Synergetic Effect of Y Zeolite and ZSM-5 Zeolite Ratios on Cracking, Oligomerization and Hydrogen Transfer Reactions
Gong Jianhong; Xu Youhao; Long Jun; Wei Xiaoli
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Synergetic Effect of Y Zeolite and ZSM-5 Zeolite Ratios on Cracking, Oligomerization and Hydrogen Transfer Reactions
Gong Jianhong; Xu Youhao; Long Jun; Wei Xiaoli
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
The objective of this study is to explore the optimum composition of Y and ZSM-5 zeolites to develop novel catalysts for obtaining lower gasoline olefins content and higher propylene yield. Five composite zeolite catalysts with varying Y zeolite/ZSM-5 zeolite ratios have been prepared in this work to investigate the synergy between the Y zeolite and ZSM-5 zeolite on the selectivity to protolytic cracking, β-scission, oligomerization, and hydrogen transfer reactions using a FCC naphtha feedstock at 480 ℃ in a confined fluidized bed reactor. Experimental results showed that the composite catalyst with a Y zeolite/ZSM-5 zeolite ratio of 1:4 had the highest protolytic cracking and β-scission ability, which was even higher than that of pure ZSM-5 catalyst. On the other hand, the catalyst with a Y zeolite/ZSM-5 zeolite ratio of 3:2 exhibited the strongest hydrogen transfer functionality while the pure Y zeolite based catalyst had the highest oligomerization ability. For all the catalysts tested, increasing conversion enhanced the selectivity to protolytic cracking and hydrogen transfer reactions but reduced the selectivity to β-scission reaction. However, no clear trend was identified for the selectivity to oligomerization when an increased conversion was experienced.
composite zeolites; protolytic cracking; β-scission; oligomerization; hydrogen transfer
1 Introduction
The increasing usage of automobiles is boosting the world-wide fuel consumption, and as a consequence, is causing increasingly heavier environmental pollution[1]. Extensive efforts have been undertaken by both the petroleum refining industry and the automobile industry to mitigate these environmental problems, for example, by adopting automotive catalytic converters and cleaner fuel. Among these strategies, tightening transportation fuel specification is a major aspect of reducing automobile emissions. Worldwide fuel specifications are showing clear trends toward lower olefins and sulfur contents without sacrificing the octane number of gasoline[2]. In China, a new specification (GB 17930—2006) to control harmful substances in unleaded gasoline has been put into effect starting January 1, 2010. It requires gasoline to contain ≤30 v% of olefins, ≤40 v% of aromatics, ≤1.0 v% of benzene and<150 mg/g of sulfur. Since the FCC gasoline currently makes up over 75% of the whole commercial gasoline pool in China, the quality of FCC gasoline will directly affect the performance of finished gasoline product. Moreover, since the olefin content in FCC gasoline is excessively high (about 40v%—60v%) due to the heavy and inferior feedstocks widely used at Chinese refineries, it is very urgent to reduce the olefin content in FCC gasoline. On the other hand, propylene is an important building block of the petrochemical industry. In recent years, propylene demand has been continuously increasing. Therefore, much effort has been focused on improving the FCC process to simultaneously produce cleaner gasoline and more propylene. This demand calls for a novel multifunctional catalyst.
Among numerous complex reactions occurring in FCC process, hydrogen transfer reactions between olefins and naphthenes to yield paraffins and aromatics have a significant impact on the product quality, in particular the olefin yield. To reduce the olefin content in FCC gasoline, hydrogen transfer reaction must be enhanced[3]. It is well known that the Y zeolite is favorable for catalyzing hydrogen transfer reaction since its steric structure can promote the transition state to perform hydride transfer. It hasbeen stressed that for the case of Y zeolite, the higher the dealumination degree, the lower the site density and the more intense the site isolation, all of which would cause bimolecular hydrogen transfer reactions to decline[4].
Using ZSM-5 zeolite as an additive to FCC catalyst to improve the gasoline octane number is well known to attain growing importance[5-6]. However, the additive is used primarily to increase the yields of C3—C5olefins. The reaction mechanisms of ZSM-5 zeolite in combination with REUSY catalysts have been described in detail[7-12]. When the ZSM-5 zeolite is used as a separate additive, the synergy between ZSM-5 zeolite and the base catalyst have been investigated[13-15].
