Synthesis of a Novel Solid Acid with both Sulfonic and Carbonyl Acid Groups and Its Catalytic Activities in Acetalization*
CHENG Yuxiao (程欲晓), ZHOU Yuyan (周宇艳), ZHANG Jidong (张继东), FEI Xudong (费旭东), MA Tengzhou (马腾洲)and LIANG Xuezheng (梁学正)
1Inspection Center of Industrial Products and Raw Materials, Shanghai Entry-Exit Inspection and Quarantine Bureau, Shanghai 200135, China
2Institute of Applied Chemistry, Shaoxing University, Shaoxing 312000, China
Synthesis of a Novel Solid Acid with both Sulfonic and Carbonyl Acid Groups and Its Catalytic Activities in Acetalization*
CHENG Yuxiao (程欲晓)1, ZHOU Yuyan (周宇艳)1, ZHANG Jidong (张继东)1, FEI Xudong (费旭东)1, MA Tengzhou (马腾洲)1and LIANG Xuezheng (梁学正)2,**
1Inspection Center of Industrial Products and Raw Materials, Shanghai Entry-Exit Inspection and Quarantine Bureau, Shanghai 200135, China
2Institute of Applied Chemistry, Shaoxing University, Shaoxing 312000, China
The novel solid acid with both sulfonic and carbonyl acid groups has been synthesized from 3-((2-sulfoethoxy) carbonyl)acrylic acid and tetraethyl orthosilicate (TEOS). The catalytic activities were investigated through the acetalization. The results showed that the novel solid acid was very efficient for the reactions with the high yields. The high acidity, high stability and reusability were the key feature of the novel solid acid. Moreover, the sulfonic and carbonyl acid groups could cooperate during the catalytic process, which improved its catalytic activities. The catalyst shows recyclability, and hold great potential for replacement of homogeneous catalysts.
solid acid with sulfonic and carbonyl acid groups, acetalization, green chemistry
1 INTRODUCTION
Acid catalysts are very important in chemical industries for production of various useful chemicals [1]. Large amounts of homogeneous acids such as sulfuric acid have been used for the purpose, which needs tedious post-treatment and produces numerous wastes [2, 3]. Various solid acids were developed to replace these acids [4-6]. Recently, carbon based solid acid catalysts attract much attention because of the high acidity and catalytic activities [7-9]. However, these acids have low Brunauer-Emmett-Teller (BET) surface with amorphous structures. The hydrophilic groups on the surface prevent incorporation of hydrophobic molecules interacted with the acid sites, which makes it unsuitable for the reactions between hydrophobic compounds. Furthermore, the amorphous structure adds the difficulty in recovery [10].
The silica materials have attracted much attention because of their adjustable porous structures and potential applications in catalysis [11, 12]. However, the pure siliceous materials are difficult to act as catalysts for little functional groups [13]. Therefore, various acid sites are introduced onto the silica structure and act as solid acid catalysts. Sulfuric acid is successfully supported on silica-gel and showed high activities for aromatic nitration [14] and synthesis of trisubstituted imidazoles [15]. The silica-included heteropolyacid catalysts show high activities for the alkylation of phenol [16]. Lewis acid supported on silica-gel is developed and used for the synthesis of benzophenones [17]. Perchloric acid is also immobilized on silica and shows high activities for the protection of hydroxyl groups [18].
Although the catalytic activities of these silica supported acid catalyst are very high for the first time, the recycled activities drop quickly, which indicates that the solid acids are not stable and the active sites are depleted from the solid substrate. It is generally known that the liquid acids such as sulfuric acid, perchloric acid, Lewis acid and heteropolyacid own relatively low molecule sizes and the active sites could pass through the silica pores and escape from the solid, which accounts for the low stability. In this work, novel solid acid was synthesized from 3-((2-sulfoethoxy) carbonyl)acrylic acid and tetraethyl orthosilicate (TEOS) (Fig. 1). 3-((2-Sulfoethoxy)carbonyl)acrylic acid was used as the functional molecule, which could supply both the sulfonic and carbonyl acid groups and could act as the active sites. Furthermore, the functional molecule owns double bonds, which could polymerize to increase the molecule size and avoid the release of acid sites. The silica offers the regular structure with high BET surface. The catalytic activities of the novel solid acid were tested through acetalization reaction. The results show that the novel solid acid owned high activity and stability for the reaction.
