Preparation and Catalytic Performance of Silica-Supported Cr(acac)3/PNP for Ethylene Tetramerization
Shao Huaiqi; Li Yafei; Gao Xianglu; Zhang Baojun; Yi Jianjun; Jiang Tao; Li Jian
(1. College of Material Science and Chemical Engineering, Tianjin University of Science & Technology, Tianjin 300457; 2. Petrochemical Research Institute, PetroChina, Beijing 100195)
Preparation and Catalytic Performance of Silica-Supported Cr(acac)3/PNP for Ethylene Tetramerization
Shao Huaiqi1; Li Yafei1; Gao Xianglu1; Zhang Baojun2; Yi Jianjun2; Jiang Tao1; Li Jian1
(1. College of Material Science and Chemical Engineering, Tianjin University of Science & Technology, Tianjin 300457; 2. Petrochemical Research Institute, PetroChina, Beijing 100195)
Chromium acetylacetonate and bis(diphenylphosphino)isopropylamine were coordinated in situ and supported on methylaluminoxane-modified silica. The catalyst structure and effects of reaction temperature, reaction pressure and Al/ Cr molar ratio on ethylene tetramerization were investigated in detail. Chromium was uniformly and firmly immobilized on the support and could not be leached off by methylaluminoxane. The supported catalyst, upon being activated with methylaluminoxane, exhibited catalytic activity of 1.70×107g/(mol Cr·h) for ethylene tetramerization to form 1-octene at a reaction temperatures of 80 ℃, a pressure of 2.0 MPa and an Al/Cr molar ratio of 300. The supported catalyst presented a good tolerance to high temperature.
ethylene tetramerization; 1-octene; Cr(acac)3/PNP; supported catalyst
1 Introduction
The growing demand for 1-hexene and 1-octene by the polymer manufacturing industry stimulates the research on highly active and selective catalysts for oligomerization of ethylene. The Cr/PNP catalyst system, which was firstly described by the BP researchers as being capable of selectively trimerizing ethylene, was later rediscovered by the Sasol researchers as ethylene tetramerization catalysts[1-2]. Up to now, the Sasol’s PNP and diphosphino catalysts of S-K Energy remain to be the only two industrially used catalytic systems of high activity and high selectivity (70%) for ethylene tetramerization to form 1-octene[2-3]. Nevertheless, their low operating temperature (45 ℃) is unfavorable for industrial application because of large heat of reaction released during ethylene tetramerization[4]. Therefore, it remains a challenging issue for improving their performance with regard to good tolerance of high temperature and higher selectivity to 1-octene or 1-hexene. Simply varying the substituents of the ligand can only result in limited improvements of the catalyst performance to meet the above-mentioned industrial needs[5-7]. It is noteworthy that immobilization of bis(imino)pyridine iron complexes on inorganic supports is proved to be an effective alternative in ethylene oligomerization[8]. Moreover, Toulhoat’s research, using grand canonical Monte Carlo simulations, predicted that the activity and the selectivity of Ti-based catalyst in ethylene oligomerization could be changed after adding zeolite[9]. Silica is an extensively used support for metallocene, Ziegler-Natta and other transition metal catalysts for ethylene polymerization[10-12]. In the present work, the silicasupported chromium diphosphinoamine catalysts were prepared, and their structures and catalytic performance for ethylene oligomerization were investigated.
