Alkylation of o-Xylene with Styrene over Modified Mordenite for Environmentally Friendly Synthesis of PXE
Kong Jie; Sheng Xiaoli; Zhou Yuming; Zhou Shijian; Zhang Zewu
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189)
Alkylation of o-Xylene with Styrene over Modified Mordenite for Environmentally Friendly Synthesis of PXE
Kong Jie; Sheng Xiaoli; Zhou Yuming; Zhou Shijian; Zhang Zewu
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189)
Effects of hydrochloride acid dealumination of mordenite (MOR) catalysts for the synthesis of 1-phenyl-1-xylyl ethane (PXE) were investigated. The structure and acidity of catalysts were characterized by XRD, BET, XRF, FT-IR,27Al NMR and NH3-TPD techniques. The catalytic performance of the acid-treated MOR zeolites was studied through using the alkylation ofo-xylene with styrene. The test results showed that the strength of remaining Brønsted acid sites increased despite the reduction of total number of acid sites after dealumination, and the micropores of HMOR were slightly enlarged coupled with the formation of secondary mesopores. Additionally, the modified HMOR zeolites showed longer catalyst life with the styrene conversion rate retained. Among the catalysts employed in this study, the modified mordenite that was dealuminated by HCl (2 mol/L) could be used repeatedly without significant loss of activity and selectivity during six catalytic runs, which have been ascribed to its specific acidity and structural properties.
mordenite; dealumination; 1-phenyl-1-xylyl ethane; alkylation
1 Introduction
Acid-catalyzed reactions such as the Friedel-Crafts alkylation are one of the important processes in organic synthesis and petrochemicals manufacture, as well as in fine chemical production[1]. The product of Friedel-Crafts alkylation of styrene witho-xylene, 1-phenyl-1-xylyl ethane (PXE), is a colorless synthetic liquid with many excellent properties suitable for various applications, e.g., solvent for pressure-sensitive record materials, plasticizer, heating medium, electric-insulating oil and high-boiling solvent[2]. Nevertheless, the aforementioned reactions involving traditional acids (e.g., H2SO4, AlCl3) are generally associated with problems of high corrosiveness, poor shape-selectivity, low hydrothermal stability and difficulties in waste acid treatment. The hastily demand for environmentally friendly catalytic technology requires the development of solid acid catalysts, thanks to their advantages such as high activity and selectivity, ease of separation and reusability.
With regard to PXE synthesis, a monumental series of solid acid catalysts such as cation-exchange resins[3], ordered mesoporous Al-MCM-41[4], silica-alumina[5], sulfated zirconia/titania[6]and HPW/SBA-15[7-8]are adopted as catalysts, all of which have promoted the catalytic activity. However, the compatibility of reusability and catalytic activity of the aforementioned catalysts is far less satisfactory. Mordenite, a two-dimensional large pore zeolite with dimensions of 0.65 nm×0.70 nm (major) may meet the above requirements, based on its acidity level and framework geometry which is beneficial to implementation of shape-selectivity reactions. However, the linear pores of mordenite despite its high-efficiency are vulnerable to carbon deposition, which could lead to its rapid catalytic deactivation[9]. So it is desirable to find out an efficient way to modify the mordenite, in order to improve its reusability on the premise that its catalytic activity could be retained or slightly decreased.
Dealumination of the mordenite was supposed to reduce the acid sites concentration, prolong the catalyst life, modify its pore structure and improve its thermal stability[10]. In recent years, a number of investigators have devoted themselves to the dealumination of mordenite. Although the dealumination of mordenite is not as thoroughly studied as that of zeolite Y, it can be achievedeither with steam treatment at high temperature[11], or by leaching with mineral acids such as HNO3and HCl[12-13], or with dibasic carboxylic acid such as acetic acid[10]. In addition, mordenite can also be dealuminated by organic agents such as the disodium salt of ethylenediaminetetraacetic acid, or mineral agents such as ammonium hexafluorosilicate and silicon tetrachloride[14]. In general, these methods are effective at bringing about large reduction in overall acidity, resulting in the improvement of catalytic activity. However, to the best of our knowledge, there is little literature referring to the study of relationship between catalyst life and dealumination of mordenite. To this end, we have set out to modify the mordenite with HCl leaching, making it appropriate for the alkylation reaction ofo-xylene with styrene.