2 EXPERIMENTAL
2.1 Apparatus and reagents
All organic reagents were commercial products of the highest purity available (>98%) and used for the reaction without further purification.
2.2 Synthesis of the novel solid acid
In the typical procedure, maleic anhydride (1.96 g, 20 mmol) was dissolved in 15 ml ethyl acetate. Hydroxyethylsulfonic acid (2.52 g, 20 mmol) was addedto the solution drop by drop. The mixture was kept stirred for 4 h at room temperature (25 °C). Then, the solvent was evaporated under reduced pressure using a rotary evaporator to obtained the colorless liquid monomer 3-((2-sulfoethoxy)carbonyl)acrylic acid with the purity over 98%. 2 g monomer, 2 g tetraethyl orthosilicate (TEOS), 0.01 g azobisisobutyronitrile (AIBN), 15 ml ethanol and 2 ml water were mixed together to form homogenous solution, which was heated to 70 °C and stirred for 12 h to form solid gel. The gel was directly dried at room temperature overnight and ground to powder. It was washed with hot water (>80 °C) and filtered until no acidity detected in the filtrate. The novel solid acid was obtained after drying at 120 °C overnight in oven.
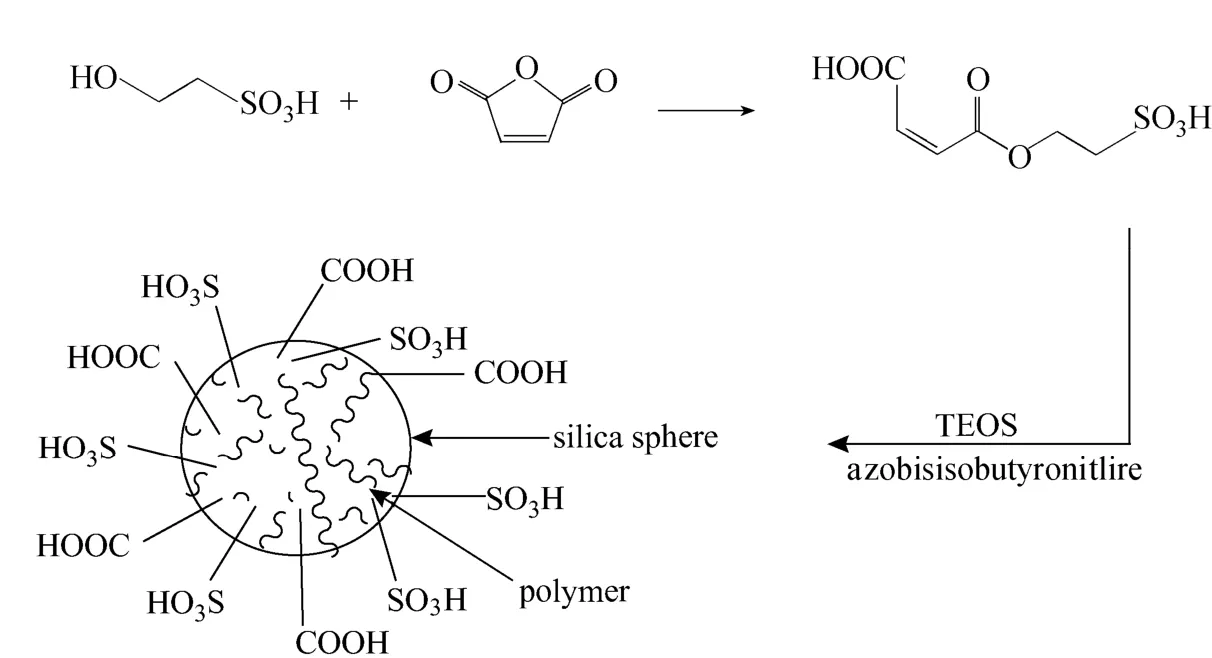
Figure 1 The synthesis route of a novel solid acid
2.3 Preparation of acetals and ketals
The typical procedure is: An aldehyde or ketone (0.1 mol), 10 ml cyclohexane, a diol (0.15 mol) and the catalyst (0.05 g) were mixed together in a three necked round bottomed flask equipped with a magnetic stirrer and a thermometer, and a Dean-Stark apparatus was used to remove the water continuously from the reaction mixture. The mixture was heated at 120 °C for the specified time as shown in Table 1. The process of reaction was monitored by gas chromatograph (GC) analysis of the small aliquots withdrawn at 0.5 h intervals. Quantitative analysis of the product was carried out on a temperature-programmed Shimadzu gas chromatograph (GC-14C) with OV-17 column and flame ionization detector (FID) using nitrogen as carrier gas. The column temperature was raised from 40 to 260 °C at a rate of 10 °C·min−1. On completion, the catalyst was recovered by filtering and washed with acetone, then dried in oven at 80 °C for about 1 h.
3 RESULTS AND DISCUSSION
3.1 Characterization of the solid acid