The ZSM-5 zeolite can be added as a separate additive, or it can be incorporated into the base FCC catalyst. Although the use of ZSM-5 zeolite as a separate additive has more flexibility, it requires the addition of two separate base catalysts and additive. Routinely adding one single FCC base catalyst containing the ZSM-5 zeolite into the FCC unit is relatively easier and more convenient to achieve for operators. In fact, catalysts containing dual zeolites have already been developed and commercialized to produce lower olefin content gasoline while increasing propylene yield and maintaining the octane number of FCC naphtha at the same time[16-17].
Since the gasoline olefinicity primarily depends on the hydrogen transfer activity of Y zeolite while light olefins production relies on the β-scission reaction taking place on the ZSM-5 zeolite, it is of interest to explore how the ZSM-5 zeolite performs in combination with the Y zeolite. Nevertheless, very few studies have been conducted to investigate the effect of Y zeolite to ZSM-5 zeolite ratio in a catalyst on protolytic cracking, β-scission, oligomerization and hydrogen transfer reactions[18]in relation to conversion. Such information will help determine the product distribution and product quality. In this work, five catalysts with various ratios of Y zeolite to ZSM-5 zeolite were prepared to investigate the effect of the Y zeolite to ZSM-5 zeolite ratio on the relative proportion of reactions such as protolytic cracking, β-scission, oligomerization and hydrogen transfer at different reactant conversions. The objective of this study is to explore the optimum composition of Y and ZSM-5 zeolites to develop novel catalysts for achieving lower olefins content in gasoline, higher propylene yield and better product slate.
2 Experimental
2.1 Feedstock and catalysts
FCC naphtha was used as the feed in this study since it is difficult to characterize regular FCC heavy feedstock such as VGO in detail. Another advantage of choosing FCC naphtha is the ability to examine all kinds of chemical reactions during catalytic cracking, such as oligomerization. The FCC naphtha used in this study was provided by the Sinopec’s Changzhou refinery with its properties given in Table 1. The composition and the distillation data were determined by PIONA and ASTM D86 analyses, respectively.
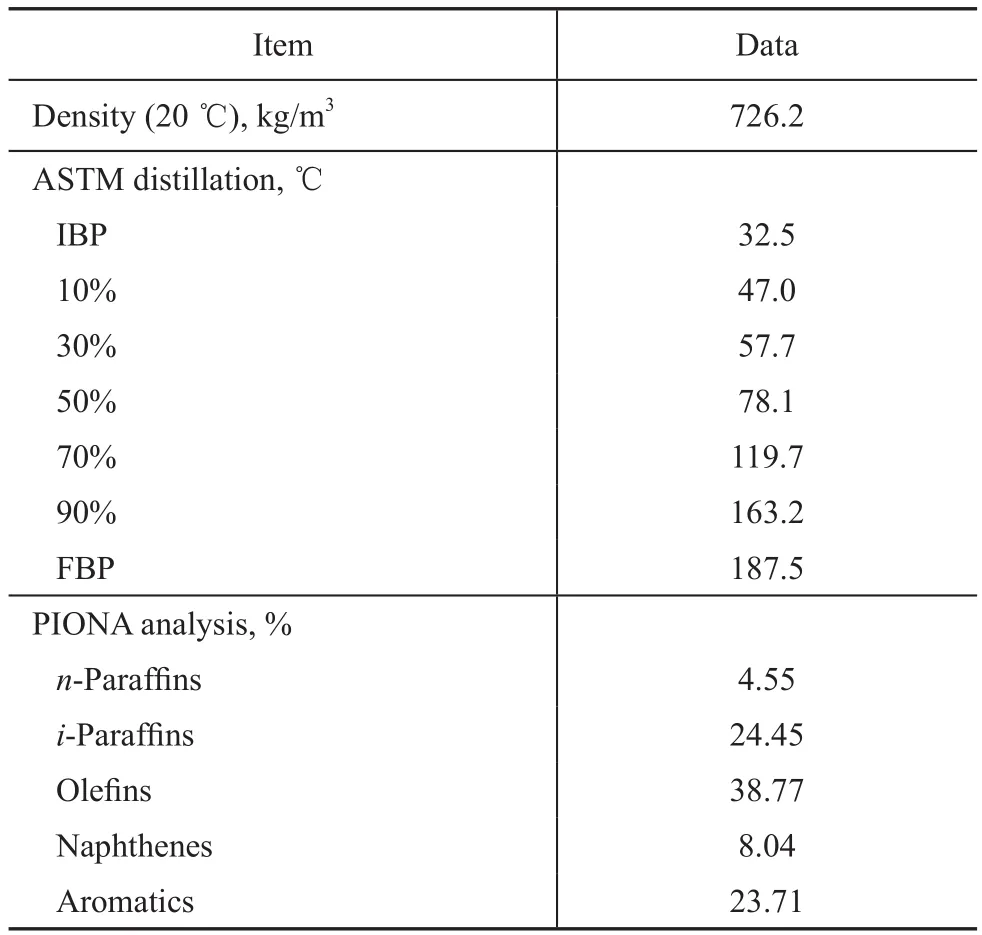
Table 1 FCC Naphtha Feed properties
Three composite zeolites based catalysts, labeled as N164, N165 and N166, have been prepared with different mixing ratios of Y zeolite to ZSM-5 zeolite. The mass ratio of Y zeolite to ZSM-5 zeolite is 4:1 in the catalyst N164, 3:2 in the catalyst N165, and 1:4 in the catalyst N166. In addition, pure Y and ZSM-5 zeolites were also prepared which were denoted as catalyst N161 and N162, respectively. The total mass concentration of zeolites in each individual catalyst was all the same. All the five catalysts have been deactivated with 100% steam at 800℃. To achieve similar MAT activity for all catalysts in order to better evaluate these catalysts in subsequent catalyticcracking tests, the aging time was set differently according to their different hydrothermal ability based on their respective zeolite composition. The characteristics of the five catalysts after deactivation are given in Table 2.