The present study was aimed to conduct acidity level and framework geometry analysis of untreated and modi fied mordenite samples, to investigate the changes of pore system and acidity responsible for increased selectivity and catalytic activity for PXE synthesis after modi fication of mordenite. Especial attention was paid to the catalyst stability and reusability. Preliminary studies have revealed the unprecedented catalyst life of the modi fied mordenite.
2 Experimental
2.1 Catalyst preparation
A commercial Na-MOR (with a Si/Al molar ratio of 12.5) was converted to NH4-MOR by three sequential ion-exchanges with 1 mol/L of NH4NO3solution at 363 K for 6 h. Prior to dealumination, the resulting sample was dried at 393 K for 12 h and then calcined at 823 K for 5 h in the air, yielding HMOR. The HMOR zeolite underwent acid treatment, using hydrochloric acid solution at about 368 K for 4 h to partially extract the alumina atom that was present in the zeolite framework of the mordenite. A study on catalyst preparation variables, including various hydrochloric acid concentrations (2, 4, and 6 mol/L) and their impact on the dealumination of mordenite, was initiated. The treatment time and solution-to-catalyst ratio (20 mL solution/g catalyst) were held constant in these experiments. Samples were washed with deionized water, dried and calcined after dealumination treatment. The as-synthesized catalysts were named HM-2, HM-4 and HM-6 respectively, based on the relevant concentration of the acid used.
2.2 Catalyst characterization
The crystallographic characterization of untreated and modified mordenite samples was carried out with an X-ray powder diffractometer (Rigaku, type RINT-Ultima III) using Cu Kα radiation. The measurements were operated at 40 kV and 30 mA, with the large-angle XRD patterns obtained in the range of 2θ= 5°—40° at a scanning rate of 2(°)/min.
The N2physical adsorption and desorption isotherms were adopted at -196 ℃ to obtain surface areas with the ASAP 2020 apparatus (Micromertics, USA) by means of the Brunauer-Emmett-Teller (BET) method. Before measurements, the samples were preheated at 120 ℃ for 3 h in flowing N2stream. Specific surface areas were determined using the BET equation, while values of the micropore surface area and the micropore volume were estimated by applying thet-plot method.
The Si/Al molar ratios of the parent and acid-treated samples were obtained by X-ray fluorescence (XRF) measurements on a Switzerland’s ARL 9800 XRF apparatus. The solid-state27Al MAS NMR spectra were collected by a Bruker DSX-400 spectrometer. The27Al NMR spectra were obtained at 10.0 kHz, using 15° pulses and a 4-seconds delay, with a total of 2 000 pulses being accumulated. IR spectra of adsorbed pyridine were recorded using a Nicolet-510P apparatus. The samples were pressed into thin wafers and placed in a Pyrex glass cell equipped with CaF2windows. The samples were pretreated in situ at 300 ℃ for 1 h under vacuum (10-6torr) and then cooled down to room temperature. Afterward, pyridine was passed over the sample for 30 min and the pyridine adsorption spectra were recorded after desorption at 150 ℃and 350 ℃ for 1 h.
Surface acidity was measured by NH3-TPD in a TP-5000 apparatus (made in China) at atmospheric pressure. The sample (150 mg) was preheated at 400 ℃ for 1 h, and then cooled down to room temperature under a flowing He stream. At this temperature, sufficient pulses of NH3were injected until adsorption saturation.
2.3 Catalytic Tests
The alkylation reactions were carried out in a continuously stirred batch reactor under reflux conditions using a three-neck 100-mL round-bottom flask equipped with a condenser. Preliminary runs were conducted with 7.50 g(0.072 1 mol) of styrene, 57.35 g (0.540 2 mol) ofo-xylene (at a molar ratio ofo-xylene to styrene=7.5:1) and 1.50 g of catalyst (20% of styrene) at 120 ℃ for 180 minutes. The required amount ofo-xylene was initially added to the reactor at the reaction temperature, followed by adding the desired amount of catalyst, and a known amount of styrene was then added to the reaction mixture at the same temperature. After the reaction, the remainingoxylene was distilled out under atmospheric pressure and then the collected remainder part was called as crude product. The crude product was analyzed with a GC-9890A gas chromatograph equipped with an OV-1 capillary column and a flame ionization detector (FID).