The dual acidic solid was white powder, with the acidity of 2.6 mmol·g−1as determined through neutralizing titration. The novel solid acid owned much higher acidity than that of the traditional heterogeneous acids such as Nafion. Moreover, the acidity could be adjusted through changing the molar ratio of the functional compounds and TEOS. The more functional molecule used, the higher acidity obtained. The highest acidity of 5.4 mmol·g−1could be achieved when 4.8 g functional compounds and 2 g TEOS was used. On the other hand, the structure and BET surface were affected by the TEOS amount. The viscous resin-like mixture instead of solid material when the mass ratio of the function molecule to TEOS was above 2.5.
The thermal stability of the novel solid acid was investigated on Mettler-Toledo Thermogravimetric Analyzer (Model SDTA851e) with heating rate of 10 °C·min−1under nitrogen atmosphere. The thermogravimetry (TG) analysis showed that the mass loss took place above 180 °C, which indicated that solid acid had much higher thermal stability than the traditional ions exchange resins (below 120 °C). The thermodesorption of chemisorbed ammonia [NH3-TPD (temperature programmed desorption)] of the solid acid was taken on the Micrometrics Chem BET (Model TPD/TPR2900, USA) with heating rate of 10 °C·min−1from 100 to 800 °C to examine the acid strength. The results showed that the solid acid owned both the weak acidity in which ammonia was desorbed below 200 °C and strong acidity in which ammonia was desorbed at 400 to 600 °C, which were quite in accord with the chemical structure of the solid acid. The BET surface area measurements were performed by Quancachrome BET equipment (Model 02108-KR-1) using nitrogen adsorption-desorption isotherms at the temperature of liquid nitrogen. The solid acid had the BET surface of 325 m2·g−1, which was quite high compared to the carbon based solid acid [7-10].
The Fourier transform infrared spectroscopy (FT-IR) of the solid acid was carried out on the Nicolet Nuxes 670 instrument using the KBr tablet compression method. The IR spectrum (Fig. 2) showed the strong sulfonic acid group absorbability at 1040 and 850 cm−1and carbonyl group (C O) absorbability at 1640 cm−1, which confirmed the dual acidic groups.FT-IR spectrum also showed that the solid acid contained resident functionalities including(1150 cm−1), and OH (3400 cm−1).
