Photocatalytical Inactivation of Enterococcus faecalis from Water Using Functional Materials Based on Natural Zeolite and Titanium Dioxide*
Cornelia Bandas (Ratiu), Corina Orha, Corina Misca, Carmen Lazau,**, Paula Sfirloagaand Sorin OlariuNational Institute for Research and Development in Microtechnologies, Bucharest 077190, Romania
2National Institute for Research and Development in Electrochemistry and Condensed Matter, Condensed Matter Department, Timisoara 300254, Romania
3Banat’s University of Agricultural Sciences and Veterinary Medicine of Timisoara, Faculty of Food Products Technology, Timisoara 300645, Romania
4Medical and Pharmaceutical University “Victor Babes”, Timisoara 300041, Romania
Photocatalytical Inactivation of Enterococcus faecalis from Water Using Functional Materials Based on Natural Zeolite and Titanium Dioxide*
Cornelia Bandas (Ratiu)1,2, Corina Orha2, Corina Misca3, Carmen Lazau2,**, Paula Sfirloaga2and Sorin Olariu41National Institute for Research and Development in Microtechnologies, Bucharest 077190, Romania
2National Institute for Research and Development in Electrochemistry and Condensed Matter, Condensed Matter Department, Timisoara 300254, Romania
3Banat’s University of Agricultural Sciences and Veterinary Medicine of Timisoara, Faculty of Food Products Technology, Timisoara 300645, Romania
4Medical and Pharmaceutical University “Victor Babes”, Timisoara 300041, Romania
The functional materials based on natural zeolite (clinoptilolite), TiO2-zeolite and Ag-TiO2-zeolite have been successfully synthesized by solid-state reaction in fast-hydrothermal conditions. The obtained functional materials were investigated by X-ray diffraction (XRD), FT-IR (Fourier transform infrared) spectroscopy, DRUV-VIS (diffuse reflectance ultraviolet-visible) spectroscopy, BET (Brunauer-Emmett-Teller) and SEM/EDX (scanning electron microscope/energy dispersive X-ray spectrometer) analyses. The XRD results indicated that the clinoptilolite structure has a good thermal stabilization after the fast-hydrothermal treatment. Also, the high specific surface area about 92.55 m2·g−1was noticed for Ag-TiO2-zeolite functional material. The presence of dopants was evidenced from EDX spectra. The enhanced bactericidal activity of Ag-TiO2-zeolite catalyst is proved through damaging of Enterococcus faecalis colonies under visible irradiation, at different material doses and irradiation times.
titanium dioxide, composite, catalytic properties, Enterococcus faecalis
1 INTRODUCTION
Microorganisms are part of the organic matter in wastewater. Streptococcus faecalis, specifically, can affect human health and are commonly found in wastewater because they are present in fecal material. Previous health and epidemiological studies by the US Environmental Protection Agency (US EPA) have demonstrated that colony-forming unit (CFU) densities of the bacterial genus Enterococcus sp. from waters are directly correlated with gastroenteritis illness rates [1, 2]. Based on these data, guidance has been issued on the maximum concentrations of these organisms that correspond to acceptable health risks [3]. The removal or inactivation of pathogenic microorganisms (among them streptococcus faecalis) is the last step in the treatment of wastewater. In order to remove Enterococcus sp. from polluted water, the bactericide activity of TiO2nanocrystals has been investigated for a long time. Photocatalysts based on TiO2have been intensively investigated in order to achieve better photocatalytic efficiency for different applications.
Recently, studies on the doping of transition metallic and non-metallic ions into TiO2and the immobilization of TiO2on supports have become attractive in the area of photocatalysis [4-7]. Different substrates have been used as catalyst support for photocatalytic inactivation of specific contaminants from water and wastewater, such as glass and stainless steel, alumina beds, activated carbon, synthetic zeolites [8-10]. Among these materials, zeolites have been chosen as support since they can delocalize band gap excited electrons of titanium dioxide (TiO2) and thereby minimize electron-hole recombination [11]. Supporting TiO2in zeolite matrix depends on several factors, such as location and migration of cations, strength of interactions between cations, and guest molecules dimensions of cavities and channels. The high photocatalytic activity of powder TiO2(mainly anatase) reveals its increasing applications towards inactivation of various water contaminants [12]. It has been suggested that fine TiO2particles should be anchored on supports (natural or synthetic zeolites) for ease of handling as well as for improving adsorption and photocatalytic efficiency [13-15]. They also serve as ion-exchangers and molecular sieves. Potential applications are expected in a number of technological fields, such as photochemistry, biology, optoelectronics, semiconducting devices and chemical sensors [16-18]. The functionalization of zeolite with TiO2can be carried out through three ways, namely solid-solid, solid-liquid and solid-gas reactions [19, 20]. Solid-state reactions between inorganic species have been studied. However, to the best of our knowledge, there are no investigations about solid-state reaction in hydrothermal conditions between TiO2nanocrystals and natural zeolite.
