Influence of Gas Density on Hydrodynamics in a Bubble Column
Tang Xiaojin; Hou Shuandi; Zhang Zhanzhu
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Influence of Gas Density on Hydrodynamics in a Bubble Column
Tang Xiaojin; Hou Shuandi; Zhang Zhanzhu
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Two kinds of gases were used to investigate the influence of gas density on the hydrodynamic characteristics in a bubble column. It can be found out that higher gas density leads to smaller bubble diameter and the flow patterns are different for the both gases. Energy balance mechanisms are analyzed by considering the gas density difference. Models were developed to predict the average bubble diameter with good accuracy.
bubble column; hydrodynamics, gas density
1 Introduction
Bubble columns are widely used in chemical and petrochemical industries where oxidation[1], hydrogenation[2], neutralization[3]and other reactions[4-5]are applied. In bubble columns, gas phase is dispersed into bubbles and liquid phase is a continuous phase. Thus, the hydrodynamic characteristics in a bubble column are significantly influenced by bubbles. Until now, bubble behaviors cannot be described clearly due to the complexity of two-phase flow, and the design and scale-up of bubble columns are still very difficult. Industrial applications of bubble columns are mainly based on a series of empirical pilot scale tests. Krishna and Sie[6]suggested that the combination of large cold model experiments and small hot model pilot tests might be helpful to the scale-up of bubble columns. For cold model experiments, air at normal pressure and ambient temperature are usually used as the gas phase. However, in most cases the density of air is not as the same as that in real reactor under high pressure so the applicability of these cold model experimental results is limited. Wilkinson, et al.[7]found out that the gas holdup increases with the increase of gas density. From this point of view, investigations on the influence of gas density on hydrodynamics are very essential for the design and scale-up of bubble columns based on the cold model experiments. In this study, the hydrodynamic characteristics in a bubble column were investigated. Two kinds of gases were used in the experiments and the influence of gas density was analyzed.
2 Experimental
The scheme of experimental setup is shown in Figure 1. The bubble column is made of glass, 1 695 mm in height and 27 mm in diameter. As shown in Figure 1, the liquid phase and gas phase are mixed prior to entering the bubble column at the bottom. In the column, the mixture of gas and liquid firstly goes upward through a bed of ceramic balls, 53 mm in height, which is used as the distributor to generate a uniform distribution of the gas phase. In the bubble column, the gas phase is dispersed into bubbles. The upward moving mixture of gas and liquid leaves the column at the overhead, and then enters a gas-liquid separator. In the separator, the gas phase is separated from the liquid phase. The gas phase leaves the separator at the top and the liquid phase leaves the separator at the bottom.
Kerosene was used as the liquid phase. Hydrogen and nitrogen were used as the gas phase, respectively. The physical properties of the experimental materials are listed in Table 1.
The experiments were conducted at ambient temperature and atmospheric pressure. The super ficial liquid velocitywas set at 0.000 12 m/s and the super ficial gas velocitywas set in the range of between 0.001 02—0.015 3 m/s.The holdup of the gas phase in the bubble column was measured by volumetric replacement method. The bubble size is measured by photographic method.

Figure 1 Experimental setup of bubble column
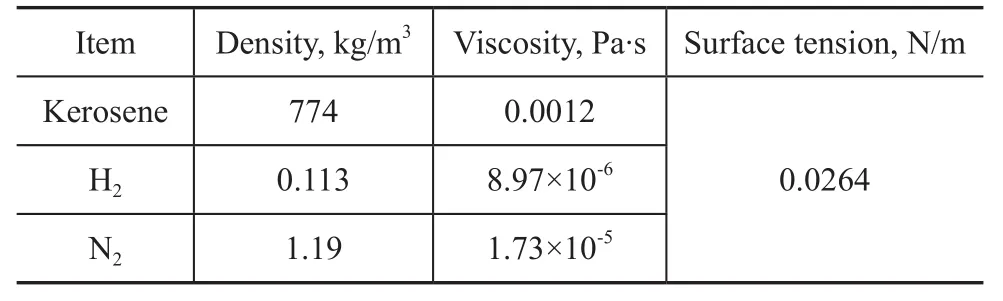
Table 1 Physical properties of experimental system
3 Results and Discussions
3.1 Gas holdup
The relationship between superficial gas velocity (ug) and gas holdupφis shown in Figure 2. It can be identified thatφincreases linearly with the increase ofugfor each kind of gas. Whenugis relatively low (<0.012 5 m/s),φof hydrogen is higher than that of nitrogen. Whenugis high enough (>0.012 5 m/s),φof nitrogen is higher than that of hydrogen.