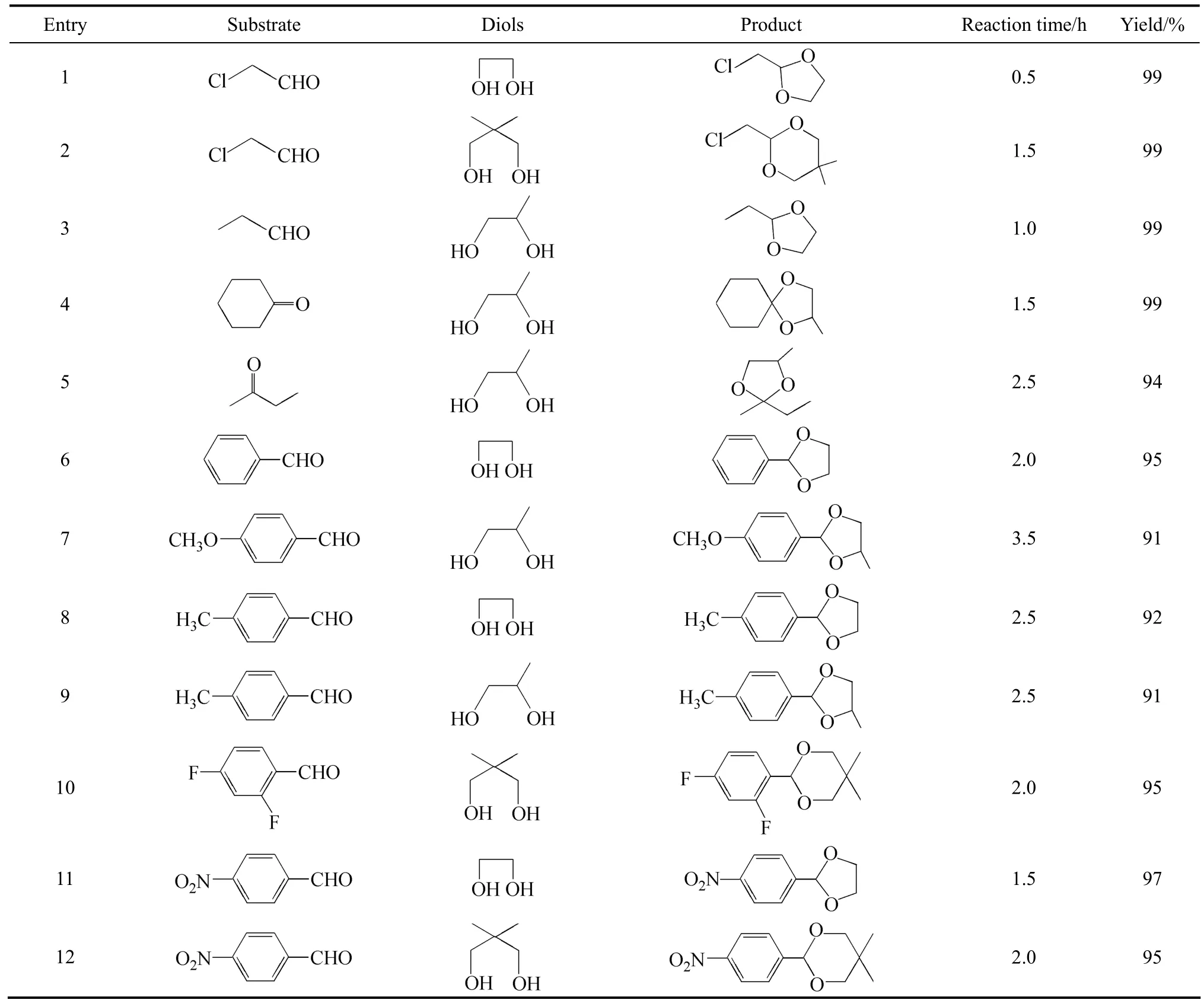
Table 1 Catalytic acetalization of various carbonyl compounds and diols
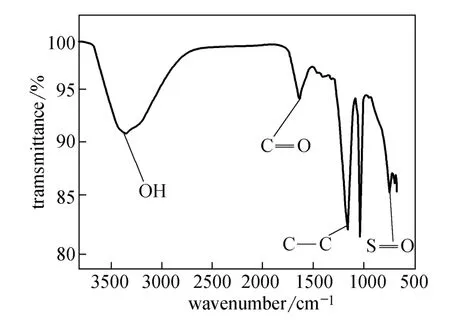
Figure 2 The IR spectrum of the solid acid
The scanning electron microscope (SEM) of the solid acid was done on the Hitachi S-4800 scanning electron microscope. The SEM images showed that the resulting particles were irregular spheres structure with the particle sizes of 2-5 μm as depicted in Fig. 3. The particle sizes decreased when the acid amount was increased and only gel molecules instead of solid precipitates formed when the acid amount was too high. The sol-gel process was catalyzed by H+ions, and more nuclei formed when more acid functional molecules were used, which resulted in smaller particle size and the particles adhered with each other. Meanwhile, the acidity of the solid acid changed with the acid functional molecule amount and the higher acidity was obtained when more functional molecules used. On the other hand, the silica structure might be destroyed when the TEOS amount was quite low so that no solid product formed then. The regular spherical structure with big dimension made the recovery ofthe solid acid quite simple without special procedure except for filtration.
