Organotemplate-free Hydrothermal Synthesis of SUZ-4 Zeolite: Influence of Synthesis Conditions*
ZHOU Hualan (周华兰), WU Yajing (吴雅静), ZHANG Wei (张伟)and WANG Jun (王军),**
1State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China
2College of Sciences, Nanjing University of Technology, Nanjing 210009, China
Organotemplate-free Hydrothermal Synthesis of SUZ-4 Zeolite: Influence of Synthesis Conditions*
ZHOU Hualan (周华兰)1, WU Yajing (吴雅静)2, ZHANG Wei (张伟)1and WANG Jun (王军)1,**
1State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China
2College of Sciences, Nanjing University of Technology, Nanjing 210009, China
Various conditions were investigated in detail for the novel organic template-free static hydrothermal synthesis of SUZ-4 zeolite in the presence of seeds. The obtained samples were characterized by XRD (X-ray diffraction), SEM (scanning electron microscope), TG (thermal gravimetric analysis), ICP (inductively coupling plasma) elemental analysis, nitrogen sorption isotherm and surface area. The results show that pure SUZ-4 zeolites with high crystallinity are obtained in a broad window of synthesis conditions: seed mass concentration 0.2%-2%, SiO2/Al2O3molar ratio 21-25, KOH/SiO2molar ratio 0.33-0.43, H2O/SiO2molar ratio 7.14-38.1, aging time 24 h, crystallization temperature 160 °C, and crystallization time 6-10 d. Also, crystallinity and size of the rod-like SUZ-4 zeolite crystals are found to alter with the conditions.
crystal growth, microporous materials, SUZ-4 zeolite, zeolite synthesis, organic template-free
1 INTRODUCTION
SUZ-4 zeolite patented by British Petroleum Company in 1992 [1] has a framework topology similar to ferrierite and ZSM-57 [1-5]. Its two-dimensional pore system consists of interconnected ten- and eight-membered ring channels which are elliptical in shape; the ten-membered rings have pore openings of 0.46×0.52 nm [2] and demonstrate as the good catalyst for many processes [6-9], including the conversion of n-hexane [6], elimination of nitrogen oxides [8], and transformation of methanol to dimethylether [9]. The synthesis of SUZ-4 zeolite, however, is still not so easy, which limits its practical application.
The synthesis of SUZ-4 zeolite is mostly reported by using a rotating hydrothermal crystallization in the presence of the organic template tetraethylammonium cation (TEA) [5, 10, 11]. The other organic templates such as quinuclidine and N,N,N,N,N,N-hexaethylpentane-diammonium bromide (Et6-diquat-5) are also used for the synthesis of SUZ-4 zeolite [1, 3]. Normally, the organic templates direct the assembly pathways of zeolite precursors and ultimately fill the pore space of a zeolite [11-14]. It has been accepted for a long time that the templating species are essential in the synthesis of zeolites, especially in the case of high-silica zeolites [15]. The use of organic templates, however, has many negative subsequences such as the environmental pollution and high energy consumption for the removal of organic templates during high-temperature calcination. Therefore, organotemplate-free method for zeolite synthesis is much desirable, for example, the organotemplate-free synthesis of ZSM-5 zeolite [16-18].
In the past decades, many efforts have been devoted to the organotemplate-free synthesis of zeolites in the presence of the additives of methyl ethyl ketone [19], methanol/ethanol [20], acetone [21], and crystal seeds [22]. It is known that the addition of crystalline seeds into the starting aluminosilicate gels can remarkably accelerate zeolite crystallization [22-25]. Moreover, the seeded hydrothermal route has been proved to be very effective in the synthesis of zeolites without organic templates involved. With the methods, zeolites of ECR-1 [26], Beta [27, 28], ZSM-34 [29], ZSM-12 [30], FER [31] and LEV [32] have been sythesized. But the synthesis of SUZ-4 zeolite without using any organic templates has not been reported up to date. Very recently, our group [33] reported the preliminary data for an organotemplate-free hydrothermal synthesis of SUZ-4 zeolite aided by SUZ-4 crystal seeds. In this study, we investigate in detail the influences of various conditions on the organotemplate-free synthesis of SUZ-4 zeolite, aiming to obtain the optimal synthesis window of the conditions.
2 EXPERIMENTAL
2.1 Materials
Potassium hydroxide (Sinopharm Chemical Reagent Co., AR); aluminum powder (99%, Sinopharm Chemical Reagent Co.); colloidal silica [40% (by mass) SiO2, Zhejiang Yuda Chem. Co., LR]; tetraethylammonium hydroxide [TEAOH, 35% (by mass) in water, AR, Jiangsu Jintan Xinan Chemical Research Institute].
2.2 Synthesis
The SUZ-4 zeolite that is used as the seed was prepared using the organic template TEAOH according to the conventional rotation method [7], followedwith a calcination at 550 °C for 5 h.