3 Results and Discussion
3.1 Relative crystalline and bulk composition of mordenite samples
The long-range crystalline HMOR structure in these zeolites was substantiated by XRD as shown in Figure 1. The diffraction patterns of the parent and acid-treated mordenite samples are consistent with the typical diffraction lines of mordenite-type zeolites, and the crystallinity of the dealuminated samples had decreased slightly to 95%, indicating to the retainable features of zeolite structure. However, a significant increase in the Si/Al molar ratio of the acid-treated samples was detected, as evidenced by XRF measurements (see Table 1). After acid leaching, the Si/Al molar ratios of the modified samples treated with 2, 4, and 6 mol/L HCl increased to 14.4, 15.2 and 17.2, respectively, with the sodium content being lower than 1 000 μg/g. So it is clear that more and more framework aluminum was pulled out of the zeolite structure as the dealumination proceeded.
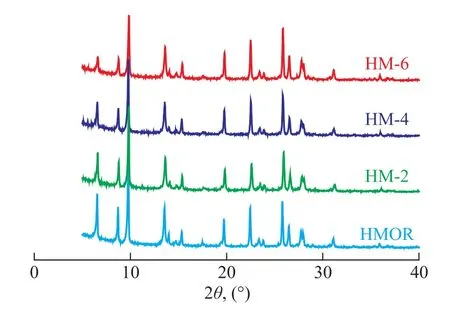
Figure 1 XRD patterns of the parent and HMOR samples treated with HCl
3.2 Nitrogen adsorption
Nitrogen adsorption-desorption isotherms of the parent and various acid-treated mordenite samples are presented in Figure 2. The isotherm of untreated mordenite sample is of type I showing an insignificant hysteresis loop, which suggests that the secondary particles piled up pores of mordenite grains or local defects formed in the process of crystallization. In comparison with HMOR, the dealuminated mordenite samples exhibit wider hysteresis loops attributed to a combination of typeⅠand type IV isotherms[15], which suggests the formation of secondary mesopores by acid treatment[16]. The mesopores of the parent HMOR were enlarged after acid treatment, and could be tuned by manipulating the concentration of the hydrochloride acid. Furthermore, the slopes of isotherms atp/p0=0.1—0.4 on the acid-treated mordenite were also slightly higher than that on the HMOR zeolite, denoting the enlargement of micropores[10].

Figure 2 Nitrogen adsorption-desorption isotherms (a) and mesopore size distribution (b) of various samples
Textural properties of the parent and acid-treated mordenite samples are shown in Table 1. It can be clearly seen that the micropore surface area increased largely after acid treatment, implying the removal of the amorphousaluminum species blocking the micropores of HMOR. Additionally, in comparison with other acid treated samples, the sample HM-2 possessed significantly higher mesoporosity, as evidenced by a mesopore volume of 0.11 cm3/g, a mesopore size of 7.6 nm, and a mesoporous surface area of 84 m2/g. Thus, the hydrochloride acid treatment of HMOR zeolite induced the formation of secondary mesopores as well as the enlargement of micropores, which was beneficial to the diffusion of reactants and products and improvement of catalytic performance.

Table 1 Textural properties of the parent and HMOR samples treated with HCl
3.3 NH3-TPD and FTIR study on pyridine adsorption
Acidic properties of parent HMOR and modified HMOR were investigated by NH3-TPD analysis, with the TPD profiles presented in Figure 3. It can be seen from Figure 3 that the samples exhibit two NH3desorption peaks at 260 ℃ and 550 ℃,corresponding to the ammonia desorption from non-zeolitic sites and the strong adsorption of NH3on Brønsted acidity sites[17], respectively. However, the intensity of desorption of ammonia decreased after acid treatment, implying the decrease in the number of acid sites. In addition, the temperature of the peak maxima shifted toward higher temperature that was related with the acid treatment, which was attributed to the increase of the strength of acid site. Especially, the strength and the amount of Brønsted acid sites of the HM-2 zeolite were the highest among the dealuminated mordenite samples. To make a point, samples were characterized by FTIR spectrometry after being activated at 400 ℃ (see Figure 4), and a mechanism for enhancement of Brønsted acid sites was proposed (see Scheme 1).