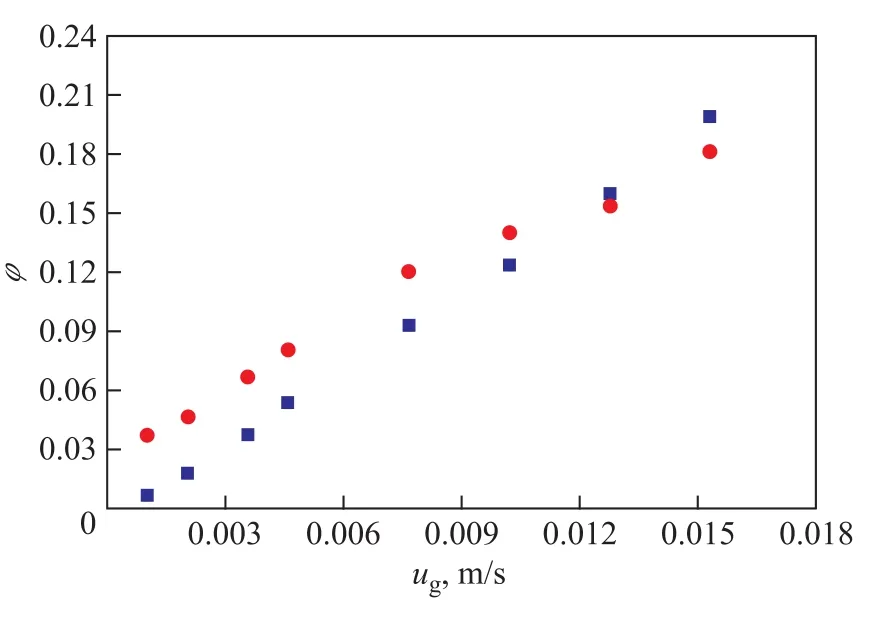
Figure 2 Relationship between ugandφ
3.2 Flow pattern
Flow pattern can be used to determine the operating regime in a bubble column. Usually, there are two flow patterns existing in the column. One is a homogeneous bubbly flow regime at low levels of gas velocity and the other is a churn-turbulent flow regime[6]. In homogeneous bubbly flow regime, the size of bubbles is uniform, while the gas holdup is low and the distance between two bubbles is quite long. In this sense, it is hard for bubbles to coalesce into big bubbles. While in the churn-turbulent flow regime, the gas velocity and the gas holdup are high. It is easy for bubbles to coalesce into big bubbles and the bubble size distribution is wide. The movement of big bubbles can lead to a turbulent flow in the column.
Figure 3 shows the flow patterns for the both gases under the same superficial gas velocity. For hydrogen, the bubble size distribution is much wider than that for nitrogen. It is easy to qualitatively conclude that the flow pattern of hydrogen is in the churn-turbulent flow regime while that of nitrogen in the homogeneous bubbly flow regime.

Figure 3 Flow patterns in bubble column (at ug=0.003 57 m/s)
A quantitative method can be used to determined the flow pattern in a bubble column by the relationship between slip velocity and gas holdup[8-9]. The slip velocityVsis defined by Eq. 1. WhenVsincreases with the increase ofφ, the flow pattern in the column is in the churn-turbulent flow regime. WhenVsdecreases with the increase ofφ, the flow pattern in the column is in the homogeneous bubbly flow regime.