In a typical synthesis of SUZ-4 zeolite by the organotemplate-free hydrothermal approach, 2.6 g of KOH was dissolved in 20.0 g of deionized water, then 0.27 g of aluminum powder was added to the KOH solution under stirring to get the homogeneous solution A. Solution B was prepared by adding 15.8 g of colloidal silica into 15.6 g of deionized water under stirring. Then, solution B was added into solution A to make a gel mixture. Afterwards, 0.5 g of the seed (1% based on the total mass of the synthesis mixture) was added to the above gel, followed with an aging at room temperature for 24 h. The molar composition of the gel was 7.9 KOH︰Al2O3︰21 SiO2︰500 H2O (SiO2/Al2O3: 21, KOH/SiO2: 0.38, H2O/SiO2: 23.8). The synthesis gel was finally transferred into a Teflon-lined autoclave and left static in the oven at 160 °C for 6 d under the autogenous pressure. The resultant was then filtrated, recovered, washed with deionized water and dried at 100 °C for 12 h. Thus obtained SUZ-4 zeolite was used as the reference material for calculating relative crystallinity of various samples synthesized under other conditions. The relative crystallinity was calculated by comparing the intensities of the ten featured X-ray diffraction (XRD) peaks for the SUZ-4 phase (2θ=7.9°, 12.0°, 15.3°, 19.0°, 19.6°, 22.75°, 23.5°, 25.0°, 25.8° and 28.7°). The investigation on synthesis conditions was performed by varying the reaction parameters as the following: SiO2/Al2O315-30, KOH/SiO20.29-0.57, H2O/SiO27.14-42.86, seed concentration 0-4% (by mass), aging time 0-24 h, crystallization temperature 140-200 °C, and crystallization time 0-12 d.
2.3 Characterization
XRD patterns were collected on a Bruker D8 ADVANCE powder diffractometer using Ni-filtrated Cu Kαradiation source at 40 kV and 20 mA, from 5° to 50° with a scan rate of 2°·min−1. The morphologies of the products were taken with a QUANTA 200 (FEI) scanning electron microscope (SEM). The Brunauer-Emmett-Teller (BET) surface area was obtained by recording the N2-sorption isotherm at the temperature of liquid nitrogen using a Micromeritics ASAP2010 analyzer. Thermal gravimetric analysis (TG) was conducted on a TA Instrument (Netzsch, TG/209/F3) operated under air atmosphere. Chemical compositions of the samples were obtained by a Jarrell-Ash 1100 inductively coupling plasma (ICP) spectrometer.
3 RESULTS AND DISCUSSION
3.1 Influence of the seed concentration
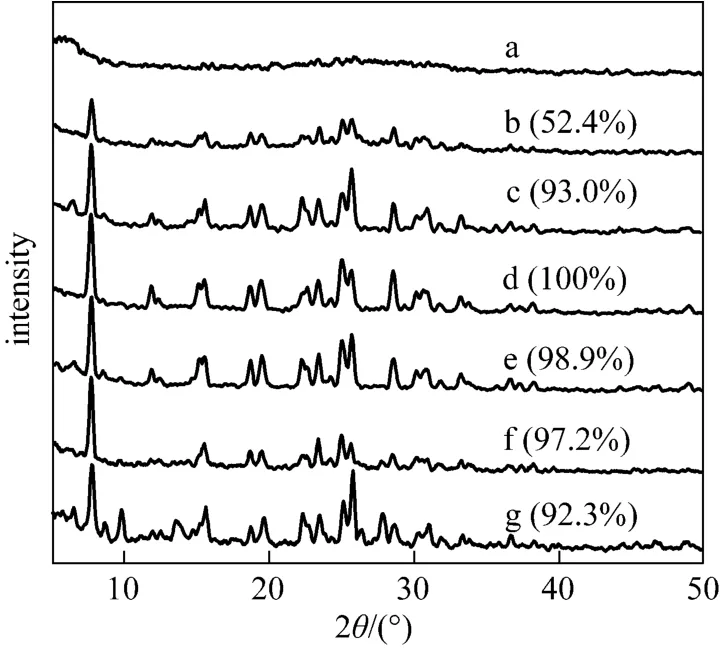
Figure 1 XRD patterns for SUZ-4 samples synthesized at 21 SiO2: Al2O3: 7.9 KOH: 500 H2O, 160 °C, 6 d, with different mass concentrations of seeds (data in parentheses are comparative crystallinities)seed mass concentration: a—0; b—0.2%; c—0.5%; d—1%; e—2%; f—4% (4 d); g—4%
The effect of seed concentration is shown in Fig. 1. It can be seen that only an amorphous product could be obtained in the unseeded system (Curve a) and low crystallinity zeolite appeared with a small amount of seed (Curve b). Curves d and e displayed a set of sharp and well resolved diffraction peaks assigned to the pure SUZ-4 phase [2], suggesting that the highly crystallized SUZ-4 zeolite can be obtained in the presence of 1%-2% (by mass) seed with a 6 d crystallization at 160 °C. When a larger mass amount of seed 4% (Curve f) was used, a highly crystallized SUZ-4 zeolite was got with a shorter hydrothermal time 4 d, whereas a 6 d hydrothermal treatment led to the detection of diffraction peaks at ca. 10° and 14° for mordenite (Curve g), a known competing zeolite phase in SUZ-4 synthesis [34].
