核壳型PS/CdS复合催化剂的制备及其光催化性能*
王 涵,许 倩,郑 星,韩文清,江 波,孙冠华 ,钟彩霞,尹华承,郑经堂
[1.中国石油大学(华东)重质油国家重点实验室,山东 青岛 266580;2.包头轻工职业技术学院,内蒙古 包头 014045;3.北京中能环科技术发展有限公司,北京 100080]
·研究论文·
核壳型PS/CdS复合催化剂的制备及其光催化性能*
王 涵1,2,许 倩1,郑 星3,韩文清2,江 波1,孙冠华1,钟彩霞2,尹华承1,郑经堂1
[1.中国石油大学(华东)重质油国家重点实验室,山东 青岛 266580;2.包头轻工职业技术学院,内蒙古 包头 014045;3.北京中能环科技术发展有限公司,北京 100080]

核-壳结构;CdS;超声化学法;合成;光催化性能
在众多杂化材料中,核壳结构因其独特的组成和排列方式,以及多重纳米粒子属性而备受科学家关注[1]。研究表明,通过设计核壳结构的核-壳,利用两者间的互补效应,可改善材料的表面及形态结构,提高材料的整体性能[2-4]。半导体CdS具有较窄的禁带宽度(2.42eV)和可见光敏感性,已广泛应用于光电领域。国内外学者通过Layer-by-Layer(LBL)[5]、微波辅助法[6]、辐照法[7]、静电自组装法[8,9]和原子转移自由基聚合(ATRP)[10]等方法制备了核壳型有机/CdS材料。
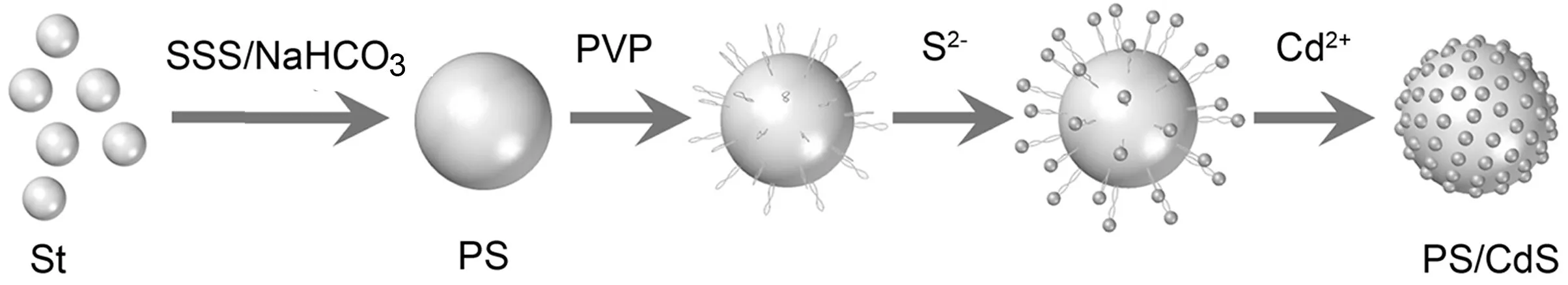
Scheme 1
超声化学法是利用超声波引发的液-固非均匀体系反应,可有效控制晶体生长和颗粒团聚,得到粒度分布窄的超细颗粒。由于该方法易于控制、周期短和效率高,已被用于制备包括核壳结构在内的多种纳米单体及复合材料,如:Au/Pd[11],Au/Ag[12],PS/ZnS[13],ZnO/CdS[14],CdSe/ZnS[15],SiO2/FePt[16],ZnO/ZnS[17]和Fe3O4/SiO2[18]。
1 实验部分
1.1 仪器与试剂
GS54T型紫外-可见分光光度计(UV-Vis);Thermo Nicolet NEXUS型红外光谱仪;X′Pert Pro MPD型X-射线衍射仪;S4800型扫描电镜;JEM-2100UHR型电子透射镜。
所用试剂均为分析纯,国药集团化学试剂有限公司;实验用水为自制去离子水。
1.2 合成
(1)单分散PS微球的制备[19]
N2保护下,在反应瓶中加入水200mL,对苯乙烯磺酸钠(SSS)35.6mg和NaHCO3140.8mg,搅拌使其混合均匀;升温至70℃,快速加入苯乙烯(St),反应30min;加引发剂过硫酸钾(KPS)288.0mg,反应28h。冷却至室温得PS乳液A。