2 Experimental
2.1 Catalyst preparation
The product bis(diphenylphosphino)isopropylamine (PNP) was synthesized by the reaction between isopropylamine and diphenylphosphine chloride according to the method described in the literature[2]. S757 silica gel (made by the Ineos Silicas Asia Pacific Ltd.) was activated at 600 ℃ for 8 h and stored in a glove drybox under nitrogen atmosphere blanketing before use. The silicasupported catalysts were prepared as follows:
S757 silica gel (1.0 g) was added to a flask in the glovedrybox. Then toluene (10 mL) was added in the flask under vigorous stirring. 0.211 2 g of Cr(acac)3(provided by the Sigma-Aldrich Co.) and 0.262 8 g of PNP dissolved in toluene (10 mL) were added into the flask at 50 ℃ under stirring and the resulting mixture was continuously stirred at 50 ℃ for 12 h. After filtration of the mixture, the solid was washed with toluene (10 mL×3) and dried under vacuum at 50 ℃ to give the supported catalyst SiCr-1. 1.0 g of S757 silica gel, 4 mL of methylaluminoxane (MAO at a concentration of 1.4 mol/L in toluene, provided by the Albemarle Corp.) and toluene (10 mL) were sequentially added in a flask under stirring and the resulting mixture was kept at 30 ℃ for 12 h. The filtered solid was washed with toluene (10 mL×3) to provide the MAO-modified S757. The MAO-modified S757 was suspended in toluene (10 mL), and was then mixed with a solution of Cr(acac)3(0.211 2 g) and PNP (0.262 8 g) in toluene (10 mL) and stirred continuously at 50 ℃ for 12 h. The filtered solid was washed with toluene (10 mL×3) and dried in vacuum at 50 ℃ to give the supported catalyst SiCr-2. Cr(acac)3(0.2112 g), PNP (0.2628 g) and MAO (4 mL) dissolved in toluene (10 mL) were added in a flask prior to vigorous stirring at 50 ℃ for 1 h, and the resulting mixture was added to the MAO-modified S757, which was suspended in toluene (10 mL) in a flask. The resulting suspension was stirred continuously at 50 ℃ for 12 h. The filtered solid was washed with toluene (10 mL×3) and dried in vacuum at 50 ℃ to give the supported catalyst SiCr-3.
2.2 Characterization of catalyst samples
The chromium contents of silica-supported catalysts were determined by atomic absorption spectrometry (AAS) using an AA-6800 atomic absorption spectrometer (made by the Shimadzu Co.) equipped with a heated graphite tube atomizer.
Scanning electron microscopy and energy dispersive X-ray spectrometry (SEM-EDS) of supported catalyst samples were obtained using a Hitachi S-4800 field emission scanning electron microscope. The sample was prepared with an ultramicrotome apparatus. At the liquid nitrogen temperature, the sample was cut off using a diamond knife from a block of cured epoxy resin, in which the supported catalyst was dispersed.
Nitrogen adsorption and desorption isotherms of catalyst samples were measured at -196 ℃ by a Quantachrome Autosorb-iQ automated gas sorption system. Samples were previously degassed at 300 ℃ for 4 h (for support) or at 60 ℃ for 12 h (for supported catalyst). Specific surface area (SBET) was calculated by the Brunauer-Emmett-Teller equation. The pore diameter (DP) was determined by peak value of pore size distribution curve. The total pore volume (VP) was determined by adsorption volume at ap/p0ratio of 0.995. The surface area and volume of micropores were calculated by thet-plot method.
The average diameter (d50) of silica and supported catalysts were analyzed by a Hydro 2000MU (A) Laser Mastersizer (made by the Malvern Instruments, Ltd.), and heptane was used as the dispersant. The thermogravimetric analyses (TGA) were carried out using a Rigaku’s PTC-10A TG-DTA system with a heating rate of 10 ℃/min up to 700 ℃ under a nitrogen flow rate of 60 mL/min.
Fourier transform infrared spectrometric (FTIR) analyses were performed using a Bruker Tenssor 27 instrument. The support sample was loaded on a KBr disc. The supported catalyst was dispersed in paraffin oil and coated between two KBr discs in a glove drybox. The two KBr discs were tightly pressed together to prevent from contacting air. The background of KBr was taken off before loading sample.
Elemental content on the surface of catalyst and binding energy of chromium analyzed by X-ray photoelectron spectroscopy (XPS) were performed using the Escalab MKII spectrometer (made by the Thermo VG Co.) with monochromatic Mg Kσ radiation (1 253.6 eV) operated at 150 W.