It has been proved that the added seed in an organotemplate-free aluminosilicate gel mixture favors crystallization started by the fast agglomeration of the small-sized particles at the seed-amorphous interface [35]. The results in Fig. 1 indicate that the seed is indispensable for organotemplate-free synthesis of SUZ-4 zeolite, and the well crystallized SUZ-4 zeolite can be obtained within the seed mass content range of 0.2%-2%.
Figure 2 (a) shows the TG curves for selected samples. For the seed without the high-temperature calcination to remove the organic template TEAOH, the initial mass loss before 200 °C is attributed to the desorption of physically adsorbed water, and the large mass loss ranging from 200 °C to 550 °C is due to the decomposition of the charge compensating TEA+cations in the micropores of SUZ-4 zeolite [11]. In contrast, for the SUZ-4 sample synthesized by the present seed-assisted organotemplate-free hydrothermal approach, no detectable mass loss happened at 200-550 °C, confirming that the SUZ-4 product as synthesized does not bear any organic species. Fig. 2 (a) also verifies that the seed itself is actually organic template-free, which excludes the possibility that a slight of organic templates left in the seed play structure-directing roles in the present synthesis.

Figure 2 (a) TG curves for SUZ-4 synthesized with 1% (by mass) seed shown in Fig. 1d, seed, and seed without the hightemperature calcination; (b) The nitrogen isotherm for SUZ-4 synthesized with 1% (by mass) seed shown in Fig. 1d a—SUZ-4 with seed in Fig. 1d; b—seed; c—seed without calcination

Figure 3 SEM images for SUZ-4 samples synthesized at 21 SiO2︰Al2O3︰7.9 KOH: 500 H2O, 160 °C, 6 d, with different mass concentrations of seeds: (a) 0.2%, (b) 0.5%, (c) 1%, and (d) 2%
Figure 2 (b) gives the N2-sorption isotherm for the SUZ-4 sample obtained from the seed-assisted organotemplate-free hydrothermal method. A steep increase appeared in the relative pressure range 10−6
The SEM images of SUZ-4 products obtained with various amounts of seeds are presented in Fig. 3. It is observed that the amount of seeds employed in the organotemplate-free hydrothermal synthesis affected the crystallization rate, as well as the crystal size. With 0.2% (by mass) seed, amorphous particles occurred surrounding the rod-like zeolite crystals [Fig. 3 (a)], which means that the aluminosilicate gels were not converted to SUZ-4 crystals completely due to a slow crystallization rate at such a low seed concentration. At higher seed mass concentrations of 0.5%, 1% and 2%, the obtained three SUZ-4 samples exhibited a well-defined rod-like morphology with average crystal sizes of 4.8 μm×0.77 μm, 4.4 μm×0.38 μm and 2.3 μm×0.29 μm, respectively. The decreased crystal size corresponds to the increased seed-amorphous interface areas available for crystal growth [23-25, 35].
3.2 Influence of the SiO2/Al2O3ratio
Usually, the SiO2/Al2O3molar ratio of 21 is used for the synthesis gel mixture in the previous organic template-aided synthesis of SUZ-4 zeolite, and it is still rather difficult to synthesize SUZ-4 zeolite with a higher SiO2/Al2O3molar ratio [11, 34]. The effect of SiO2/Al2O3ratio on the crystallization of SUZ-4 zeolitewithout organic templates was investigated in this work, with the XRD patterns for the obtained SUZ-4 samples shown in Fig. 4. It can be seen that pure SUZ-4 zeolites could be obtained from the synthesis gels with SiO2/Al2O3ratios of 21 and 25. Note that the crystallization of the sample with a SiO2/Al2O3ratio of 25 took a longer time of 10 d compared with 6 d for the ordinary SiO2/Al2O3ratio of 21. The corresponding SiO2/Al2O3ratios analyzed by ICP for the final solid products were 21.4 and 23.9, respectively. The surface areas for the two samples with SiO2/Al2O3ratios of 21 and 25 were measured to be 324 and 290 m2·g−1, respectively. The decrease of the surface area at the SiO2/Al2O3ratio of 25 may be due to the lower crystallinity, as shown by Curves b and c in Fig. 4.

Figure 4 XRD patterns for SUZ-4 samples synthesized at Al2O3︰7.9 KOH: 500 H2O, 160 °C, 1% (by mass) seed, with different SiO2/Al2O3molar ratios and crystallization times (the data in parentheses are comparative crystallinities)a—SiO2/Al2O3: 15, 6 d; b—SiO2/Al2O3: 21, 6 d;c—SiO2/Al2O3: 25, 10 d; d—SiO2/Al2O3: 30, 10 d
When the SiO2/Al2O3ratio was as low as 15, the XRD Pattern a in Fig. 4 showed a much lowered crystallinity of SUZ-4 phase, together with the diffraction peak at ca. 5.6° that is assignable to an impurity phase of perlialite. On the other hand, at the much higher SiO2/Al2O3ratio of 30, the 10 d crystallization seemed cause a comparatively high crystallinity of SUZ-4 zeolite, but with the concomitance of mordenite impurity [34].