Table 2 Characteristics of the aged catalysts used in this work
2.2 Apparatus
Catalytic cracking tests under atmospheric pressure were carried out in a confined fluidized bed reactor shown in Figure 1.
A weighed FCC naphtha feed was mixed with dispersed steam before being pumped into the preheater. The preheated mixture was then introduced into the reactor for taking part in the catalytic cracking reactions. After reaction, the entrained hydrocarbons on the coked catalyst were removed through steam stripping, while the hydrocarbon products were cooled down and separated into liquid and gas samples.
An Agilent 6890 gas chromatograph equipped with the ChemStation software was used to measure the volume percentage of components in the gas product. The ideal gas law was used to convert the volume data into mass percentages. The liquid product was analyzed by two methods. The first method used a simulated distillation gas chromatograph to analyze the mass fraction of gasoline, LCO and heavy oil. In the second method, PIONA analysis was used to determine the composition of the gasoline fraction. PIONA analysis is a multi-dimensional analysis to determinen-paraffins,i-paraffins, olefins, naphthenes, and aromatics in gasoline by gas chromatograph. It uses traps and multiple columns to separate aromatics, olefins and saturates, while temperature programming is used to separate paraffins and naphthenes by carbon number[19]. The coked catalyst was regenerated with pure oxygen gas to restore its activity. The flue gas, after having measured its volume, was analyzed by an Agilent 7890 gas chromatograph for determining the composition of CO and CO2to calculate the coke yield.

Figure 1 Schematic diagram of confined fluidized bed reactor
The FCC operation was conducted under the conditions shown below. The cracking temperature was set at 480 ℃;the catalyst to oil mass ratio varied between 3 and 9 to achieve different conversion rates; and the weight hourly space velocity (WHSV) varied from 6 h-1to 20 h-1.
Blank tests showed that the thermal cracking of FCC naphtha under the selected operating conditions was negligible.
Since FCC naphtha was used as the feedstock in this study, the conversion was de fined as 100 minus the gasoline yield in the product. The product selectivity was defined as the product yield divided by the conversion.
3 Results and Discussion
The cracking ability of the five catalysts prepared in this work is given in Table 3. It can be seen from Table 3 that the N162 catalyst (pure ZSM-5 zeolite) had the highest ability to crack gasoline feedstock with a highest conversion under the same operating conditions. The cracking ability of other studied catalysts decreased in the following order: N161>N164>N166>N165.