Figure 3 NH3-TPD profiles of the parent and HMOR samples treated with HCl

Table 2 NH3-TPD results of different samples

Scheme 1 Proposed mechanism for enhancement of Brønsted acid sites
It can be seen from Figure 4 that the background of all the mordenite samples showed two distinct hydroxyl stretching bands at 3 610 cm-1and 3 730 cm-1, respectively. The intensity of the low-frequency stretching (3 610 cm-1) responsible for Brønsted acid sites decreased[18], and the intensity of the high-frequency stretching (3 730 cm-1), which was assigned to the vibration of SiOH groups capable of making Brønsted acid sites stronger (see Scheme 1), increased. And it can be explained by the fact that the hydroxyl nests formed by dealumination will polarize the electrons away from the protons, resulting in the enhancement of acid strength.
Brønsted and Lewis acidities of the HM-X samples were probed with pyridine, and the results are shown in Figure 5 and Figure 6. The peaks at 1 547 cm-1and 1 457 cm-1can be assigned to the Brønsted acid sites and Lewis acid sites, respectively. While the signal at 1 491 cm-1indicated an overlap of these two types of acid sites, it was found that upon dealumination the number of strong acid sites increased with a simultaneous decrease of the total number of Brønsted acidity. The sample HM-2 possessed the largest and stronger Brønsted acid sites, which were in compliance with the results of NH3-TPD analysis. Marcelo, et al. reported similarly that the hydrochloric acid leaching on mordenite increased the strength of Brønsted acidity sites and raised the selectivity for target product[19].

Figure 5 Infrared spectra of samples after pyridine adsorption followed by desorption at 423K

Figure 6 Infrared spectra of samples after pyridine adsorption followed by desorption at 623K
3.427Al MAS NMR spectrometric analysis
As shown in Figure 7, the27Al NMR spectra of the parent HMOR and treated samples all showed two peaks centering at chemical shift of 0 and 54 ppm, which corresponded to the extra-framework (EFW) octahedral aluminum and the framework (FW) tetrahedral aluminum, respectively[20]. In comparison with the parent HMOR, the27Al NMR signals of acid-treated samples at 54 ppm and 0 were both weakened. Particularly, the HM-2 zeolite underwent slightly changes with the FW aluminum species and possessed barely detectable EFW aluminum species. In addition, the samples HM-4 and HM-6 possessed more EFW aluminum species than the sample HM-2, which was attributed to the formation of EFW aluminum species during the removal of FW aluminum species off the crystal lattices of zeolite. This confirms the above characterization results that acid treatment of moderate leads to the enlargement of micropores and formation of secondary mesopores in the external surface of zeolite due to dredging of pore systems, while the enhancement of acidity is resulted from the exposure of more acid sites.

Figure 727Al MAS NMR spectra of the parent and treated HMOR
3.5 Alkylation of styrene witho-xylene over modified mordenite
Alkylation ofo-xylene and styrene over the parent and treated HMOR catalysts was carried out to discovertheir difference in catalytic performance, with the results shown in Table 3. It can be seen from Table 3 that comparatively higher PXE yield and selectivity are achieved by all the acid catalysts, which can be indexed to their linear pore structure and appropriate acid sites concentration. It is noteworthy to mention that Na-MOR showed poor catalytic performance compared to other catalysts even under the same reaction conditions, indicating that the presence of acid sites is beneficial to the synthesis of PXE. However, all the modified catalysts exhibit an increase in PXE yield and selectivity compared to the parent zeolite irrespective of their lessened amount of acid sites, which can be explained by the previous report[17]that PXE is mainly produced on strong Brønsted acid sites. Among the HMOR catalysts dealuminated with HCl of different concentrations, the sample HM-2 showed the best catalytic performance, displaying that both pore system and acidity play an important role in the generation of PXE during the alkylation process.