The SEM images for SUZ-4 products with different SiO2/Al2O3ratios are illustrated in Fig. 5. The samples with SiO2/Al2O3ratios of 21 and 25 exhibited rod-like crystalline morphologies, whereas the rods were thicker with the higher silica amount. For the two samples with the much low SiO2/Al2O3ratio of 15 and the high ratio of 30, impurities could be observed besides the rod-like SUZ-4 crystals, in agreement with the XRD results in Fig. 4.
3.3 Influence of the aging time
Before hydrothermal crystallization a synthesis mixture needs generally an aging treatment. Fig. 6 (a) presents the XRD patterns for the samples obtained with different aging times, and the corresponding crystallization curve is plotted in Fig. 6 (b). Though the peaks for SUZ-4 zeolite were detectable without aging, its crystallinity is at a very low level. A longer aging time is favorable to the formation of zeolite nuclei on the seed surface, accelerating the crystallization and enhancing the crystallinity [33]. It is drawn from Fig. 6 that 24 h is the optimal aging time to create a fully crystallized SUZ-4 zeolite.
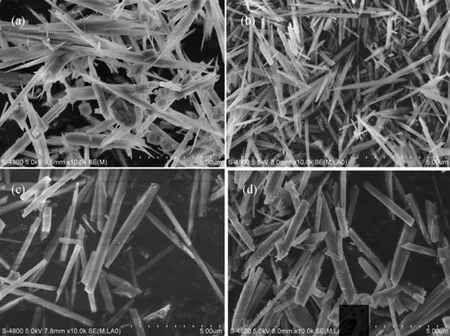
Figure 5 SEM images for SUZ-4 samples synthesized at Al2O3︰7.9 KOH: 500 H2O, 160 °C, 6 d, 1% (by mass) seed, with different SiO2/Al2O3molar ratios and crystallization times: (a) SiO2/Al2O3: 15, 6 d, (b) SiO2/Al2O3: 21, 6 d; (c) SiO2/Al2O3: 25, 10 d; and (d) SiO2/Al2O3: 30, 10 d

Figure 6 (a) XRD patterns and (b) crystallization curve for SUZ-4 samples synthesized at 21 SiO2︰Al2O3︰7.9 KOH︰500 H2O, 160 °C, 6 d, 1% (by mass) seed, with different aging times
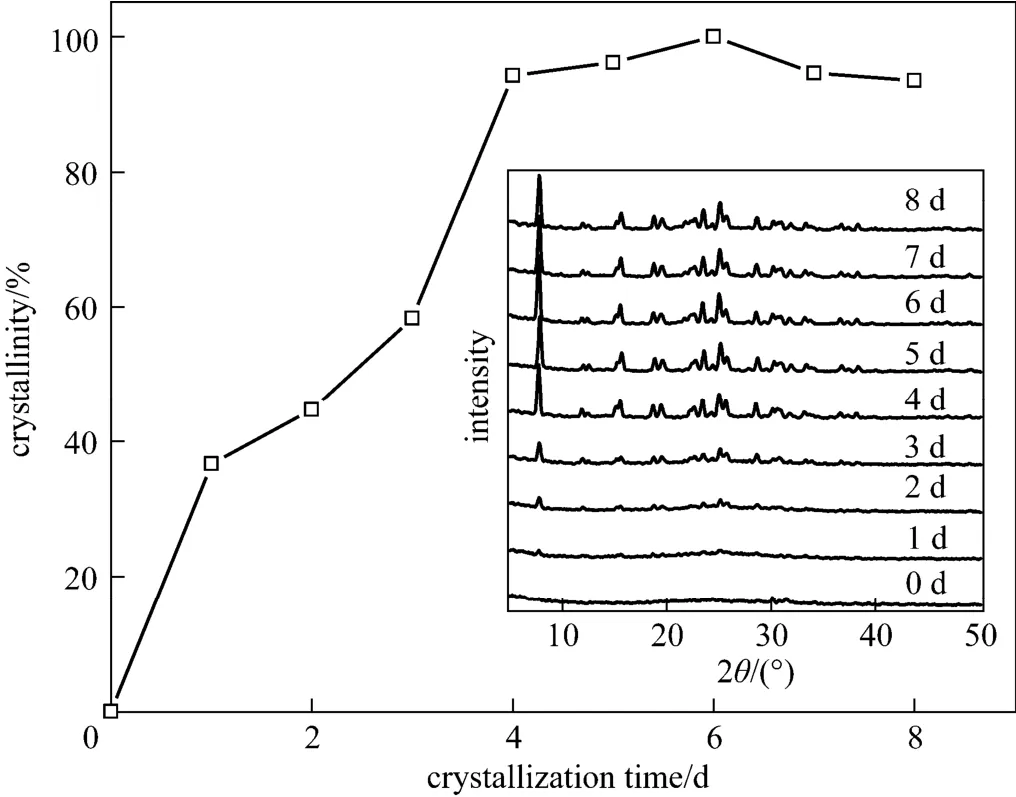
Figure 7 Crystallization curve for the samples obtained at various crystallization time with the synthesis gel composition 7.9 KOH︰Al2O3︰21 SiO2︰500 H2O, 160 °C and 1% (by mass) seed (Insertions are the XRD patterns)
3.4 Influence of the crystallization time
Figure 7 plots the crystallization curve for the samples obtained at various crystallization time with the synthesis gel composition 7.9 KOH︰Al2O3︰21 SiO2︰500 H2O, based on the inserted XRD patterns. It can be seen that crystallinity of the obtained SUZ-4 zeolites increased gradually with the prolongation of crystallization time, and when the time was beyond 4 d the crystallinity reached a level above 90%. The highest crystallinity was obtained at 6 d.
