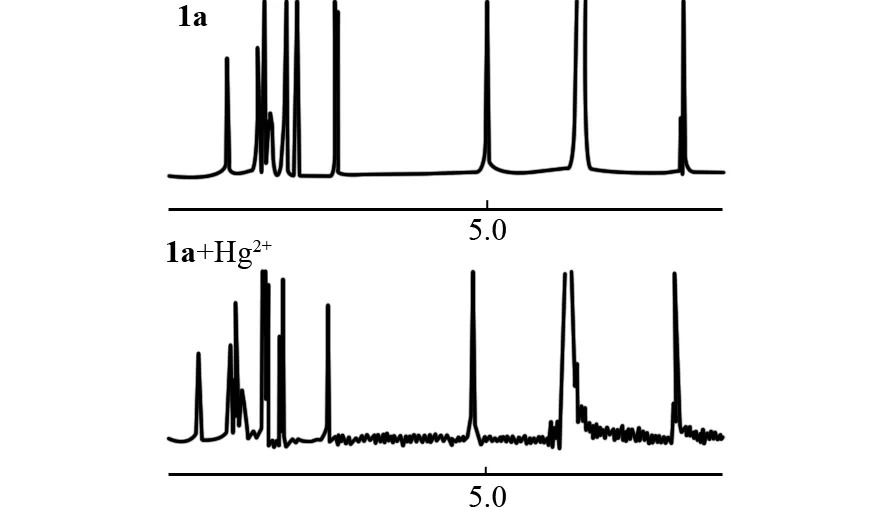
Fig.6 1H NMR spectra of 1a (4.0 mmol/L) in the presence of 1.0 equiv. of Hg2+ in D2O-DMSO-d6 (1∶1, V/V)
To further elucidate the binding mode of sensor1awith Hg2+ion, the1H NMR titration experiments were carried out in D2O-DMSO-d6(1∶1,V/V). The spectral differences are depicted in Fig.6. The results showed that down-field shifts were observed for most of the protons of sensor1a. The protons of methlene of sensor1awere shifted downfield by 0.17 in the presence of 1.0 equiv. of Hg2+. The results suggested the binding of sensor1ato Hg2+formed a rigid conjugation system through interactions with amide group and the nitrogen of pyridine (Fig.6).
3 Conclusion
In conclusion, we designed and synthesized a new fluorescent sensor1aat a significantly high yield. The experiments showed a higher sensitivity and selectivity of sensor1atoward Hg2+than those of other tested metal ions via the PET process (Fig.4) in H2O-DMSO (9∶1,V/V). The readily accessible synthetic sensor presented here was distinguished in terms of synthetic simplicity, efficient synthetic route, low detection limit for the determination of Hg2+. The results indicated that the carbonyl group and the nitrogen of pyridine could affect the binding of Hg2+to the sensor. This type fluorescence responding behavior of the sensor could serve as a new potential platform for commercial uses and significant developments for future sensor systems.
[1]SOLID P D, ZHAO J X. Sensing mercury for biomedical and environmental monitoring [J]. Sensors, 2009, 9: 5446-5459.
[2]YAO W Y , XU K X , KONG H J, et al. Novel naphthalene-based fluorescent chemosensors for Cu2+and Fe3+in aqueous media [J]. Supramol Chem, 2013, 25: 146-150.
[3]CHENG P F , XU K X , YAO W Y, et al. Novelfluorescent chemosensors based on carbazole for Cu2+and Fe3+in aqueous media [J]. J Lumin, 2013, 143: 583-586.
[4]ZHAO Y, ZHONG Z J. Tuning the sensitivity of a foldamer-based mercury sensor by its eolding energy [J]. J Am Chem Soc, 2006, 128: 9988-9989.
[5]YU C, TSENG W. Colorimetric detection of mercury(II) in a high salinity solution using gold nanoparticlescapped with 3-mercaptopropionate acid and adenosine monophosphate [J]. Langmuir, 2008, 24: 12717-12722.
[6]KELLER L O, KALLIS K T, FIEDLER H L J. Nano-fin based mercury-sensor for environmental surveillance [J]. Nanosci Nanotechnol, 2010, 10: 5921-5925.
[7]GOSWAMI S, AICH K, SEN D. Acridine-based switching on fluorescence sensor for Cd2+functioning in absolute aqueous media [J]. Chem Lett, 2012, 41: 863-865.
[8]CHO Y, LEE S S, JUNG J H. Recyclable fluorimetric and colorimetric mercury-specific sensor using porphyrin-functionalized Au@SiO2core/shell nanoparticles [J]. Analyst, 2010, 135: 1551-1555.
[9]ZHOU Y, ZHU C, GAO X, et al. Hg2+-Selective ratiometric and “off-on” chemosensor based on the azadiene-pyrene derivative [J]. Org Lett, 2010, 12: 2566-2569.
[10]CHENG P F, XU K X, YAO W Y, et al. Novel fluorescent chemosensors based on tryptophan unit for Cu2+and Fe3+in aqueous solution [J]. Chem Res Chin Univ, 2013, 29: 642-646.
[11]孔华杰,祖福丽,李 茜,等. 水溶液中对Cr3+高选择性荧光化学传感器的研究[J]. 化学研究, 2014, 25(1): 93-96.
[12]YANG X, GE J, XU Q, et al. A selective, sensitive probe for mercury(ii) ions based on oxazine-thione [J]. Tetrahedron Lett, 2011, 52: 2492-2495.
[13]NOLAN E M, LIPPARD S J. Tools and tactics for the optical detection of mercuric ion [J]. Chem Rev, 2008, 108: 3443-3480.
[14]JEONG Y, YOON J. Recent progress on fluorescent chemosensors for metal ions [J]. Inorg Chim Acta, 2012, 381: 2-14.
[15]WANICHACHEVA N, PRAPAWATTANAPOL N, LEE V S, et al. Hg2+-induced self-assembly of a naphthalimide derivative by selective “turn-on” monomer/excimer emissions [J]. J Lumin, 2013, 134: 686-690.
[16]LERAY R I, VALEUR B. Lead and mercury sensing by calixarene-based fluoroionophores bearing two or four dansyl fluorophores [J]. Chem Eur J, 2004, 10: 4480-4490.
[17]KUMAR M, KUMAR R, BHALLA V. A reversible fluorescent Hg2+/K+switch that works as keypad lock in the presence of F-ion [J]. Chem Commun, 2009: 7384-7386.
[18]VALEUR B, POUGET J, BOURSON J, et al. Tuning of photoinduced energy transfer in a bichromophoric coumarin supermolecule by cation binding [J]. J Phys Chem, 1992, 96: 6545-6549.
date:2014-03-06.
Supported by Technology Department of Henan Province (132300410055, 132102210388) and the Education Department of Henan Province of China (14A150050).
Biography:ZU Fuli (1988-), female, postgraduate, majoring in supramolecular chemistry and molecular biological sensors.*
, E-mail: xukx@henu. edu. cn.