Figure 4 shows the relationship betweenandφ. It can be found out from Figures 3 and 4 that in the whole ex-perimental range, the flow pattern of hydrogen is exactly in the churn-turbulent flow regime and that of nitrogen in the homogeneous bubbly flow regime.
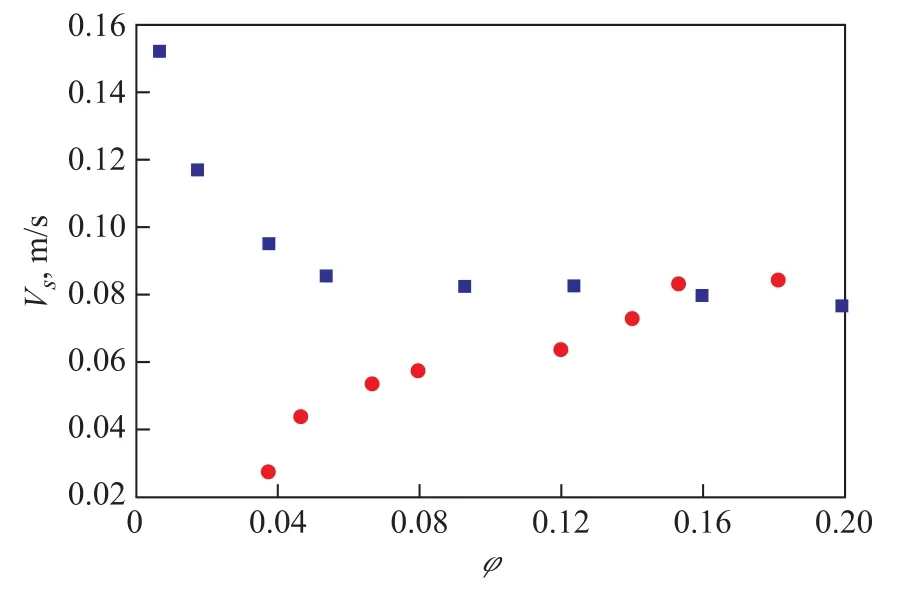
Figure 4 Relationship between Vsandφ
3.3 Average bubble diameter
In a bubble column, the average bubble diameter is usually calculated by Eq. 2 as the Sauter mean diameterd32.
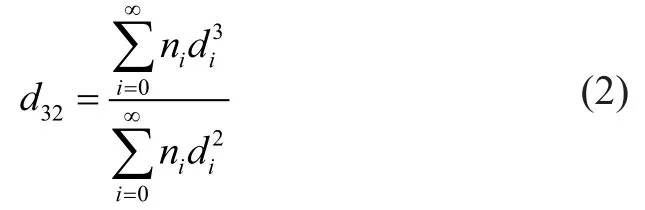
The relationship betweend32andugis shown in Figure 5. The value ofd32increases with the increase ofugfor both gases. Under the same gas velocity, the value ofd32of hydrogen is higher than that of nitrogen.
According to the theory of isotropic turbulence, the average bubble diameter is determined by the balance of mechanical energy and surface energy in a bubble column. Moreover, the average bubble diameter is proportional to the characteristic turbulent length. The process of gas being dispersed into bubbles is also a process for energy conversion from mechanical energy to surface energy. The parameter energy dissipation rateεcan be used to measure the level of mechanical energy input andεcan be calculated by Eq. 3, in whichρgis the density of gas phase,ρLis the density of liquid phase. It can be found from Eq. 3 that under the same physical propertiesεis the only function (ug/φ) that is assumed to be much less than 1. Figure 6 shows the relationship betweend32andug/φwhich can be used to measure the level ofε.

It can be found from Figure 6 that the value ofd32increases with the increase ofεfor hydrogen while the value ofd32decreases with the increase ofεfor nitrogen. Because the relationship for the case of hydrogen is opposite to that for the case of nitrogen, there might be different energy conversion mechanisms relating to both gases. For nitrogen, it is assumed that there is a balance between kinetic energy and surface energy. In this sense, the Weber number (We) in the column is constant as shown in Eq. 4. In Eq. 4,σmeans the surface tension,uis the characteristic turbulent velocity as shown in Eq. 5. By combining Equations 4 and 5,d32can be obtained from Eq. 6. By fitting the experimental data, the parameterKin Eq. 6 is determined to be 21.55. Figure 7 shows the comparison between the experimental and calculated values ofd32. It can be found out that Eq. 6 could predict the value ofd32of nitrogen with good accuracy.