2.3 Leaching test
Leaching test was used to determinate whether the chromium species on the catalyst could be leached out under the same condition used by the oligomerization reaction system in the absence of ethylene. Under nitrogen blanketing 0.020 g of the supported catalyst was suspended in a flask with 6 mL of cyclohexane, to which 0.2 mL of MAO solution was then added. The flask was maintained at 50 ℃ for 30 min and then quenched by HCl/ethanol (10%) mixture. Subsequently, the slurry was filtered and the effluent was concentrated to dryness. The residue was dissolved in 10 mL of distilled water prior to analyzing the chromiumcontent by AAS. The content of leached chromium element was calculated based on the chromium content in the effluent.
2.4 Ethylene tetramerization
Ethylene tetramerization was carried out in a 0.5 L autoclave. After evacuating and purging of the autoclave three times with nitrogen then two times with ethylene (provided by the Tianjin Summit Specialty Gases Co.), the autoclave was charged with 0.1 L of cyclohexane and mechanically stirred in the ethylene atmosphere. When the desired reaction temperature was reached, MAO and the supported catalyst, which was suspended in 2 mL of cyclohexane, were injected into the reactor. After 30 min of reaction, the reaction solution was quickly cooled down to 20 ℃and then quenched with the HCl/ethanol (10%) mixture. The catalyst activity was calculated based on the increase of product mass. The product concentration was analyzed by the HP-7890 gas chromatograph equipped with a HP-5 capillary column (30 m×0.25 mm).
3 Results and Discussion
3.1 Characterization of silica-supported catalyst
The chromium content in the bulk and elemental contents on the surface of three silica-supported catalysts are presented in Table 1. It is noticed that the chromium element has been already loaded onto silica. The content of leached chromium element was equal to 52.3% and 85.9% from the catalyst samples SiCr-1 and SiCr-3, respectively, indicating that chromium was not firmly immobilized on silica and could be easily washed off by MAO solution.
This result means that the catalysts SiCr-1 or SiCr-3 would simultaneously catalyze the ethylene tetramerization reaction while functioning as homogeneous and supported catalysts, so the two catalysts will not be further studied in the present work. In contrast, the content of leached chromium from the catalyst SiCr-2 was only 4.2%. Therefore, the issue of leached chromium from this supported catalytic system seems to be practically negligible, which implies that the supported catalyst would catalyze the oligomerization reaction while mainly functioning as a heterogeneous catalyst. In addition, the existence of aluminum, nitrogen and phosphorus on the catalyst surface has revealed that the MAO and PNP are already loaded onto the silica.
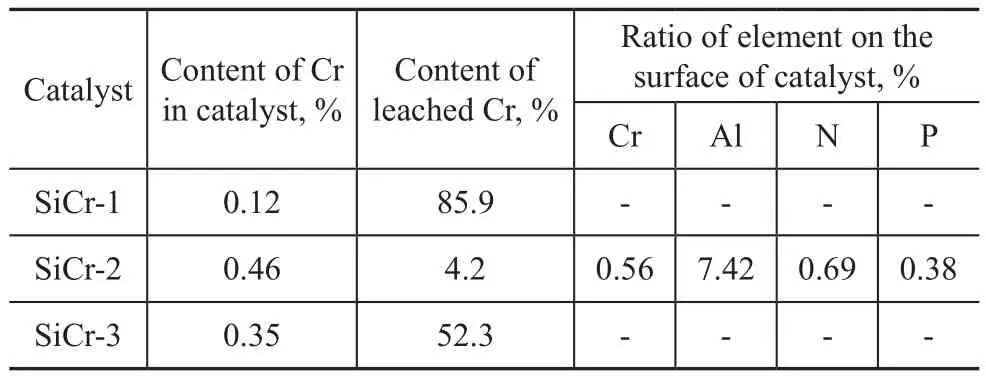
Table 1 Elemental content of the silica-supported catalysts
Figure 1 illustrates the patterns of distribution of aluminum and chromium elements on the catalyst SiCr-2 determined by SEM-EDS. Aluminum and chromium species are uniformly distributed despite slightly higher density of aluminum and chromium content at the edge of the particle. The uniform distribution of chromium species implies that the ethylene tetramerization reaction can uniformly occur on the surface of the catalyst particle upon being activated by MAO.