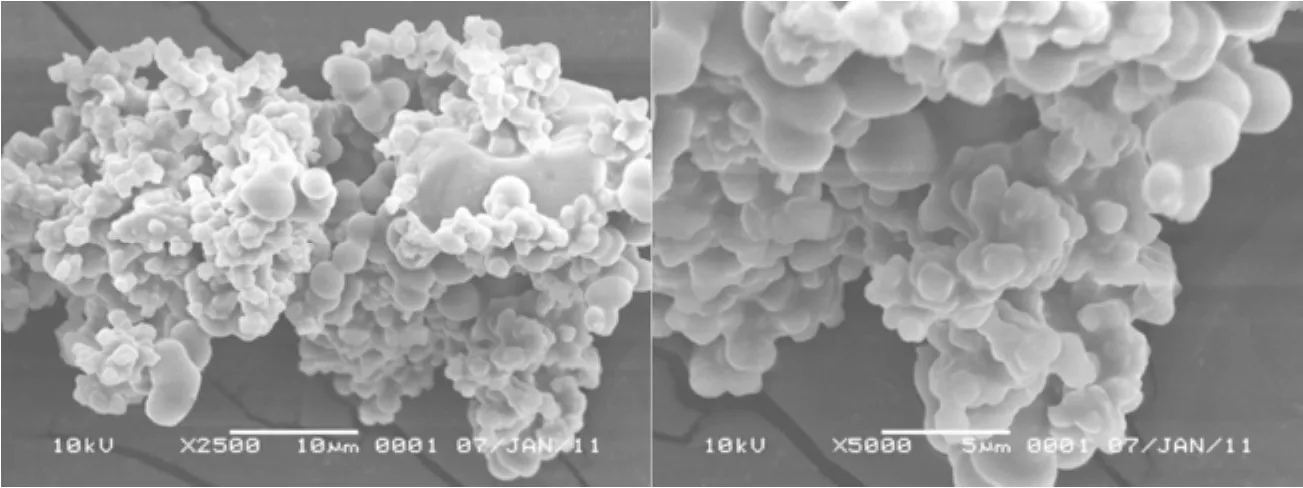
Figure 3 The SEM images of the novel solid acid
3.2 Catalytic procedure for the synthesis of acetals and ketals
The catalytic activities of the novel solid acid were investigated through the acetalization of various carbonyl compounds with diols (Table 1). The reactions were difficult to carried out when no catalyst was used, and only the aldehydes with high reactivities showed the yield of 63% in 12 h (Entry 1). Other reactants obtained the yield below 40% under the same reaction conditions. As was anticipated, the solid acid showed high activities for all the reactions. Aliphatic aldehydes transformed to the corresponding acetals with very high yield under the reaction conditions (Entries 1-3). The neopentyl glycol with high steric hindrance was also efficiently acetalized (Entry 2). The ketalization reaction of cyclohexanone worked very well with the active sites and the equal activities as the aliphatic aldehydes (Entry 4) were demonstrated. The ring structure fixed the carbonyl group of cyclohexanone, which made the carbonyl groups easier to be attacked by the acid sites. The linear ketones such as butanone owned relatively low activity for the alkyl chain on both sides of carbonyl added the steric hindrance (Entry 5). Aromatic aldehydes such as benzaldehyde could also be acetalized with high yields (Entry 6). The aromatic ring attached to the carbonyl group reduced the nucleophilicity greatly. The solid acid was also still very active for the reaction with the yield of 95% in 2.0 h. The groups attached to the aromatic ring affected the reactivities greatly. The aromatic aldehydes with electron-donating groups such as methyl or methoxyl groups showed relatively low activity, which might reduce the nucleophilicity of the carbonyl groups (Entries 7-9). On the other hand, electron-withdrawing groups enhanced the reactivities and the aromatic aldehydes with F and nitro groups showed higher activities (Entries 10-12). Different diols such as 1,2-ethanediol, 1,2-propylene glycol and neopentyl glycol were all efficiently transformed to the acetals smoothly. The conversions reduced as follows: 1,2-ethanediol>1,2-propylene glycol>neopentyl glycol for the steric hindrance of the diols. The result further confirmed that the solid acid owned high activity and wide applicability for the acetalization and ketalization.
3.3 The recycled activity of the solid acid