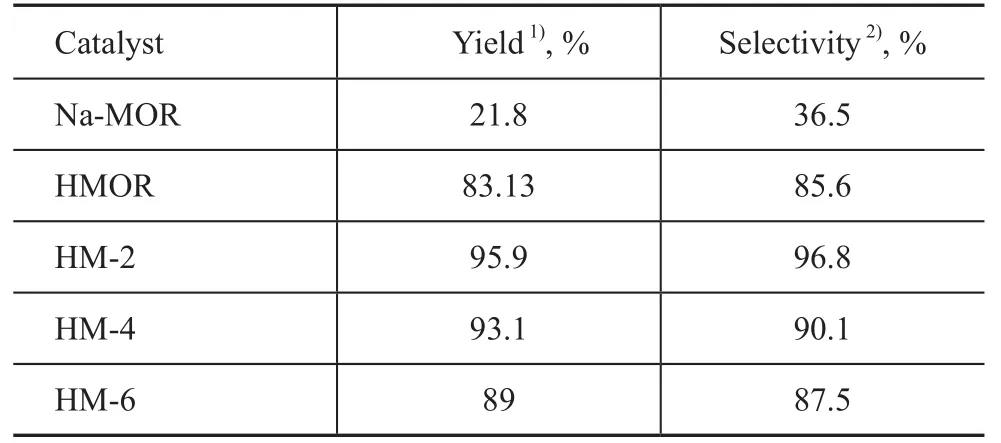
Table 3 Activity of various mordenite catalysts in alkylation ofo-xylene with styrene
During the process of alkylation reaction, small amounts of styrene oligomers and some heavy substitutes were formed as by-products (Scheme 2). The activity of HMOR catalyst was improved by dealumination due to its enhanced strong Brønsted acid sites, and this enhancement of activity raises the yield of PXE and by-products at the same time. But the increase of PXE yield is larger than that of the by-products yield, thus improving the selectivity for PXE along with the enhancement of the catalytic activity by dealumination. The number of acid sites decreased after dealumination, but the yield of byproducts increased in spite of the decreased activity, so there must be new active sites on which by-products are produced over some deficient sites (Scheme 3) that are formed upon dealumination. This is quite similar to the results in the report made by Kwak that the heavy oligomers and major by-products are formed mainly on the external surface sites of the mordenite catalyst[17].
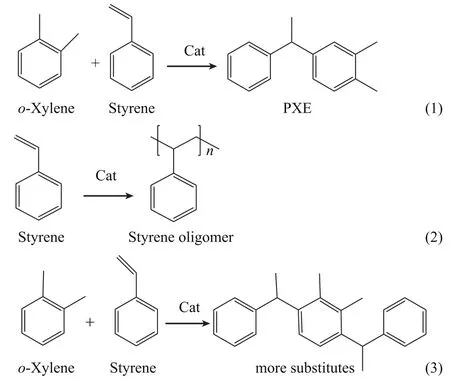
Scheme 2 Reaction scheme of alkylation of o-xylene with styrene over a heterogeneous catalyst

Scheme 3 Formation of external mesopores by HCl dealumination of HMOR samples
It is proposed that a fast diffusion rate can retard coke formation. The stability of mordenite can be attributed to the modification of pore structures. While the activity and selectivity for PXE were affected by the micropores, the catalytic stability of the mordenite samples could be correlated to their mesopore sizes (Scheme 4). The acid treatment enlarged the micropores and mesopores of the parent HMOR, resulting in an improved catalytic activity and stability. The excessive HCl treatment, however, could destroy the pore system to deteriorate the catalytic performance. In general, the enlarged mesopores could improve diffusivity of coke precursors and oligomer byproducts, thereby enhancing catalytic stability and PXE selectivity. This phenomenon is in agreement with the early report made by Sun and Prins[21]that mesoporous zeolites exhibit enhanced catalytic activity in the alkylation reaction due to diffusion alleviation of large molecular size.