Figure 1 SEM-EDS images of the catalyst SiCr-2
The textural properties of the silica and the catalyst SiCr-2 are presented in Table 2. In comparison with the sample S757, the particle size and the specific surface area of the MAO-modified S757 are almost unchanged, but the microporous surface area and the pore volume are dramatically decreased. Such changes indicate that most of micropores are blocked by MAO, which is coated on the mesoporous channels, accompanied by production of new
mesopores. After MAO-modified S757 is loaded with Cr(acac)3/PNP to form the catalyst SiCr-2, the surface area of the catalyst SiCr-2t is dramatically decreased (79 m2/g), while its reduction in surface area, microporous volume and pore diameter is insignificant (equating to 4 m2/g, 0.004 cm3/g and 0.6 nm, respectively). The results indicate that the Cr/PNP complex mainly entered the smaller mesopores.
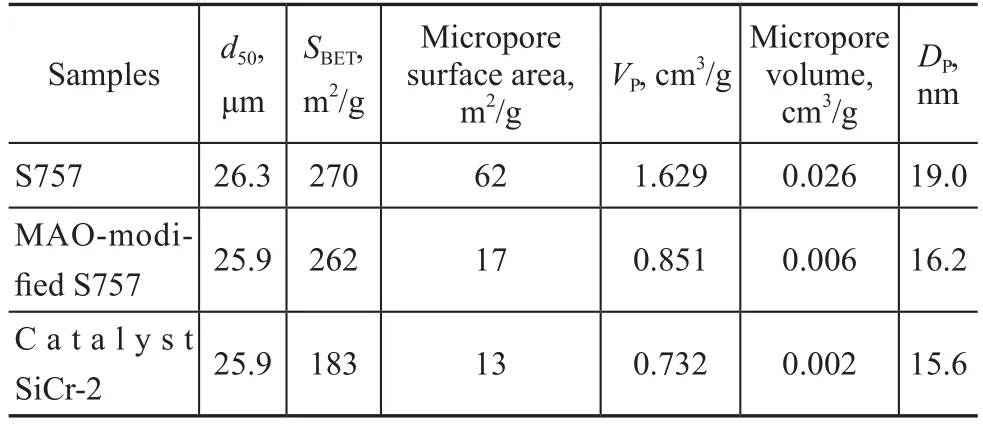
Table 2 Textural properties of the support and supported catalyst
The interaction between the silica and the Cr(acac)3/PNP was investigated by means of thermal gravimetric analysis. Figure 2 shows the thermograms of the mixture of the Cr(acac)3with one molar equivalent of PNP and SiCr-2. There was no weight loss found at the endothermic peak of 124 ℃ (Figure 2(a)), which was inferred as the melting heat of PNP. The weight loss (64.4 %) at 308 ℃ was caused by the decomposition of PNP. Correspondingly, the exothermic peaks at 503 ℃ and 542 ℃ were attributed to the decomposition of Cr(acac)3as compared with the relatively low decomposition temperature of pure Cr (acac)3(405 ℃)[13]. The disappearance of the peak at 124 ℃ in Figure 2 (b) suggests no free PNP molecules in the supported catalyst SiCr-2. Only strong exothermic peak at 462 ℃ accompanied by obvious weight loss could imply a further complexation between Cr(acac)3/PNP and the support. The change of the decomposition temperature reveals a strong interaction between the Cr(acac)3/PNP and the support, which was not destroyed by MAO.