After reactions, the catalyst was recovered by filtration, and its activity was investigated through the acetalization of benzaldehyde and 1,2-ethenediol (Entry 6 in Table 1) carefully. Although the high acidities were obtained and showed high activities for the first time for other silica supported solid acids as reported in Refs. [14-18], the active sites covered on the silica surface released from the solid acid easily and the recycled activities dropped quickly. The novel solid acid owned much higher stability and the yields remained unchanged even after the sample had been recycled for a sixth time (Fig. 4). The sample composition of the novel solid acid remained unchanged after recycled for five times according to the results of the element analysis. Furthermore, the filtrate from catalyst recovery showed almost no activity for the reactions and the element analysis of the filtrate showed almost no element sulfur, which further confirmed the high stability of the solid acid.
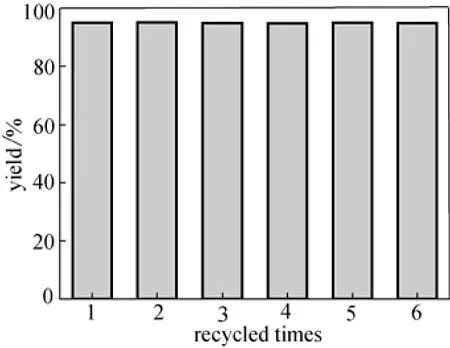
Figure 4 The recycled activity of the catalyst
3.4 The comparison of the catalytic activities
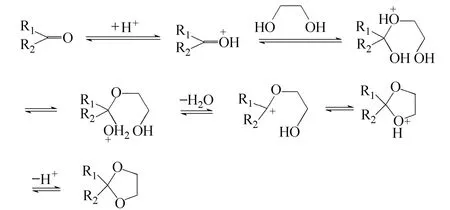
Figure 5 The reaction kinetics of acetalization
A comparative study on the catalytic activities of the novel solid acid with the reported catalysts were taken through the reaction between benzaldehyde and 1,2-ethanediol (Table 2). Amberlyst-15 was obtained from Fluck with the acidity of 0.8 mmol·g−1. H2SO4has relatively high activities. Although H2SO4owned strong acidity and the H+dissociated quite easily, both the acetalization and other side reactions including carbonization and oxidation could be activated by the strong acid sites (Entry 2). After tedious post treatment, the acetal product with deep color was obtained. The traditional carbon based solid acid with only sulfonic acid group also owned relatively high activities for the reaction [9] (Entry 4). The butyl acid with the single carbonyl acid group showed low activities and the results indicated that the low acid strength of carbonyl acid groups was not active enough to catalyze the reaction (Entry 3). As to the widely-used Amberlyst-15 with only sulfonic group, the catalytic activities was still low. (Entry 5). The novel solid acid showed the best activities of all. The solid acid owned high acidity and BET surface, which made the acid sites more accessible by the reactants compared to the other solid acid (Entries 4, 5). The reaction route was shown in Fig. 5. It can be seen that the H+should be given to the carbonyl groups at beginning to initiate the reaction and afterwards it was dissociated from the product to complete the catalytic cycle. The novel solid acid owned the dual acid sites. The strong acid sites from sulfonic acid groups gave the H+at the beginning to initiate the reaction and H+was absorbed by the low acid sites from carbonyl acid groups in the end to complete a catalytic cycle. The dual acidic groups cooperated during the catalytic process, whichimproved the activities. The novel solid acid held great potential for the green chemical processes.