3.5 Influence of the crystallization temperature
The crystallization temperature was changed from 140 to 200 °C to measure its influence on the synthesis, with the XRD results shown in Fig. 8. At a low temperature of 140 °C, amorphous products were observed without any diffraction peaks if employing the standard 6 d crystallization time (XRD pattern not shown). Only with a much longer crystallization time of 12 d, SUZ-4 zeolite could be generated (Curve a). It is known that the increase of temperature can enhance the solubility of silicate species, leading to the rapid growth of zeolite crystals [34]. Therefore, 160 °C could cause the formation of well crystallized SUZ-4 zeolite (Curve b). However, at the high temperatures of 180 °C and 200 °C, SUZ-4 zeolites were produced even with a much shorter crystallization time of 2 d, but with the concomitance of mordenite impurity (Curves c and d) [34]. Consequently, 160 °C is selected as the most favorable crystallization temperature for the synthesis of SUZ-4 zeolite.
3.6 Influence of the KOH/SiO2ratio

Figure 8 XRD patterns for SUZ-4 samples synthesized with 21 SiO2︰Al2O3︰7.9 KOH︰500 H2O, 1% (by mass) seed, at different crystallization temperatures (the data in parentheses are comparative crystallinities)a—140 °C, 12 d; b—160 °C, 6 d; c—180 °C, 2 d; d—200 °C, 2 d
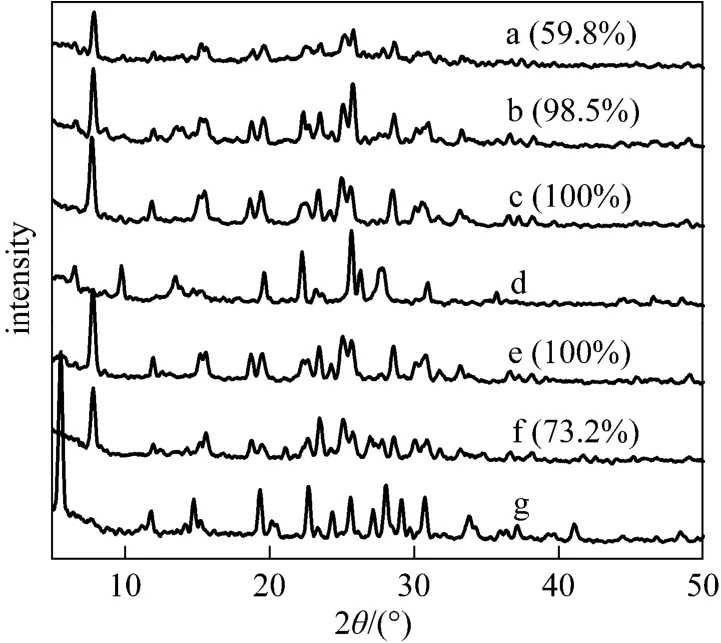
Figure 9 XRD patterns for SUZ-4 samples synthesized at 21 SiO2︰Al2O3︰500 H2O, 160 °C, 6 d, 1% (by mass)seed, with different KOH/SiO2molar ratios (the data in parentheses are comparative crystallinities) a—0.29; b—0.33; c—0.38; d—NaOH/SiO2: 0.38; e—0.43; f—0.48; g—0.57
The alkalinity of starting gels is an important parameter to influence the crystallization of zeolite, thus KOH/SiO2molar ratios were varied from 0.29 to 0.57 to evaluate the influence of gel’s alkalinities. The XRD patterns in Fig. 9 indicate that SUZ-4 zeolite was obtained when the KOH/SiO2ratio was in the range 0.33-0.43. At a low alkalinity (KOH/SiO2: 0.29), a poorly crystallized SUZ-4 phase was observed because the low concentration of hydroxyl ions could not depolymerize the silica source to provide sufficient solubilized aluminosilicate species to form nuclei [36]. On the contrary, a high alkalinity (KOH/SiO2: 0.48) made the crystallinity lowered remarkably, probably due to the dissolution of the already formed zeolite nuclei in the presence of excess hydroxyl ions [36]. KOH/SiO2up to 0.57 caused the formation of pure perlialite crystals (Curve g). Moreover, NaOH was also attempted as the alkaline source in comparison with KOH. It can be seen in Curve d that mordenite was the only crystallite produced, which is in agreement with the previous result that K+is a necessity for creating the SUZ-4 phase [11]. Accordingly, the optimal KOH/SiO2ratio of 0.33-0.43 is crucial for creating the pure phase of SUZ-4 zeolite.