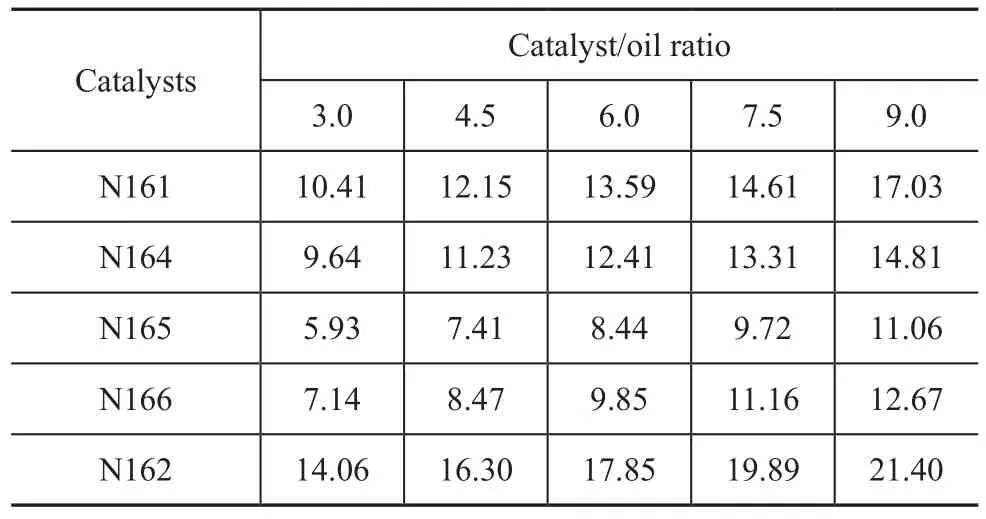
Table 3 Cracking ability of different catalysts %
3.1 Protolytic cracking
It is well known that the introduction of ZSM-5 zeolite into the catalyst composition should increase the ratio of protolytic reaction versus β-scission cracking, and would have an important impact on the product distribution since protolytic cracking is the initiation of paraffins cracking[20]. Since the product distribution obtained by pure protolytic cracking is very similar to that obtained by a radical-type mechanism with high H2, CH4, C2concentrations[21], dry gas (H2, and C1—C2hydrocarbons) can be viewed as the characteristic product of protolytic cracking. The profile of dry gas selectivity against conversion therefore can be used to characterize the impact of protolytic cracking reaction.
The profile of the dry gas selectivity versus conversion is presented in Figure 2.

Figure 2 Dry gas selectivity profile versus conversion
Although the N162 catalyst exhibited the highest conversion, it was observed from Figure 2 that the N162 catalyst did not show accordingly the highest selectivity of dry gas at a constant conversion. Instead, the N166 catalyst had the highest dry gas selectivity. The N161 catalyst showed the lowest dry gas selectivity, which was understandable since the Y zeolite has weaker protolytic cracking property as compared with the ZSM-5 zeolite according to the literature information.
The reason why the N162 catalyst did not show the highest protolytic cracking ability might be related to the feedstock and the zeolite composition of the catalyst in this study. The feedstock (FCC naphtha) contains both paraffins and olefins. So the protolytic cracking reaction could not come into play completely on this feedstock. In fact, olefins might have contributed considerably to the initiation because olefinic double bonds could be easily attacked by the Brönsted acid sites of the catalyst to form carbenium ions. Once the carbenium ions formed, the catalytic cracking reactions could proceed with the transfer of a hydride ion from a reactant molecule to an adsorbed carbenium ion, which was favorable for the Y zeolite due to its steric structure. Hydride transfer can result in new paraffin production which could be the reactant of protolytic cracking. So with FCC naphtha used as the feedstock, the synergetic effect of the Y and ZSM-5 zeolites (Y/ZSM=1:4) in the N166 catalyst might be contributed to its highest dry gas selectivity at a constant conversion.
It can be seen from Figure 2 that among the three catalysts containing composite zeolites (N164, N165, and N166) the dry gas selectivity increased with an increasing ZSM-5 ratio in the composite catalyst, indicating that the ZSM-5 zeolite did increase the probability of the protolytic cracking.