Scheme 4 Proposed mechanism for improved catalytic performance of dealuminated mordenite
One of the most important advantages of heterogeneous catalysis over the homogeneous counterpart is the possibility of reusing the catalyst by simple filtration, without loss of activity. The catalytic reusability of the HM-2 and HMOR catalysts was evaluated by carrying out the reaction with used catalyst under the optimized conditions. After each run, the catalyst was recovered by filtration, followed by drying, and then calcination at 550 ℃ for 1 h before reuse. It can be seen from Figure 8 that only 5% reduction in the activity was observed after 6 runs with the HM-2 catalyst. In contrast, the deactivation of HMOR catalyst was much faster and its PXE yield dropped to 47% after the sixth reaction cycle. The outstanding durability of the HM-2 catalyst may be attributed to the removal of acid sites, which are usually responsible for the deactivation in alkylation reaction. On the other hand, the enhancement of strong Brønsted acid sites and formation of secondary mesopores may be another reason, as acid located at the external surface or mesopore walls of the hierarchical zeolites can catalyze reactions involving bulky molecules that cannot enter the zeolite micropores[22]. Absolutely, the decrease of product yield arising from catalysts loss experienced during separation and transfer of catalysts into the next reaction cycle cannot be excluded. It can be concluded from the above results that the acid treatment is applicable to catalytic stability improvement of mordenite in order to retain its characteristics for high PXE selectivity.

Figure 8 Catalytic stability of the HM-2 and HMOR catalysts in the alkylation ofo-xylene with styrene
4 Conclusions
The effects of hydrochloric acid during dealumination of mordenite were investigated. And the dealuminated mordenite was adopted for environmentally friendly synthesis of PXE that demonstrated an unprecedented stability and improved PXE selectivity. It was found out that the HM-2 catalyst showed a stable styrene conversion approaching 100% with PXE yield approaching 90.54% even after the sixth run, confirming that the catalytic property of a dealuminated mordenite catalyst was fully regenerative. The improved stability of the acid-treated mordenite can be attributed to the improved diffusivity of coke precursors and oligomer by-products due to enlarged mesopores and the enhancement of strong Brønsted acid sites.
Acknowledgments:The authors are grateful to the financial supports of the National Natural Science Foundation of China (Grant No. 21306023, 21376051, 21106017 and 51077013), the Fund Project for Transformation of Scientific and Technological Achievements of Jiangsu Province (Grant No. BA2011086), the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20100092120047), the Key Program for the Scientific Research Guiding Fund of Basic Scientific Research Operation Expenditure of Southeast University (Grant No. 3207043101) and the Instrumental Analysis Fund of Southeast University.
Refenrences
[1] Dong H J, Shi Li. Alkylation of toluene witht-butyl alcohol over zeolite catalysts [J]. Ind Eng Chem Res, 2010, 49(5): 2091-2095
[2] Dixit A B, Yadav G D. Deactivation of ion-exchange resin catalysts. Part I: Alkylation ofo-xylene with styrene [J].React Funct Polym, 1996, 31(3): 237-250
[3] Sato A, Shimizu I, Mesalito G. Aralkylation: The United States, US 4289918A[P], 1981-09-15
[4] Dang F M, Zhen X P, Niu C G, et al. Preparation of mesoporous molecular sieve Al-MCM-41 and the catalytic synthesis of diphenylethane [J]. Chinese Journal of Applied Chemistry, 2009, 26(12): 1404-1408 (in Chinese)
[5] Sato A, Shimizu I. Wet oxidation of wastes with circulation: The United States, US 4144279 [P], 1979-03-13.