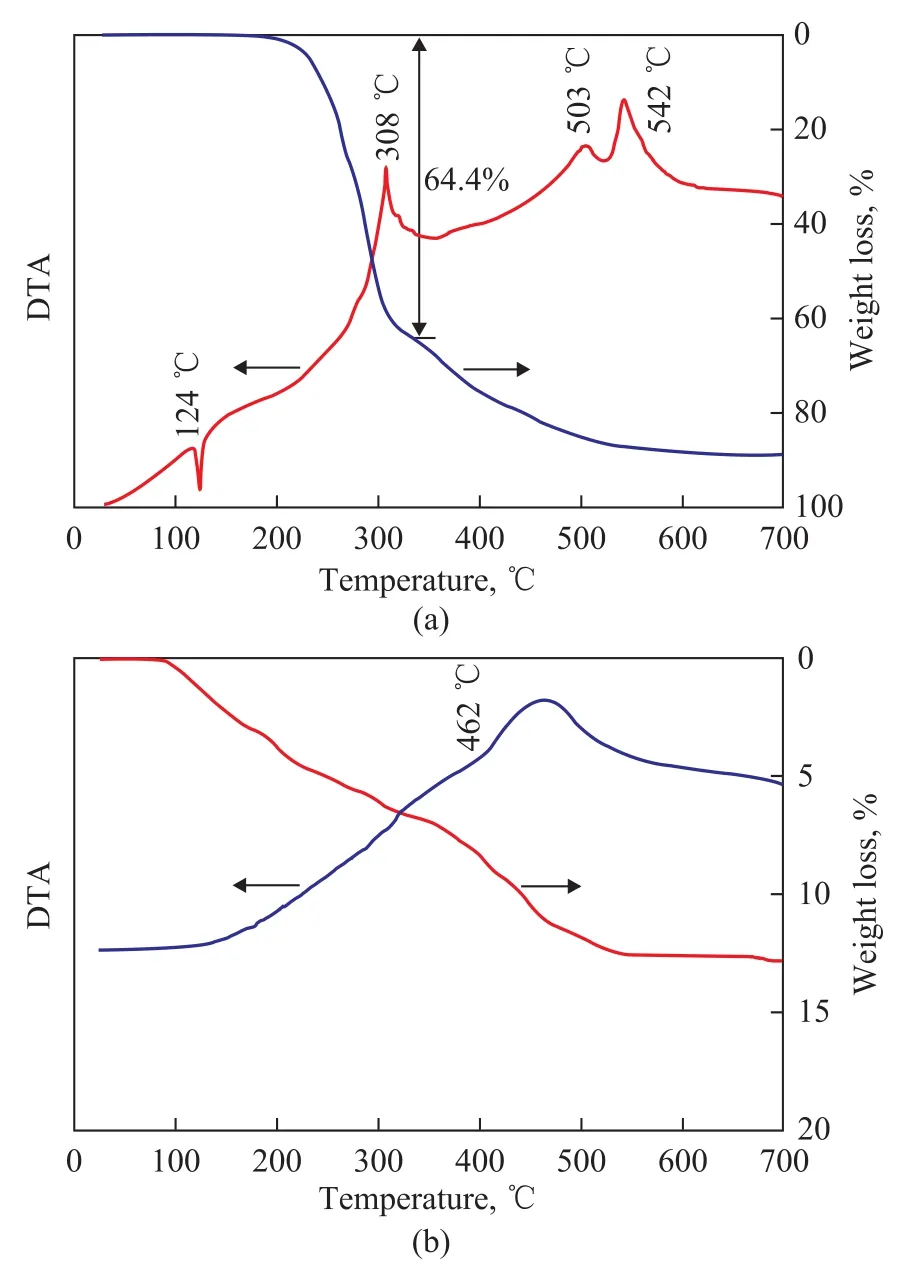
Figure 2 Thermograms of Cr(acac)3/PNP (a) and SiCr-2 (b)
The FTIR spectrometry was carried out to characterize the chemical properties of the interaction between Cr(acac)3/PNP and the support, as shown in Figure 3. When S757 silica gel was modified by MAO, the absorption peak of Si-OH at 975 cm-1disappeared, indicating to the formation of Al-O-Si framework through the interaction between the Si-OH and Al-Me, while the vibration of Si-O-Si, which shifted from 1 110 cm-1to 1 077 cm-1, may be taken as an indication for the effective incorporation of Al in the framework of the support[14-15]. After the immobilization of Cr(acac)3/PNP on MAO-modified S757, the asymmetrical C = C stretching vibration shifted from 1 519 cm-1to 1 536 cm-1[13], which revealed that a stronger interaction existed between the Cr(acac)3/PNP and the MAO-modified S757, which was consistent with the TGA result shown in Figure 2(b), suggesting an effective complexation between Cr(acac)3/PNP and the MAO-modified S757 silica. This result is similar to the observation made by Rabeah, et al.[16], who used 100 equivalents of modified MAO as
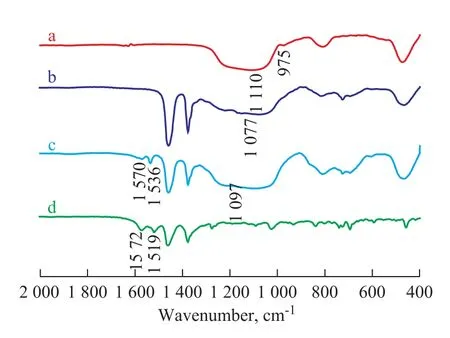
Figure 3 FTIR spectra of S757 (a), MAO-modified S757 (b), SiCr-2 (c) and Cr(acac)3/PNP (d)
reaction agent of Cr(acac)3and PNP to form Cr(CH3)2PNP. In our present work, the acetylacetate was not entirely displaced by methyl groups due to the reduced reactivity of the immovable MAO anchored on the surface of silica.
The XPS spectra of chromium on the surface of the catalyst SiCr-2 are shown in Figure 4. Referring to the binding energy of 577.9 eV of Cr 2p3/2in Cr(acac)3[17], the peak at 577.0 eV of Cr 2p3/2indicates that the oxidation state of chromium on the catalyst SiCr-2 is partially reduced from Cr3+to a lower oxidation state.
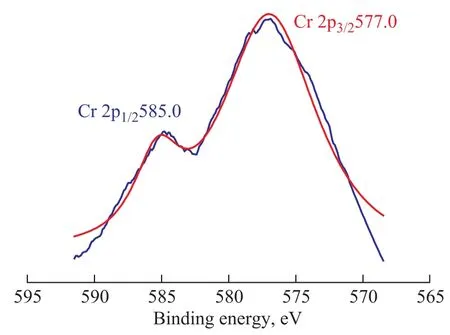
Figure 4 Cr 2p XPS spectrum of catalyst SiCr-2
3.2 Catalytic performance