It should be pointed out that the dry gas selectivityvs. conversion profile using FCC naphtha as the feedstock is different from the case using VGO as the feedstock. Figure 2 clearly shows that the dry gas selectivity continuously increases as the conversion increases with FCC naphtha feedstock used in this study. However, when using VGO as the feedstock as studied by Gong,et al.[22], the dry gas selectivity at first decreases and then increases showing a minimum value as conversion increases[22]. The reason was that the initial reaction with VGO feed could only be the protolytic cracking reaction, resulting in a high dry gas selectivity at low conversion. As conversion increases, bimolecular reactions become predominant to cause the decrease of dry gas selectivity. An explanation has been presented by Gong,et al.[22]
3.2 β-scission
Since FCC naphtha was used as the feedstock in this study, the products of β-scission should be mainly C3and C4hydrocarbons. The LPG selectivity was therefore used to examine β-scission reaction occurring over different catalysts[15]. The profile of LPG selectivity with conversion over different catalysts is given in Figure 3. It shows that the LPG selectivity, and thus the selectivity of β-scission, decreased with an increasing conversion, which might be ascribed to the enhancement of hydrogen transfer reaction with the increase of conversion as mentioned in Section 3.4. It can also be found out from Figure 3 that the N166 catalyst showed the highest β-scission ability while the N161 catalyst with pure Y zeolite exhibited the lowest β-scission selectivity at a constant conversion. The β-scission ability of other three catalysts decreased in the following order: N162>N165>N164. Upon comparing Figure 3 and Figure 2, one can see a slight difference in ranking of the five catalysts in terms of their protolytic cracking ability and β-scission ability. It seems that the protolytic cracking property of the N165 catalyst is greater than that of the N162 catalyst, which shows an opposite trend in terms of the β-scission ability.

Figure 3 Profile of LPG selectivity with conversion
The profile of propylene yield with conversion over different catalysts is presented in Figure 4, which shows an increasing propylene yield with the increase in conversion for all five catalysts. For those three catalysts (N164, N165, and N166) containing composite zeolites, the propylene yield increased as the ratio of ZSM-5 zeolite in the catalysts increased at a constant conversion. But the propylene yield remained almost constant upon comparing the catalyst N166 with the catalyst N162 at the same conversion. This indicates that further increasing the ratio of ZSM-5 beyond 80% cannot further enhance the propylene yield. An optimum ratio of ZSM-5 zeolite to Y zeolite, equating to 4:1 in this study, exists for the propylene production.
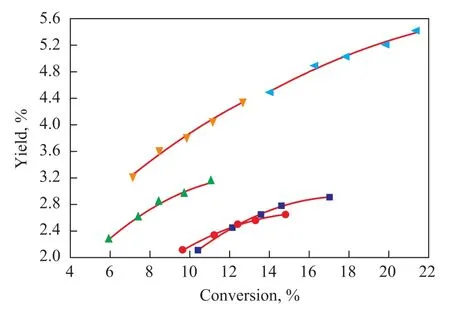
Figure 4 Profile of propylene yield with conversion for different catalysts
Figure 5 presents the profile of propylene selectivity with conversion over different catalysts, which shows adecreasing propylene selectivity with the increase in conversion. The reason may be the same as the case for LPG selectivity.

Figure 5 Profile of propylene selectivity with conversion
3.3 Oligomerization
Oligomerization is often applied to convert light alkenes into fuels, so it should be avoided as much as possible if the aim of FCC process is to maximize light alkene production. Since FCC naphtha was used as the feedstock in this study, so LCO could be treated as the oligomers. The selectivity for oligomers against conversion for all catalysts is presented in Figure 6. For all the catalysts, the oligomerization selectivity decreased in the following order: N161>N164>N162>N165>N166. It was found out that for those three catalysts containing composite zeolites, at a constant conversion the oligomerization selectivity decreased as the ratio of ZSM-5 to Y zeolite increased. This might be due to the fact that the oligomerization reactions proceed on the large external surface areas or in the mesopores of the zeolites[23]. But for the catalyst N162 containing pure ZSM-5 zeolite, its oligomerization selectivity was not the poorest one. This might be caused by shape selective cracking over the ZSM-5 zeolite to produce significant amount of light olefins, which as reactants were favorable for proceeding secondary oligomerization reaction. It was noticed that for catalysts N161, N164 and N162, the oligomerization selectivity nearly decreased with an increasing conversion. This could be explained by taking into account that secondary cracking of oligomers might occur as the conversion increased. The oligomerization selectivity, which increased with the conversion observed on catalysts N165 and N166, might be related with the extent of oligomerization selectivity that still did not reach the maximum[23]due to lower conversion.