不加PS乳液A,用类似的方法制得CdS。
1.3 光催化活性
在反应瓶中加入RhB(10mg·mL-1)100mL和c(1)25.0mg,于暗室吸附反应30min使其达到物理吸附平衡。在800W·m-2的35W氙灯冷光源照射下,每隔一定时间取样,共反应70min。离心,取上层清液于554nm处测定其吸光度(A)。
2 结果与讨论
2.1 反应机理
表面活性剂PVP由疏水骨架与亲水侧链组成。其结构中无酸性质子,但有两个电子基中心(-C=O和吡咯环)。由于N原子的空间限制,-C=O被认为是最有利的连接位点[20]。因此,PVP一端的非极性亚甲基与PS聚合物接枝共聚,硫代乙酰胺(TAA)在超声过程中缓慢释放S2-与伸出PS粒子表面的PVP另一端极性丁内酰胺基团的-C=O配位键合,使镉源提供的Cd2+与S2-仅在PS微球表面反应生成CdS,直至形成均匀包覆层并逐渐增厚。PVP作为偶联剂,通过增强壳层与内核之间的相互作用,成功地将PS与CdS复合起来形成杂化的三维核壳结构PS/CdS纳米复合材料1,如Scheme 1所示。
2.2 反应条件优化
以1的形貌,即1的SEM和TEM照片为指标,对其反应条件进行优化,寻找最佳的反应条件。