3.7 Influence of the H2O/SiO2ratio
The water content of a synthesis system is also known as a key condition for the hydrothermal synthesis of zeolite. Fig. 10 shows the XRD patterns for the samples synthesized with various H2O/SiO2ratios of 7.14-42.86. The pure and well crystallized SUZ-4 zeolite could be obtained even from the very concentrated gel with the low H2O/SiO2ratio of 7.14. A broad window of H2O/SiO2ratios 7.14-38.1 was observed for the synthesis of SUZ-4 zeolite. However, at the much lower H2O/SiO2ratios of less than 7.14, the viscosity of the aluminosilicate gel is too large for substrates to diffuse freely to form SUZ-4 crystalline [37, 38]. Also, the much diluted gel mixture (H2O/SiO2: 42.86) could not give pure SUZ-4 zeolite, which is ascribed to the very slow nucleation rate arising for the longer distance between nutrients in this diluted solution [39].
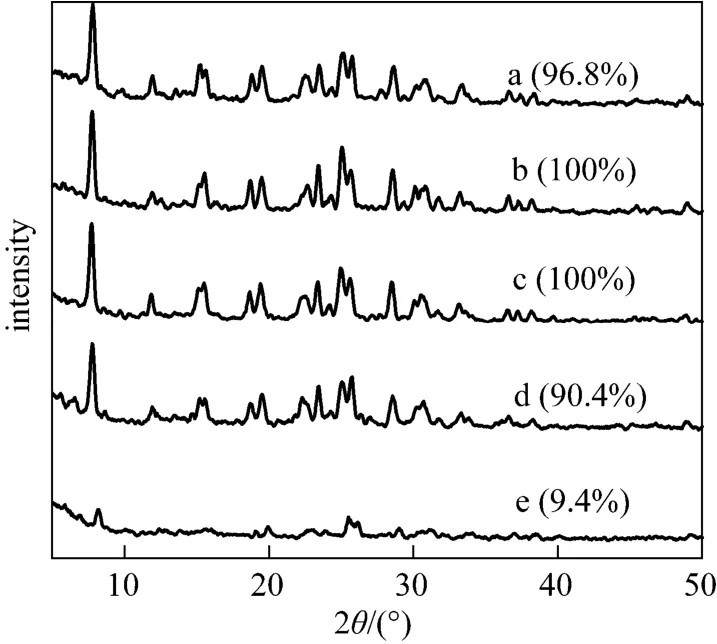
Figure 10 XRD patterns for SUZ-4 samples synthesized at 21 SiO2︰Al2O3︰7.9 KOH, 160 °C, 6 d, 1% (by mass) seed, with different H2O/SiO2ratios (the data in parentheses are comparative crystallinities) a—7.14; b—14.28; c—23.81; d—38.10; e—42.86
4 CONCLUSIONS
Pure and highly crystallized SUZ-4 zeolite can be synthesized by the organotemplate-free static hydrothermal route in the presence of the seed under following conditions: SiO2/Al2O3: 21-25 mol·mol−1, KOH/SiO2: 0.33-0.43 mol·mol−1, H2O/SiO2: 7.14-38.1 mol·mol−1, seed mass concentration=0.2%-2%, aging time = 24 h, crystallization temperature=160 °C, and crystallization time=6-10 d. The optimal condition (SiO2/Al2O321, KOH/SiO20.38, H2O/SiO223.8, seed mass concentration 1%, aging 24 h, 160 °C and 6 d) results in the SUZ-4 product with BET surface area 324 m2·g−1, micropore volume 0.13 cm3·g−1, mesopore diameter 2.26 nm and the size of rod-like crystals around 4.4 μm×0.38 μm.
REFERENCES
1 Barri, S.A.I., “Crystalline (metallo) silicates and germanates-SUZ-4”, US Pat. 5118483 (1992).
2 Lawton, S.L., Bennett, J.M., Schlenker, J.L., Rubin, M.K., “Synthesis and proposed framework topology of zeolite SUZ-4”, J. Chem. Soc. Chem. Commun., (11), 894-896 (1993).
3 Paik, W.C., Shin, C.H., Hong, S.B., “Synthesis of zeolites P1 and SUZ-4 through a synergy of organic N,N,N,N′,N′,N′-hexaethylpentanediammonium and inorganic cations”, Chem. Commun., (17), 1609-1610 (2000).
4 Paik, W.C., Shin, C.H., Lee, J.M., Ahn, B.J., Hong, S.B., “A novel method for incorporation of heteroatoms into the framework of ordered mesoporous silica materials synthesized in strong acidic media”, J. Phys. Chem. B, 105 (41), 9994-10000 (2001).