Figure 5 Relationship between d32and ug
As already mentioned above, the energy conversion mechanism for hydrogen is different from that for nitrogen. Considering that the density of hydrogen is only one tenth of that of nitrogen, the energy input level is much lower than that of nitrogen. Thus, hydrogen can hardly form little bubble system which needs more energy in the column and the bubbles are relatively larger than the case of nitrogen. In this way, it is assumed that there is a balance between potential energy and surface energy. The Bond number (Bo) in the column is constant as shown in Eq. 7. In Eq. 7,ηis the characteristic turbulent length. By combining Eqs. 7 and 8, Eq. 9 is obtained to calculatethe value ofd32. By fitting the experimental data, the parameterCin Eq. 9 is derived to be 0.009. Figure 8 shows the comparison between the experimental and calculated values ofd32. It can be found out that Eq. 9 could well predict the tendency on the value ofd32of hydrogen.


Figure 6 Relationship between d32and (ug/φ)
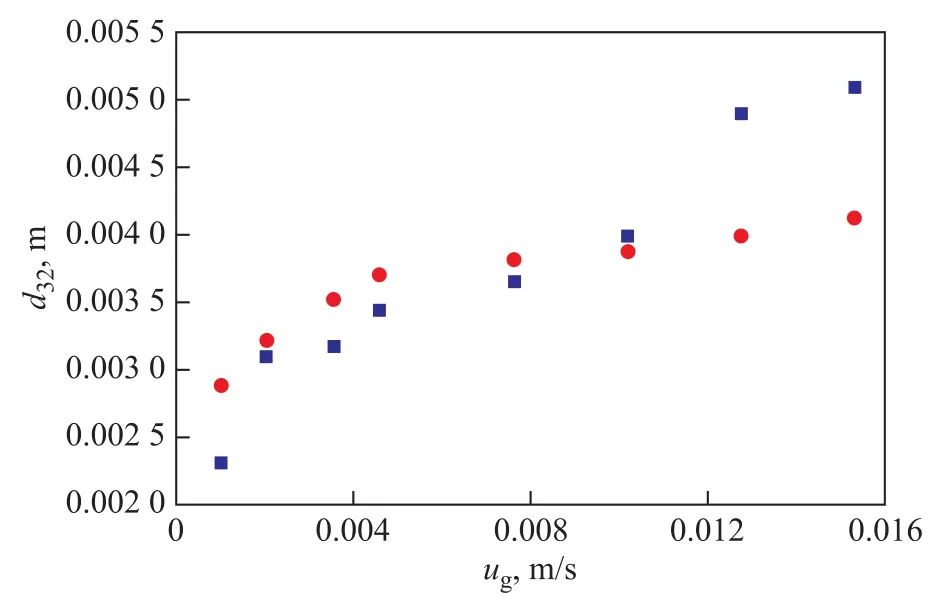
Figure 7 Comparison between experimental and calculated values of d32(of nitrogen)
3.4 Influence of gas density on hydrodynamics
From the discussion in the previous parts, we can learn that gas density has an important influence on the hydrodynamic characteristics in the bubble column. Higher density leads to smaller bubble diameter and homogeneous bubbly flow regime. For most bubble columns, the energy input is mainly dependent on the gas phase, and so the gas density determines the level of mechanical energy input by the gas phase to some extent. From this point of view, the energy balance mechanism in the column is determined by the gas density and also the flow pattern is determined.