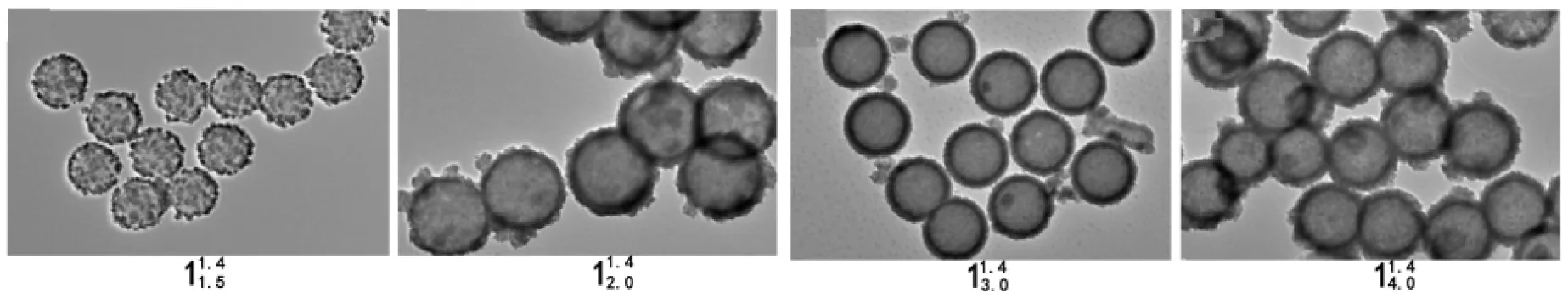


(1)r


由此可见,PS微球表面包覆了纳米CdS粒子,形成了核壳结构;当r=1.4,可得到完整均一包覆的1,即最佳的r=1.4。
(2)t


2.3 表征
(1)FT-IR

ν/cm-1

(2)XRD

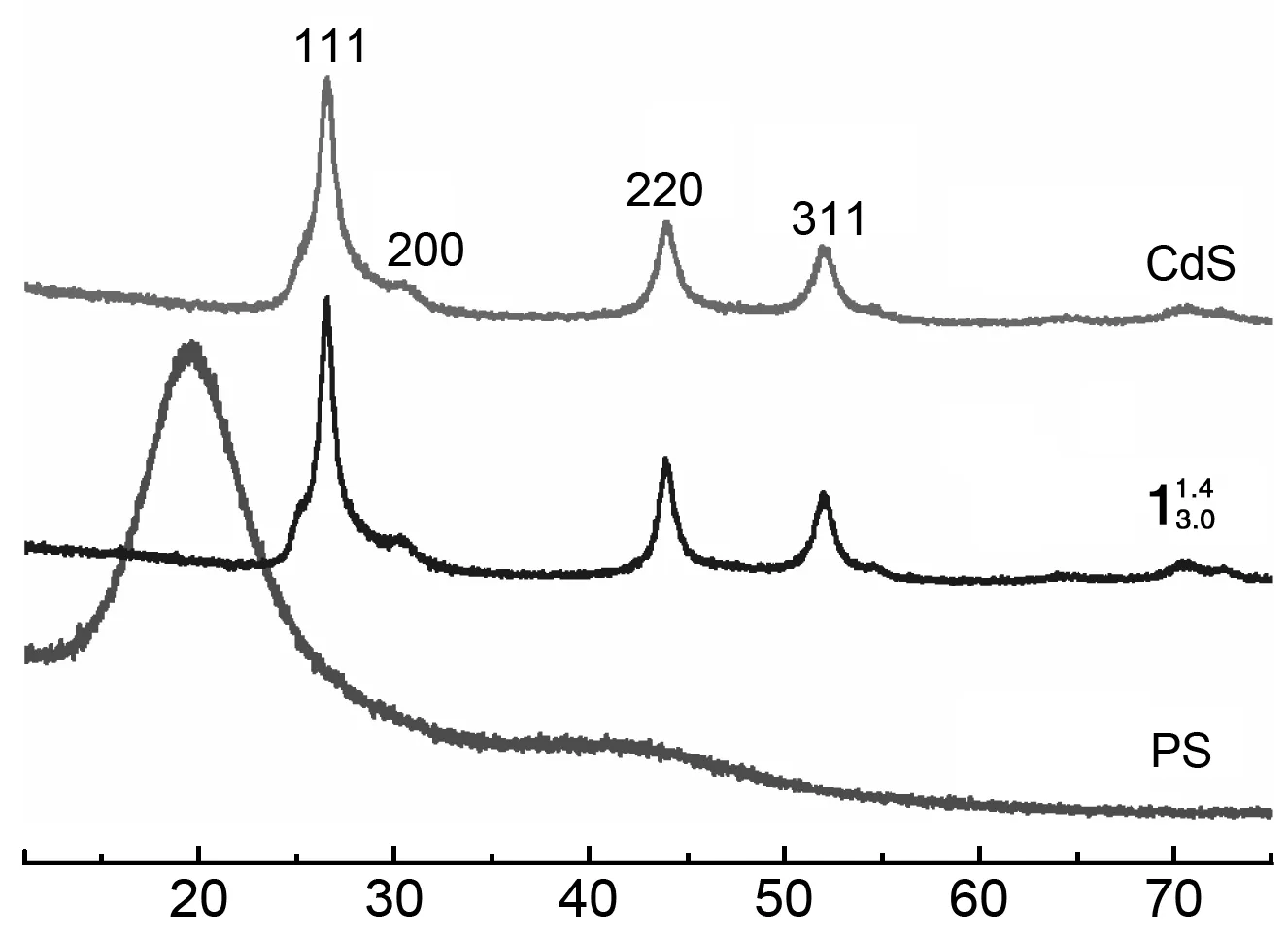
2θ/(°)

图 5 PS和1的SEM;1的TEM和EDS谱图Figure 5 SEM images of PS and 1,TEM and EDS patterns of 1
(3)SEM,TEM和EDS
2.4 光催化性能