The activity and selectivity of the homogeneous Cr(acac)3/PNP and the supported catalyst SiCr-2 in ethylene oligomerization under different reaction conditions are presented in Table 3. The similar products distribution was found during ethylene oligomerization catalyzed by the Cr(acac)3/PNP (entry 1) and the catalyst SiCr-2 (entry 2) at 50 ℃ using 300 equivalents of MAO as the cocatalyst, while the catalytic activity of SiCr-2 is lower than that of Cr(acac)3/PNP resulted probably from the steric hindrance on the surface of silica[18]. In the absence of the activator MAO, the catalyst SiCr-2 displayed very low activity (entry 3) at 50 ℃, suggesting that the chromium active sites were largely inactivated by MAO supported on the silica. By comparison, the catalyst SiCr-2 exhibited high catalytic activity after being activated with MAO (entry 4) under the same condition, indicating that the actual active site Cr-CH3was generated via displacement of acetylacetate ligand in situ in the course of reaction[16]. Because the mesopore size of the catalyst SiCr-2 is far larger than the diameter of MAO[19], the chromium active sites are believed to be uniformly distributed in the pore channels of the support and would mainly fill up the mesopores.
When ethylene oligomerization was carried out at 80 ℃ (entry 2), both the activity and the selectivity toward 1-octene achieved by the catalyst Cr(acac)3/PNP decreased compared to those realized by the same catalyst at 50 ℃ (entry 1), which is in compliance with the results reported by Walsh, et al.[4]In contrast, the activity of the catalyst SiCr-2 significantly soared when the temperature increased from 50 ℃ (entry 4) to 70 ℃ (entry 5) and to 80 ℃ (entry 6), which is even much higher than that achieved by the homogeneous catalyst Cr(acac)3/PNP at 50 ℃ (entry 1). This phenomenon may be attributed to the fact that the catalyst SiCr-2 is more stable at high temperature than the catalyst Cr(acac)3/PNP[8]. Meanwhile, the selectivity toward 1-hexene and the combined selectivity toward 1-hexene and 1-octene on the catalyst SiCr-2 significantly increased with a slightly reduced yield of 1-octene, C6cyclics andpolyethylene.