Figure 6 LCO selectivity against conversion
3.4 Hydrogen transfer
Because of its direct link with product quality and catalyst performance, hydrogen transfer is a critical issue for FCC reactions. Efforts have been devoted to the characterization of hydrogen transfer catalyst properties through different approaches and to the elucidation of factors governing the reaction[24]. Different indexes or product distribution parameters such asi-butane yield[25], and the ratio of butanes to butenes[26]have been used to estimate the hydrogen transfer performance of a given catalyst or series of catalysts. In this study the ratio of butanes to butenes was used to characterize the hydrogen transfer ability of five catalysts studied.
The profile of the butanes/butenes ratio against conversion over five catalysts is presented in Figure 7. The ratio of butanes to butenes over all these catalysts increased greatly with an increasing conversion, indicating to the increasing rate of hydrogen transfer reaction. It is known that the Y zeolite based catalyst has higher hydrogen transfer ability as compared to the ZSM-5 zeolite based catalyst which is also shown in Figure 7. One would think that catalysts with composite zeolites (Y/ZSM-5) should have less hydrogen transfer ability when the ratio of ZSM-5 zeolite to Y zeolite in the catalyst increases. This is not the case based on this study. As shown in Figure 7, when the ZSM-5 to Y zeolite ratio is less than 2/3, thehydrogen transfer ability of the composite zeolite catalyst increases with an increasing ZSM-5 zeolite to Y zeolite ratio at a constant conversion, and is higher than that of pure Y zeolite based catalyst. But when the ZSM-5 zeolite to Y zeolite ratio in the catalyst exceeds a specified value (i.e., 2/3 in this study), the hydrogen transfer ability of the composite zeolite based catalyst declines appreciably, with an activity lying between the pure Y zeolite based catalyst and the pure ZSM-5 zeolite based catalyst.
Such results for the impact of Y/ZSM-5 composite zeolites on hydrogen transfer in this study are different from what reported by I. P. Dzikh[15]who used heptane as the feed. The difference might be caused by the fact that Dzikh,et al. used a mixture of USHY+HZSM-5 zeolites instead of Y/ZSM-5 zeolites applied in this study.
Hence, to enhance the hydrogen transfer ability of the catalyst containing composite zeolites (ZSM-5+Y), an effective method is to raise the ZSM-5 to Y zeolite ratio moderately so long as it does not exceed a certain value (i.e., 2/3 in this study).
This could be explained as follows. Under a certain ZSM-5 zeolite/Y zeolite ratio, the amount of ZSM-5 zeolite may be not enough to affect the hydrogen transfer ability of Y zeolite. However, the presence of ZSM-5 zeolite can enhance the production of light olefins and in turn the hydrogen transfer since light olefins are the reactant of hydrogen transfer reaction. Increasing ZSM-5 concentration in the composite zeolite catalyst can therefore improve its ability in hydrogen transfer. Further increase in the ZSM-5 concentration, however, will make the steric limitation in the ZSM-5 pores predominant to restrict thereby the bimolecular hydrogen transfer reaction. Another possible cause of the observation in this study might be relevant to the catalyst composition. In this study, the Y and ZSM-5 zeolites are incorporated simultaneously in a catalyst with a short distance for reactant molecules to diffuse between the Y and ZSM-5 zeolites. The synergetic effect of the Y and ZSM-5 zeolites on hydrogen transfer reaction might be different from other researcher’s work in which the Y zeolite based catalyst is mechanically mixed with the ZSM-5 based additives.

Figure 7 The ratio of butanes to butenes against conversion
Hydrogen transfer reaction has an important impact on the composition of FCC gasoline in the product, especially on the olefin content. The olefin content of FCC gasoline in the product against conversion is depicted in Figure 8. It can be seen from Figure 8 that the olefin content with conversion profile can fairly reflect the hydrogen transfer property of different catalysts. The olefin content decreased with an increasing conversion due to the enhancement of hydrogen transfer reaction. In addition, the FCC gasoline olefins variation trend agreed well with the hydrogen transfer ability of different catalysts presented in Figure 7. For example, as shown in Figure 7, the N162 catalyst showed the weakest hydrogen transfer ability while the N165 catalyst exhibited the strongest ability. Accordingly, the olefin content in gasoline was the highest on the N162 catalyst while the lowest olefins content was seen on the N165 catalyst at a constant conversion.