5 Strohmaier, K.G., Afeworki, M., Dorset, D.L., “The crystal structures of polymorphic SUZ-4”, Z. Kristallogr., 221, 689-698 (2006).
6 Lukyanov, D.B., Zholobenko, V.L., Dwyer, J., Barri, S.A. I., Smith, W.J., “On the structural, acidic and catalytic properties of zeolite SUZ-4”, J. Phys. Chem., 103 (1), 197-202 (1999).
7 Asensi, M.A., Camblor, M.A., Martinez, A., “Zeolite SUZ-4: Reproducible synthesis, physicochemical characterization and catalytic evaluation for the skeletal isomerization of n-butenes”, Micropor. Mesopor. Mater., 28, 427-436 (1999).
8 Subbiah, A., Cho, B.K., Blint, R.J., Gujar, A.C., Price, G.L., Yie, J.E.,“NOxreduction over metal-ion exchanged novel zeolite under lean conditions: Activity and hydrothermal stability”, Appl Catal B Envir., 42, 155-178 (2003).
9 Jiang, S., Hwang, Y.K., Jhung, S.H., Chang, J.S., Hwang, J.S., “Zeolite SUZ-4 as selective dehydration catalyst for methanol conversion to dimethyl ether”, Chem Lett., 33 (8), 1048-1049 (2004).
10 Gujar, A.C., Moye, A.A., Coghill, P.A., Teeters, D.C., Roberts, K.P., Price, G.L., “Raman investigation of the SUZ-4 zeolite”, Micropor. Mesopor. Mater., 78, 131-137 (2005).
11 Gujar, A.C., Price, G.L., “Synthesis of SUZ-4 in the K+/TEA+system”, Micropor. Mesopor. Mater., 54, 201-205 (2002).
12 Barrer, R.M., Denny, P.J., “Hydrothermal chemistry of the silicates. Part IV. Nitrogenous aluminosilicates”, J. Chem. Soc., 971-982 (1961).
13 Davis, M.E., Lobo, R.F., “Zeolite and molecular sieve synthesis”, Chem. Mater., 4, 756-768 (1992).
14 Lawton, S.L., Rohrbaugh, W.J., “The framework topology of ZSM-18, a novel zeolite containing rings of three (Si,Al)-O species”, Science., 247, 1319-1322 (1990).
15 Fyfe, C.A., Darton, R.J., Mowatt, H., Lin, Z.S., “Efficient, low-cost, minimal reagent syntheses of high silica zeolites using extremely dense gels below 100 °C”, Micropor. Mesopor. Mater., 144, 57-66 (2011).
16 Li, H.X., Xiang, S.H., Wu, D.M., Liu, Y.T., Zhang, X.S., Liu, S.S.,“Study on the synthesis of zeolite ZSM-5”, Chem. J. Chin. Univ., 2 (4), 517-519 (1981).
17 Wang, F.S., Cheng, W.C., Zhang, S., “Synthesis of inorgano-ammonium high silica zeolites of ZSM series”, Chin. J. Catal., 2 (4), 282-287 (1981).
18 Shiralkar, V.P., Clearfield, A., “Synthesis of the molecular sieve ZSM-5 without the aid of templates”, Zeolites, 9, 363-370 (1989).
19 Narita, E., Sato, K., Okabe, T., “A convenient method for crystallization of zeilite ZSM-5 by using seed crystals in acetone/water mixture system”, Chem. Lett., 13 (7), 1055-1058 (1984).
20 Plank, C.J., Rosinski, E.J., Rubin, M.K., “Method for producing zeolites”, US Pat. 4175114 (1979).
21 Narita, E., Yatabe, N., Okabe, T., “Synthesis and crystal growth of zeolite ZSM-5 from sodium aluminosilicate systems free of organic templates”, Ind. Eng. Chem. Prod. Res. Dev., 24 (4), 507-512 (1985).
22 Edelman, R.D., Kudalkar, D.V., Ong, T., Warzywoda, J., Thompson, R.W., “Crystallization phenomena in seeded zeolite syntheses”, Zeolites, 9, 496-502 (1989).
23 Lu, B., Tsuda, T., Oumi, Y., Itabashi, K., Sano, T., “Direct synthesis of high-silica mordenite using seed crystals”, Micropor. Mesopor. Mater., 76, 1-7 (2004).
24 Lu, B., Yakushi, Y., Oumi, Y., Itabashi, K., Sano, T., “Control of crystal size of high-silica mordenite by quenching in the course of crystallization process”, Micropor. Mesopor. Mater., 95, 141-145 (2006).
25 Dutta, P.K., Bronic, J., “Mechanism of zeolite formation: Seed-gel interaction”, Zeolites, 14, 250-255 (1994).
26 Song, J.W., Dai, L., Ji, Y.Y., Xiao, F.S., “Organic template free synthesis of aluminosilicate zeolite ECR-1”, Chem. Mater., 18 (12), 2775-2777 (2006).