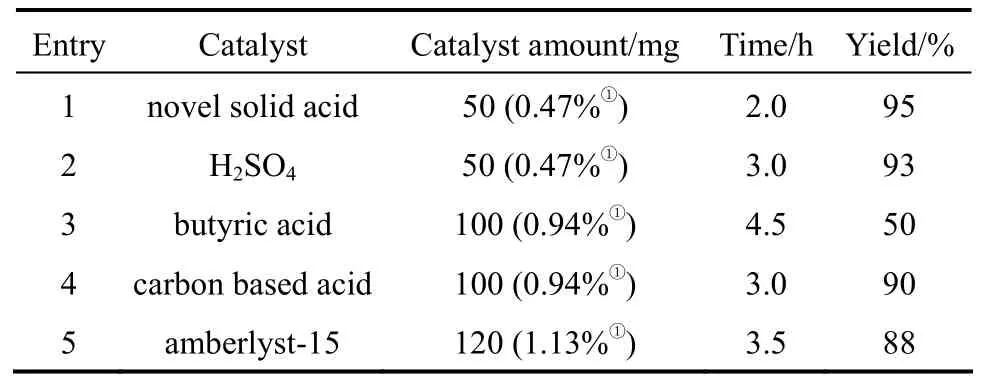
Table 2 The activity comparison of different catalysts
4 CONCLUSIONS
The novel solid acid with both sulfonic and carbonyl acid groups has been synthesized from 3-((2-sulfoethoxy)carbonyl)acrylic acid and tetraethyl orthosilicate (TEOS). The novel solid acid shows high activities for acetalization with the average yields over 90%. The catalyst has the advantages of high acidity, high BET surface, low cost and high thermal and chemical stability, and hold great potential for the replacement of the homogeneous catalysts in green chemical processes.
REFERENCES
1 Anastas, P.T., Kirchhoff, M.M., “Origins, current status, and future challenges of green chemistry”, Acc. Chem. Res., 35, 686-694 (2002).
2 Anastas, P.T., Zimmermann, J.B., “Design through the 12 principles of green engineering”, Environ. Sci. Technol., 37, 94A-101A (2003). 3 Clark, J.H., “Solid acids for green chemistry”, Acc. Chem. Res., 35, 791-797 (2002).
4 Shu, Q., Gao, J., Liao, Y., Wang, J., “Reaction kinetics of biodiesel synthesis from waste oil using a carbon-based solid acid catalyst”, Chin. J. Chem. Eng., 19, 163-168 (2011).
5 Misono, M., Acad, C.R., “Heterogeneous catalysis”, Sci. Ser. Iic., 3, 471-475 (2000).
6 Okuhara, T., “Water-tolerant solid acid catalysts”, Chem. Rev., 102, 3641-3666 (2002).
7 Hara, M., Yoshida, T., Takagaki, A., Takata, T., Kondo, J.N., Hayashi, S., Domen, K., “A carbon material as a strong protonic acid”, Angew. Chem. Int. Ed., 43, 2955-2958 (2004).