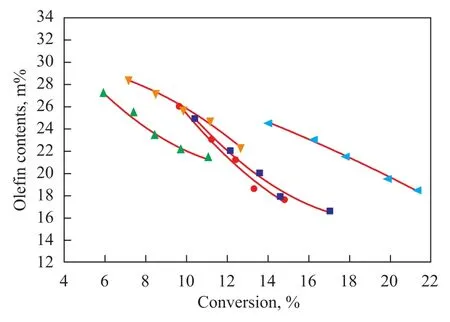
Figure 8 FCC gasoline olefin contents against conversion
4 Conclusions
Five composite catalysts containing different ratios of Y to ZSM-5 zeolites have been prepared to examine the synergic effect of Y+ZSM-5 zeolites on protolytic crack-ing, β-scission, oligomerization and hydrogen transfer reactions. The following conclusions can be drawn:
1) Though it is known that the addition of ZSM-5 zeolite into the catalyst enhances the protolytic cracking, the pure ZSM-5 zeolite based catalyst did not exhibited the highest dry gas selectivity.
2) Among all catalysts tested, the catalyst containing composite zeolites with a Y/ZSM-5 ratio of 1:4 gave the strongest ability of protolytic cracking and β-scission along with the highest dry gas and LPG selectivity.
3) Pure Y zeolite based catalyst had the lowest protolytic cracking and β-scission ability, but the strongest oligomerization ability.
4) The hydrogen transfer property of the tested composite catalysts increased with an increasing ZSM-5 concentration when the ratio of ZSM-5 zeolite/ Y zeolite was less than a certain value (i.e., ZSM-5:Y=2:3 in this study). When the ZSM-5/Y ratio exceeded that level, the hydrogen transfer ability of the composite zeolites based catalyst would decrease greatly.
Acknowledgement:The authors would like greatly appreciate the financial support from the National Key Technology R&D Program (2012BAE05B01) of China, and Dr. Xu Yun and Dr. Liu Yujian for offering the catalyst samples.
[1] Zhang P Q, Guo X W, Guo H C, et al. Study of the performance of modified nano-scale ZSM-5 zeolite on olefins reduction in FCC gasoline[J]. J Mol Catal A, 2007, 261(2): 139-146
[2] Long J, Lin W, Qiu Z H, et al. Catalyst CGP-1 for MIPCGP process to increase cleaner gasoline and propylene production [J]. Stud Surf Sci Catal, 2007, 166: 55-66
[3] Biswas J, Maxwell I E. Recent process and catalyst-related developments in fluid catalytic cracking[J]. Appl Catal, 1990, 63(1): 197-258
[4] Pine L A, Maher P J, Wachter W A. Prediction of cracking catalyst behavior by a zeolite unit cell size model[J]. J Catal, 1984, 85(2): 466-476
[5] Scherzer J. Octane-enhancing, zeolitic FCC catalysts: Scientific and technical aspects[J]. Catal Rev Sci Eng, 1989, 31(3): 215-354
[6] Creighton J E, Edwards G C, Rajagopalan K, et al. Strategies for catalytic octane enhancement in an FCC unit[J]. ACS Preprints, Division of Petrol Chem, 1987, 32(3/4): 617-620
[7] Donnelly S P, Mizrahi S, Sparrell P T, et al. How ZSM-5 works in FCC [J]. ACS Preprints, Division of Petrol Chem, 1987, 32(3/4): 621-626
[8] Buchanan J S, Adewuyi Y G. Effects of high temperature and high ZSM-5 additive level on FCC yields and gasoline composition[J]. Appl Catal A, 1996, 134(2): 247-262
[9] Buchanan J S. Gasoline selective ZSM-5 FCC additives: Model reactions of C6—C10olefins over steamed 55:1 and 450:1 ZSM-5[J]. Appl Catal A, 1998, 171(1): 57-64
[10] Adewuyi Y G, Klocke D J, Buchanan J S. Effects of highlevel additions of ZSM-5 to a fluid catalytic cracking (FCC) REUSY catalyst [J]. Appl Catal A, 1995, 131(1): 121-133
[11] Adewuyi Y G. Compositional changes in FCC gasoline products resulting from high-level additions of ZSM-5 zeolite to REUSY catalyst [J]. Appl Catal A, 1997, 163(1/2): 15-29