Table 3 Activity and selectivity of Cr(acac)3/PNP and SiCr-2 during ethylene oligomerization
It should be noted that the activity and selectivity toward 1-octene on the catalyst SiCr-2 are decreased, whereas the selectivity toward 1-butene is obviously increased, when the Al/Cr molar ratio is decreased from 300 (entry 6) to 100 (entry 7). These results can be probably explained as insuf ficient activation of chromium species at lower Al/Cr molar ratio. Similarly, an Al/Cr molar ratio of 300 is sufficient for activation of chromium species in the catalyst Cr(acac)3/PNP[2]. With the Al/Cr molar ratio increasing from 300 (entry 6) to 500 (entry 8), the catalytic system displays a decreasing trend of the catalytic activity. This is expected that excess amount of MAO may interfere with the formation of active Cr species and/or the overreduction of Cr species[20].
In addition, it is remarkable that the selectivity toward 1-octene on the catalyst SiCr-2 picked up 11 % as the ethylene pressure increased from 1.0 MPa to 4.0 MPa (as shown in entries 6, 9, and 10). Similar trend was found on the catalyst Cr(acac)3/PNP[2]with its selectivity toward 1-octene increased by 8% upon increasing the pressure from 30 bar to 45 bar. This trend is attributed to sensitivity of the silica-supported Cr-based catalyst to ethylene pressure due to the fact that the solubility and the transfer of ethylene through the pore channels are enhanced with higher ethylene pressure.
4 Conclusions
The catalyst Cr(acac)3/PNP was in situ immobilized on MAO-modified silica, and the resulted silica-supported catalysts were investigated on ethylene oligomerization under different reaction conditions, such as varied reaction temperatures, ethylene pressures and Al/Cr ratios. Compared with the homogeneous catalyst Cr(acac)3/PNP, the silica-supported catalyst shows higher activity at higher temperature (80 ℃) and better combined selectivity toward 1-hexene and 1-octene. Importantly, the enhanced high temperature tolerance of the silica-supported catalyst makes itself commercially applicable.
Acknowledgments:This work was supported by the National Natural Science Foundation of China (U1162114), the Petro-China Innovation Foundation (2012D-5006-0501), the Tianjin Municipal Education Commission of China (20110505), the Natural Science Foundation of Tianjin (12JCQNJC06000) and the Program for New Century Excellent Talents in University (NCET-07-0142).