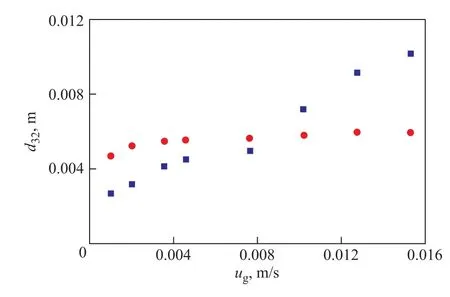
Figure 8 Comparison between experimental and calculated values of d32(of hydrogen)
In industrial bubble columns, a high density of gas phase resulted from the high operating pressure leads to small bubbles and possibly homogeneous bubbly flow regime. Actually, it is expected that the transfer processes (mass or heat) should be enhanced to create a churn-turbulent flow regime. Thus, high superficial gas velocity should be provided to raise the gas holdup, promote bubble coalescence, produce larger bubbles, and form the churn-turbulent flow regime.
4 Conclusions
Hydrogen and nitrogen were used to investigate the hydrodynamics in a bubble column. Based on the experimental data, the influence of gas density on the hydrodynamic characteristics was analyzed. Higher gas density leads to smaller bubble diameter and the flow pattern is in the homogeneous bubbly regime. Energy balance mechanisms are different due to the density difference between hydrogen and nitrogen. Models were developed to predict the average bubble diameter based on the energy balance mechanisms.
Acknowledgment:This work was financially supported by the National Key Basic Research Development Program “973”Project (2012CB224806) of China.
[1] Lucas M S, Peres J A, Puma G L. Treatment of winery wastewater by ozone-based advanced oxidation processes(O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics[J]. Separation and Puriif cation Technology, 2010, 72(3): 235-241
[2] Herrmann U, Emig G. Liquid phase hydrogenation of maleic anhydride to 1,4-butanediol in a packed bubble column reactor [J]. Ind Eng Chem Res, 1998, 37(3): 759-769
[3] Tokumura M, Baba M, Znad H T, et al. Neutralization of the acidified seawater effluent from the flue gas desulfurization process:φexperimental investigation, dynamic modeling, and simulation[J]. Ind Eng Chem Res, 2006, 45(18): 6339-6348
[4] Deckwer W D, Alper E. Katalytische suspensions reacktoren[J]. Chem Eng Tech, 1980, 52(3): 219-258
[5] Shah Y T, Kelkar B G, Godbole S P, et al. Design parameter estimation for bubble column reactors[J]. AIChE J, 1982, 28(3): 353-379
[6] Krishna R, Sie S T. Design and scale-up of the Fischer-Tropsch bubble column slurry reactor[J]. Fuel Processing Tech, 2000, 64(1/3): 73-105
[7] Wilkinson P M, Spek A P, Dierendonck L L. Design parameters estimation for scale-up of high-pressure bubble columns[J]. AIChE Journal, 1992, 38(4): 544-554
[8] Tang X, Luo G, Wang J. Mechanism analysis on the twophase flow characteristics in coalescence-dispersion pulsedsieve-plate extraction columns[J]. Ind Eng Chem Res, 2008, 47(23): 9724-9727
[9] Simonnet M, Gentric C, Olmos E, et al. Experimental determination of the drag coefficient in a swarm of bubbles[J]. Chem Eng Sci, 2007, 62(3): 858-866
Recieved date: 2014-01-15; Accepted date: 2014-01-29.
Telephone: +86-10-82369270, E-mail: tangxj.ripp@sinopec.com.
- 中国炼油与石油化工的其它文章
- Catalytic Cracking of Cycloparaffins Admixed with Olefins: 1. Single-Event Microkinetic (SEMK) Modeling
- Synthesis of Environmentally Friendly Magnesium Linoleate Detergent
- Alkylation of o-Xylene with Styrene over Modified Mordenite for Environmentally Friendly Synthesis of PXE
- ZrOCl2·8H2O: An Efficient and Cheap Catalyst for Esterification of Free Fatty Acids to Methyl Esters
- Dispersion Performance of Methanol-Diesel Emulsified Fuel Prepared by High Gravity Technology
- Preparation and Catalytic Performance of Silica-Supported Cr(acac)3/PNP for Ethylene Tetramerization