[6] Wang Y P, Qu R J, Wang C H. Synthesis of diphenylethane by magnetic nanograde solid superacid SO42−/TiO2[J]. Appl Chem Ind, 2009, 38(6): 822-826
[7] Sheng X L, Zhou Y M, Zhang Y W, et al. Highly active and green aminopropyl-immobilized phosphotungstic acid on mesoporous LaSBA-15 for alkylation ofo-xylene with styrene [J]. Catal Lett, 2012, 142(3): 360-367
[8] Sheng X L, Zhou Y M, Zhang Y W, et al. Immobilization of 12-tungstophosphoric acid on LaSBA-15 and its catalytic activity for alkylation ofo-xylene with styrene [J]. Chem Eng J, 2012, 179: 295-301
[9] Sugi Y, Tawada S, Sugimura T, et al. Shape-selective isopropylation of biphenyl over H-mordenites: Relationships of bulk products and encapsulated products in the pores [J]. Appl Catal A: Gen, 1999, 189(2): 251-261
[10] Chung K H. Dealumination of mordenites with acetic acid and their catalytic activity in the alkylation of cumene [J]. Microporous Mesoporous Mater, 2008, 111(1-3): 544-550
[11] Xue H, Huang X, Zhan E, et al. Selective dealumination of mordenite for enhancing its stability in dimethyl ether carbonylation [J]. Catal Commun, 2013, 37: 75-79
[12] Viswanadham N, Kumar M. Effect of dealumination severity on the pore size distribution of mordenite [J]. Microporous Mesoporous Mater, 2006, 92(1-3): 31-37
[13] Song C. Shape-selective isopropylation of naphthalene over H–mordenite catalysts for environmentally friendly synthesis of 2,6-dialkylnaphthalene [J]. Academie des Sciences-Series IIC-Chemistry, 2000, 3(6): 477-496
[14] Merlen E, Alario F. Catalyst based on dealuminated mordenite and its use for dismutation and/or transalkylation of aromatic hydrocarbons: The United States, US 5929296[P]. 1998-04-08
[15] Lu R Q, Gu J, Tan B, et al. Physicochemical and catalytic properties of steam treated phosphorus-modified- HZSM-5 zeolites [J]. J Fuel Chem Technol, 2004, 32(4): 504-509 (in Chinese)
[16] Ajot H, Lynch J, Raatz F, et al. Formation of secondary pores in zeolites during dealumination: Influence of the crystallographic structure and of the Si/Al ratio [J]. Stud Surf Sci Cat, 1991, 62: 583-590
[17] Kwark B S, Kim T J. Synthesis of 1-phenyl-1-xylyl ethane by Friedel–Crafts alkylation of xylene withа-methylbenzyl alcohol over mordenite [J]. Catal Lett, 1999, 59: 55-60
[18] Ghosh A K, Curthoys G. Acidity of dealuminated mordenites studied by infrared spectroscopy [J]. J Chem Soc, Faraday Trans 1, 1983, 79: 805-813
[19] Boveri M, Márquez-Álvarez C, Laborde M Á, et al. Steam and acid dealumination of mordenite: Characterization and influence on the catalytic performance in linear alkylbenzene synthesis [J]. Catal Today, 2006, 114(2/3): 217-225
[20] Engelhardt G, Michel D. High-Resolution Solid-State NMR of Silicates and Zeolites [B]. New York: Wiley, 1987
[21] Sun Y, Prins R. Friedel-Crafts alkylation over hierarchical zeolite catalysts [J]. Appl Catal A: Gen, 2008, 336(1/2): 11-16
[22] Shetti V N, Kim J, Srivastava R, et al. Assessment of the mesopore wall catalytic activities of MFI zeolite with mesoporous/microporous hierarchical structures [J]. J Catal, 2008, 254(2): 296-303
Recieved date: 2013-10-22; Accepted date: 2013-12-30.
Professor Zhou Yuming, Telephone: +86-25-52090617; Fax: +86-25-52090617; E-mail: ymzhou@seu.edu.cn.
- 中国炼油与石油化工的其它文章
- Catalytic Cracking of Cycloparaffins Admixed with Olefins: 1. Single-Event Microkinetic (SEMK) Modeling
- Synthesis of Environmentally Friendly Magnesium Linoleate Detergent
- ZrOCl2·8H2O: An Efficient and Cheap Catalyst for Esterification of Free Fatty Acids to Methyl Esters
- Influence of Gas Density on Hydrodynamics in a Bubble Column
- Dispersion Performance of Methanol-Diesel Emulsified Fuel Prepared by High Gravity Technology
- Preparation and Catalytic Performance of Silica-Supported Cr(acac)3/PNP for Ethylene Tetramerization