[1] Carter A, Cohen S A, Cooley N A, et al. High activity ethylene trimerisation catalysts based on diphosphine ligands[J]. Chem Commun, 2002(8): 858-859
[2] Bollmann A, Blann K, Dixon J T, et al. Ethylene tetramerization: A new route to produce 1-octene in exceptionally high selectivities[J]. J Am Chem Soc, 2004, 126(45): 14712-14713
[3] Han T K, Ok M A, Chae S S, et al. Ethylene tetramerization catalyst systems and method for preparing 1-octene using the same: WO Patent, 2008/088178[P]. 2008-07-24
[4] Walsh R, Morgan D H, Bollmann A, et al. Reaction kinetics of an ethylene tetramerisation catalyst[J]. Appl Catal A: Gen, 2006, 306: 184-191
[5] Blann K, Bollmann A, de Bod H, et al. Ethylene tetramerisation: subtle effects exhibited by N-substituted diphosphinoamine ligands[J]. J Catal, 2007, 249(2): 244-249
[6] Licciulli S, Thapa I, Albahily K, et al. Towards selective ethylene tetramerization[J]. Angew Chem Int Ed, 2010, 49(48): 9225-9228
[7] Shaikh Y, Albahily K, Sutcliffe M, et al. A highly selective ethylene tetramerization catalyst[J]. Angew Chem Int Ed, 2012, 51(6): 1366-1369
[8] Guo C, Xu H, Zhang M, et al. Immobilization of bis(imino) pyridine iron complexes onto mesoporous molecular sieves and their catalytic performance in ethylene oligomerization[J]. Catal Commun, 2009, 10(10): 1467-1471
[9] Toulhoat H, Fomena M L, de Bruin T. Computational study of the effect of confinement within microporous structures on the activity and selectivity of metallocene catalysts for ethylene oligomerization[J]. J Am Chem Soc, 2011, 133(8): 2481-2491
[10] Silveira F, Alves M C M, Stedile F C, et al. Effect of the silica texture on the structure of supported metallocene catalysts[J]. J Mol Catal A: Chem, 2009, 298(1/2): 40-50
[11] Luo H, Tang R, Gao K. Studies on the formation of new, highly active silica-supported Ziegler-Natta catalyst for ethylene polymerization[J]. J Catal, 2002, 210(2): 328-339
[12] Ma Z, Sun W, Zhu N, et al. Preparation of silicasupported late transition metal catalyst and ethylene polymerization[J]. Polym Int, 2002, 51(4): 349-352
[13] Dittmar A, Herein D. Microwave plasma assisted preparation of disperse chromium oxide supported catalysts Adsorption and decomposition of chromium acetylacetonate[J]. Surf Coat Tech, 2009, 203(8): 992-997
[14] Yang H, Deng Y, Du C, et al. Novel synthesis of ordered mesoporous materials Al-MCM-41 from bentonite[J]. Appl Clay Sci, 2010, 47(3/4): 351-355
[15] Anunziata O A, Beltramone A R, Cussan J. Synthesis at atmospheric pressure and characterization of highly ordered Al, V, and Ti-MCM-41 mesostructured catalysts[J]. Catal Today, 2008, 133-135: 891-896
[16] Rabeah J, Bauer M, Baumann W, et al. Formation, operation and deactivation of Cr catalysts in ethylene tetramerization directly assessed by operando EPR and XAS[J]. ACS Catal, 2013, 3(1): 95 102
[17] Srivastava S, Badrinarayanan S, Mukhedkar A J. X-ray photoelectron spectra of metal complexes of substituted 2, 4-pentanediones[J[. Polyhedron, 1985, 4(3): 409-414
[18] Liu C, Tang T, Huang B. Zirconocene catalyst well spaced inside modified montmorillonite for ethylene polymerization: role of pretreatment and modification of montmorillonite in tailoring polymer properties[J]. J Catal, 2004, 221(1): 162-169
[19] Babushkin D E, Brintzinger H H. Activation of dimethyl zirconocene by methylaluminoxane (MAO) - size estimate for Me-MAO-anions by pulsed field-gradient NMR[J]. J Am Chem Soc, 2002, 124 (43): 12869-12873
[20] Jiang T, Ning Y, Zhang B, et al. Preparation of 1-octene by the selective tetramerization of ethylene[J]. J Mol Catal A: Chem, 2006, 259: 161-165
Recieved date: 2013-09-26; Accepted date: 2013-11-04.
Prof. Jiang Tao, Telephone: +86-22-60601230; E-mail: jiangtao@tust.edu.cn.
- 中国炼油与石油化工的其它文章
- Catalytic Cracking of Cycloparaffins Admixed with Olefins: 1. Single-Event Microkinetic (SEMK) Modeling
- Synthesis of Environmentally Friendly Magnesium Linoleate Detergent
- Alkylation of o-Xylene with Styrene over Modified Mordenite for Environmentally Friendly Synthesis of PXE
- ZrOCl2·8H2O: An Efficient and Cheap Catalyst for Esterification of Free Fatty Acids to Methyl Esters
- Influence of Gas Density on Hydrodynamics in a Bubble Column
- Dispersion Performance of Methanol-Diesel Emulsified Fuel Prepared by High Gravity Technology