27 Xie, B., Song, J., Ren, L.M., Ji, Y., Li, J., Xiao, F.S., “Organotemplate-free and fast route for synthesizing beta zeolite”, Chem. Mater., 20 (14), 4533-4535 (2008).
28 Xie, B., Zhang, H.Y., Yang, C.G., Liu, S.Y., Ren, L.M., Zhang, L., Meng, X.J., Yilmaz, B., Muller, U., Xiao, F.S., “Seed-directed synthesis of zeolites with enhanced performance in the absence of organic templates”, Chem. Commun., 47, 3945-3947 (2011).
29 Zhang, L., Yang, C.G., Meng, X.J., Xie, B., Wang, L., Ren, L.M., Ma, S.J., Xiao, F.S., “Organotemplate-free syntheses of ZSM-34 zeolite and Its heteroatom-substituted analogues with good catalytic performance”, Chem. Mater., 22 (10), 3099-3107 (2010).
30 Kamimura, Y., Tanahashi, S., Itabashi, K., Sugawara, A., Wakihara, T., Shimojima, A., Okubo, T., “Crystallization behavior of zeolite beta in OSDA-free, seed-assisted synthesis”, J. Phys. Chem. C, 115 (3), 744-750 (2011).
31 Zhang, H.Y., Guo, Q., Ren, L.M., Yang, C.G., Zhu, L.F., Meng, X.J., Li, C., Xiao, F.S., “Organotemplate-free synthesis of high-silica ferrierite zeolite induced by CDO-structure zeolite building units”, J. Mater. Chem., 21, 9494-9497 (2011).
32 Zhang, H.Y., Yang, C.G., Zhu, L.F., Meng, X.J., Yilmaz, B., Muller, U., Feyen, M., Xiao, F.S., “Organotemplate-free and seed-directed synthesis of levyne zeolite”, Micropor. Mesopor. Mater., 155, 1-7 (2012).
33 Zhang, W., Wu, Y.J., Gu, J., Zhou, H.L., Wang, J., “Organotemplate-free route for synthesizing SUZ-4 zeolite under static hydrothermal condition”, Mater. Res. Bull., 46, 1451-1454 (2011).
34 Worathanakula, P., Trisuwana, D., Phatrukb, A., Kongkachuichay, P.,“Effect of sol-gel synthesis parameters and Cu loading on the physicochemical properties of a new SUZ-4 zeolite”, Colloids Surf. A Physicochem. Eng. Aspects., 377, 187-194 (2011).
35 Wu, Y.J., Ren, X.Q., Lu, Y.D., Wang, J., “Crystallization and morphology of zeolite MCM-22 influenced by various conditions in the hydrothermal synthesis”, Micropor. Mesopor. Mater., 112, 138-146 (2008).
36 Eapen, M.J., Reddy, K.S.N., Shiralkar, V.P., “Hydrothermal crystallization of zeolite beta using tetraethylammonium bromide”, Zeolites, 14, 295-302 (1994).
37 Kim, Y.C., Jeong, J.Y., Hwang, J.Y., Kim, S.D., Kim, W.J., “Influencing factors on rapid crystallization of high silica nano-sized zeolite Y without organic template under atmospheric pressure”, J Porous Mater., 16, 299-306 (2009).
38 Kim, S.D., Noh, S.H., Seong, K.H., Kim, W.J., “Compositional and kinetic study on the rapid crystallization of ZSM-5 in the absence of organic template under stirring”, Micropor. Mesopor. Mater., 72, 185-192 (2004).
39 Gu, J., Wu, Y.J., Wang, J., Lu, Y.D., Ren, X.Q., “In situ assembly of ZSM-5 nanocrystals into micro-sized single-crystal-like aggregates via acid-catalyzed hydrolysis of tetraethyl orthosilicate”, J. Mater. Sci., 44, 3777-3783 (2009).
10.1016/S1004-9541(14)60019-7
2012-03-22, accepted 2012-10-10.
* Supported by the National Natural Science Foundation of China (20976084, 21101094, 21136005).
** To whom correspondence should be addressed. E-mail:junwang@njut.edu.cn
 Chinese Journal of Chemical Engineering2014年1期
Chinese Journal of Chemical Engineering2014年1期
- Chinese Journal of Chemical Engineering的其它文章
- Steam Reforming of Dimethyl Ether by Gliding Arc Gas Discharge Plasma for Hydrogen Production*
- Interaction Analysis and Decomposition Principle for Control Structure Design of Large-scale Systems*
- Influence of Design Margin on Operation Optimization and Control Performance of Chemical Processes*
- Photocatalytical Inactivation of Enterococcus faecalis from Water Using Functional Materials Based on Natural Zeolite and Titanium Dioxide*
- Enhancing Structural Stability and Pervaporation Performance of Composite Membranes by Coating Gelatin onto Hydrophilically Modified Support Layer*
- Measurement and Modeling for the Solubility of Hydrogen Sulfide in Primene JM-T*