[12] Madon R J. Role of ZSM-5 and ultrastable Y zeolites for increasing gasoline octane number[J]. J Catal, 1990, 129(1): 275-287
[13] Hollander M A D, Wissink M, Makkee M L, et al. Synergy effects of ZSM-5 addition in fluid catalytic cracking of hydrotreated flashed distillate[J]. Appl Catal A, 2002, 223(1/2): 103-119
[14] Zhao X, Harding R H. ZSM-5 additive in fluid catalytic cracking. 2. Effect of hydrogen transfer characteristics of the base cracking catalysts and feedstocks[J]. Ind Eng Chem Res, 1999, 38(10): 3854-3859
[15] Dzikh I P, Lopes J M, Lemos F, et al. Mixing effect of USHY+HZSM-5 for different catalyst ratios on the n-heptane transformation[J]. Appl Catal A, 1999, 176(2): 239-250
[16] Katoh S, Nakamura M, Skocpol B. Reduction of olefins in FCC gasoline[J]. Stud Surf Sci Catal, 2001, 134: 141-152
[17] Qiu Z H, Lu Y B, Li C Y. FCC catalyst with high LPG yield and lower gasoline olefin content [J]. Stud Surf Sci Catal, 2004, 149: 297-304
[18] Corma A, Miguel P J, Orchilies A V. The role of reaction temperature and cracking catalyst characteristics in determining the relative rates of protolytic cracking, chain propagation, and hydrogen transfer[J]. J Catal, 1994, 145(1): 171-180
[19] Buchanan J S, Nicholas M E. Analysis of olefinic gasoline with multidimensional gas chromatography[J]. J Chro-matogr Sci, 1994, 32(5):199-203
[20] Haag W O, Dessau R M. Duality of mechanism for acidcatalyzed cracking[C]//Basel V C. Proceedings of the 8thInternational Congress on Catalysis, Berlin. Dechema: Frankfurt-am-Main, 1984, 305-316
[21] Corma A, Orchilles A V. Current views on the mechanism of catalytic cracking [J]. Micropo Mesopor Mater, 2000, 35-36: 21-30
[22] Gong J H, Long J, Xu Y H. Protolytic cracking in Daqing VGO catalytic cracking process[J]. J Fuel Chem Technol, 2008, 36(6): 691-695
[23] Pater J P G, Jacobs P A, Martens J A. Oligomerization of Hex-1-ene over acidic aluminosilicate zeolites, MCM-41, and silica-alumina Co-gel catalysts: A comparative study[J]. J Catal, 1999, 184(1): 262-267
[24] Sedran U A. Laboratory testing of FCC catalysts and hydrogen transfer properties evaluation[J]. Catal Rev Sci Eng, 1994, 36(3): 405-431
[25] Gates B C, Katzer J R, Schuit G C A. Chemistry of Catalytic Processes[M]. New York: McGraw-Hill, 1979,
[26] Corma A, Faraldos M, Martinez A, et al. Hydrogen transfer on USY zeolites during gas oils cracking: influence of the adsorption characteristics of the zeolite catalysts[J]. J Catal, 1990, 122(2): 230-239
Received date: 2014-02-24; Accepted date: 2014-08-05.
Dr. Gong Jianhong, Telephone: +86-10-82369228; E-mail: gongjh.ripp@sinopec.com.
- 中国炼油与石油化工的其它文章
- Preparation of Tungsten Film and Its Tribological Properties under Boundary Lubrication Conditions
- Effect of Stirring on Oil-Water Separation in Rare Earth Mixer-Settler
- Spray Characteristics Study of Combined Trapezoid Spray Tray
- Viscoelastic Characteristics of Asphalt Binders at Softening Point Temperature
- Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
- Preparation of Spherical MgCl2/SiO2/THF-Supported Late-Transition Metal Catalysts for Ethylene Polymerization