Time/min

λ/nm


3 结论
以乙酸镉和硫代乙酰胺为壳层,PVP为偶联剂,聚苯乙烯微球为内核,用超声化学法制备了核壳型PS/CdS纳米复合材料1。1的内核平均粒径约260nm,CdS壳层厚度为10nm~30nm。考察了反应时间和物料比对1微观形貌的影响。合成1的最佳反应条件为:Cd(Ac)228mmol,n(Cd2+)∶n(S2-)=1.0∶1.4,聚乙烯比咯烷酮为偶联剂超声反应3h。1光催化罗丹明降解反应结果表明,70min脱色率达到100%。
[1] Ghosh Chaudhuri R,Paria S.Core/shell nanoparticles:Classes,properties,synthesis mechanisms,characterization,and applications[J].Chemical reviews,2011,112(4):2373-2433.
[2] Radmilovic V,Ophus C,Marquis E,etal.Highly monodisperse core-shell particles created by solid-state reactions[J].Nature materials,2011,10(9):710-715.
[3] Zhang J,Tang Y,Lee K,etal.Nonepitaxial growth of hybrid core-shell nanostructures with large lattice mismatches[J].Science,2010,327(5973):1634-1638.
[4] Sanchez C,Julián B,Belleville P,etal.Applications of hybrid organic-inorganic nanocomposites[J].Journal of Materials Chemistry,2005,15(35-36):3559-3592.
[5] Zhang S,Zhu Y,Yang X,etal.Fabrication of core-shell latex spheres with CdS/polyelectrolyte composite multilayers[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2005,264(1):215-218
[6] Wang Y,Wang G,Wang H,etal.Microwave-assisted fabrication of PS@ CdS core-shell nanostructures and CdS hollow spheres[J].Chemistry Letters,2007,36(5):674.
[7] 王峰.辐射法制备具有核壳结构的CdS/PSt 纳米复合微球[J].安徽建筑工业学院学报:自然科学版,2006,13(5):81-83
[8] Rogach A,Susha A,Caruso F,etal.Nano-and microengineering:3-Dcolloidal photonic crystals prepared from sub-μm-sized polystyrene latex spheres pre-coated with luminescent polyelectrolyte/nanocrystal shells[J].Advanced Materials,2000,12(5):333-337
[9] Sherman R L,Ford W T.Semiconductor nanoparticle/polystyrene latex composite materials[J].Langmuir,2005,21(11):5218-5222.
[10] 王政,高原,王佳瑜,等.结合表面引发的原子转移自由基聚合和气/固反应制备CdS纳米微粒/聚苯乙烯核壳微球[J].高等学校化学学报,2008,29(7):1452-1455.
[11] Mizukoshi Y,Fujimoto T,Nagata Y,etal.Characterization and catalytic activity of core-shell structured gold/palladium bimetallic nanoparticles synthesized by the sonochemical method[J].The Journal of Physical Chemistry B,2000,104(25):6028-6032.
[12] Anandan S,Grieser F,Ashokkumar M.Sonochemical synthesis of Au-Ag core-shell bimetallic nanoparticles[J].The Journal of Physical Chemistry C,2008,112(39):15102-15105.
[13] Breen M,Dinsmore A,Pink R,etal.Sonochemically produced ZnS-coated polystyrene core-shell particles for use in photonic crystals[J].Langmuir,2001,17(3):903-907.
[14] Gao T,Li Q,Wang T.Sonochemical synthesis,optical properties and electrical properties of core/shell-type ZnO nanorod/CdS nanoparticle composites[J].Chemistry of materials,2005,17(4):887-892.
[15] Murcia M J,Shaw D L,Woodruff H,etal.Facile sonochemical synthesis of highly luminescent ZnS-shelled CdSe quantum dots[J].Chemistry of materials,2006,18(9):2219-2225.
[16] Wang K P L,Yu Lin Zhong,Ming Lin,etal.Bifunctional FePt core-shell and hollow spheres:Sonochemical preparation and self-assembly[J].In Chem Mater,2007:2566-2572.
[17] Jun Geng,Bo Liu,Lang Xu,etal.Facile route to Zn-based Ⅱ-Ⅵ semiconductor spheres,hollow spheres,and core/shell nanocrystals and their optical properties[J].Langmuir,2007(23):10286-10293.
[18] Anne L M,Sergei I N,Karine G,etal.Sonochemical approach to the synthesis of Fe3O4@SiO2core-shell nanoparticles with tunable properties[J].ACS nano,2008,2(5):847-856.
[19] LI S Z J,YANG W Y.A new synthesis process and characterization of three-dimensionally ordered macroporous ZrO2[J].Materials Letters,2007,61(26):4784-4786.
[20] Gupta P,Thilagavathi R,Chakraborti A K,etal.Role of molecular interaction in stability of celecoxib-PVP amorphous systems[J].Molecular Pharmaceutics,2005,2(5):384-391.
[21] Wu D,Ge X,Zhang Z,etal.Novel one-step route for synthesizing CdS/polystyrene nanocomposite hollow spheres[J].Langmuir,2004,20(13):5192-5195.
[22] 卢南,孙瑾,程兴,等.CdS的制备及其光催化性能研究[J].广州化工,2013,40(22):74-76.
[23] Zhuang J,Dai W,Tian Q,etal.Photocatalytic degradation of RhB over TiO2bilayer films:Effect of defects and their location[J].Langmuir,2010,26(12):9686-9694.
SynthesisandPhotocatalysisActivityofPS/CdSCore-ShellStructureCompositePhotocatalyst
WANG Han1,2,XU Qian1,ZHENG Xing3,HAN Wen-qing2,JIANG Bo1,SUN Guan-hua1,ZHONG Cai-xia2,YIN Hua-cheng1,ZHENG Jing-tang1
(1.State Key Laboratory of Heavy Oil Processing,China University of Petroleum,Qingdao 255580,China;2.Baotou Light Industry and Vocational Technical College,Baotou 014045,China;Beijing ZNHK Science and Technology Development Co.,LTD,Beijing 100080,China)
A core-shell structure composite photocatalysts(PS/CdS,1)was synthesized by facile ultrasonic method,using PS as the model and PVP as the coupling agent.The structures and photocatalysis activity were characterized by UV-Vis,FT-IR,XRD,SEM,TEM and EDS.Effects of reaction time and mole ratio on the microstructure of1were investigated.The optimum reaction conditions of1were as follows: Cd(Ac)2was 28mmol,n(Cd2+)∶n(S2-)=1.0∶ 1.4and reaction time was 3h.The results of photocatalyzing RhB degradation indicated that the decolourization ratio was 100% after 70min.
core-shell structure;CdS;ultrasonic method;synthesis;photocatalysis
2014-07-10
国家自然科学基金资助项目(21376268,21176260);国家重点基础研究发展计划(973)资助项目(2011CB605703);泰山学者资助计划(ts20130929)
王涵(1975-),女,汉族,内蒙古赤峰人,博士研究生,讲师,主要从事新型环保材料的研究。E-mail: wanghan960070@126.com
郑经堂,博士生导师,E-mail: jtzheng03@163.com;郑星,E-mail: znhk113@163.com
O631.3;TQ426.6
A
1005-1511(2014)06-0725-05