8 Toda, M., Takagaki, A., Okamura, M., Kondo, J.N., Hayashi S., Domen, K., Hara, M., “Green chemistry: Biodiesel made with sugar catalyst”, Nature, 438 (7065), 178 (2005).
9 Okamura, M., Takagaki, A., Toda, M., Kondo, J.N., Domen, K., Tatsumi, T., Hare, M., Hayashi S., “Acid-catalyzed reactions on flexible polycyclic aromatic carbon in amorphous carbon”, Chem. Mater., 18, 3039-3045 (2006).
10 Nakajima, K., Okamura, M., Kondo, J.N., Domen, K., Tatsumi, T., Hayashi, S., Hara, M., “Amorphous carbon bearing sulfonic acid groups in mesoporous silica as a selective catalyst”, Chem. Mater., 21, 186-193 (2009).
11 Xia, Y., Mokaya, R., “On the hydrothermal stability of mesoporous aluminosilicate MCM-48 materials”, J. Phys. Chem. B, 107, 6954-6960 (2003).
12 Li, C., “Chiral synthesis on catalysts immobilized in microporous and mesoporous materials”, Catal. Rev., 46, 419-492 (2004).
13 Wang, Y.J., Caruso, F., “Mesoporous silica spheres as supports for enzyme immobilization and encapsulation”, Chem. Mater., 17, 953-961 (2005).
14 Riego, J.M., Sedin, Z., Zaldívar, J.M., Marziano, N.C., Tortato, C.,“Sulfuric acid on silica-gel: An inexpensive catalyst for aromatic nitration”, Tetrahedron Lett., 37, 513-516 (1996).
15 Shaabani, A., Rahmati, A., “Silica sulfuric acid as an efficient and recoverable catalyst for the synthesis of trisubstituted imidazoles”, J. Mol. Catal. A Chem., 249, 246-248 (2006).
16 Izumi, Y., Hisano, K., Hida, T., “Acid catalysis of silica-included heteropolyacid in polar reaction media”, Appl. Catal. A Gen., 181, 277-282 (1999).
17 Khadilkar, B.M., Borkar, S.D., “Synthesis of benzophenones using silica-gel supported Lewis acid catalyst”, Tetrahedron Lett., 38, 1641-1642 (1997).
18 Shaterian, H. R., Shahrekipoor, F., Ghashang, M., “Silica supported perchloric acid (HClO4-SiO2): A highly efficient and reusable catalyst for the protection of hydroxyl groups using HMDS under mild and ambient conditions”, J. Mol. Catal. A Chem., 272, 142-151 (2007).
CATALYSIS, KINETICS AND REACTION ENGINEERING
Chinese Journal of Chemical Engineering, 22(3) 312—317 (2014)
10.1016/S1004-9541(14)60048-3
2012-05-27, accepted 2013-03-14.
*Supported by the Chinese National General Administration of Quality Supervision, Inspection and Quarantine (2012IK048, 2011IK041) and the National Natural Science Foundation of China (21103111).
**To whom correspondence should be addressed. E-mail: liangxuezheng@126.com
 Chinese Journal of Chemical Engineering2014年3期
Chinese Journal of Chemical Engineering2014年3期
- Chinese Journal of Chemical Engineering的其它文章
- A Bi-component Cu Catalyst for the Direct Synthesis of Methylchlorosilane from Silicon and Methyl Chloride
- Hydrodynamics and Mass Transfer of Oily Micro-emulsions in An External Loop Airlift Reactor
- Effects of Shape and Quantity of Helical Baffle on the Shell-side Heat Transfer and Flow Performance of Heat Exchangers*
- A Contraction-expansion Helical Mixer in the Laminar Regime*
- Preparation and Characterization of Sodium Sulfate/Silica Composite as a Shape-stabilized Phase Change Material by Sol-gel Method*
- Determination of Transport Properties of Dilute Binary Mixtures Containing Carbon Dioxide through Isotropic Pair Potential Energies