In this paper, functional materials based on natural zeolite modified with undoped TiO2and Ag-doped TiO2were synthesized by solid-state reaction by a novel fast-hydrothermal method, which has the advantage of significantly reduced synthesis-cycle time from 6-8 h (classical hydrothermal) to 15-30 min(fast-hydrothermal) [21]. Undoped TiO2/Ag-doped TiO2nanocrystals were previously synthesized by this method [21]. X-ray diffraction, FT-IR (Fourier transform infrared) spectroscopy, DRUV-VIS (diffuse reflectance ultraviolet-visible) spectroscopy, BET (Brunauer-Emmett-Teller) and SEM/EDX (scanning electron microscope/ energy dispersive X-ray spectrometer) analysis were used for the morpho-structural characterization of functional materials. The antibacterial performances of functional materials under visible irradiation were determined against Enterococcus faecalis, specific contaminant in river water.
2 MATERIALS AND METHODS
Romanian zeolitic mineral from Mirsid, used as support for doping TiO2, was supplied by CEMACON Company, Romania. This natural mineral was powdered and sieved with a Multilab sieve shaker. The grain size selected to carry out the experiments was between 0.8-1.2 mm with the mass composition of 62.20% SiO2, 11.65% Al2O3, 1.30% Fe2O3, 3.74% CaO, 0.67% MgO, 3.30% K2O, 0.72% Na2O, 0.28% TiO2.
2.1 Synthesis of functional materials
The functional materials syntheses are performed in two steps: firstly the undoped and Ag-doped TiO2nanocrystals were obtained by the fast-hydrothermal method [21], and secondly the functional materials based on natural zeolite in sodium form were modified with undoped TiO2and Ag-doped TiO2by solid-state reaction in fast-hydrothermal conditions. For obtaining of functional materials based on undoped and doped TiO2was used the pure anatase form of TiO2. Anatase TiO2exhibits higher photocatalytic activity than rutile TiO2due to its conduction band position which demonstrates stronger reducing power.
The preparation of the chemically modified natural zeolite presumes two steps to reach acid form (H form) by using 2 mol·L−1HCl solution and then sodium form (Na form) with 2 mol·L−1NaNO3solution for a more efficient ion exchange. Consequently, Na forms of clinoptilolite are expected to remove other cations easily in ion-exchange applications. For the same reason, Na form is most frequently prepared for research in clinoptilolite [11]. Functional materials based on natural zeolite modified with undoped and Ag-doped TiO2(TiO2-zeolite, Ag-TiO2-zeolite) were successfully synthesized by solid-state reaction in fast-hydrothermal conditions, in aqueous solution. The fast-hydrothermal installation consists of a quartz autoclave, with a reasonably thick protective steel jacket submerged in thermo-stated silicon oil bath. Thus, the increasing synthesis temperature inside the autoclave is achieved in only 1-2 min. The premature nucleation is avoided, and all the crystallization takes place in isothermal conditions. Therefore, the transitory processes are almost totally avoided, and also important electrical energy savings are obtained. The method presumes mixing of natural zeolite in Na form with 50 ml distilled water and undoped TiO2or Ag-doped TiO2nanocrystals (2%, by mass) under continuous stirring for 4 h. The obtained solutions were introduced in a quartz autoclave with 50% fullness, for 30 min to 150 °C. After 30 min the autoclave was removed from the oil bath and submerged for 1 h in a water bath for fast cooling, then solution was filtered, the solid product was washed, and dried at 60 °C for 5 h.
2.2 Characterization methods
The crystallinity of the prepared samples was measured by X-ray diffraction using PANalytical X’PertPRO MPD Diffractometer with Cu tube. The bond vibration was analyzed by IR (infrared) spectrometry using a Jasco FT/IR-430 spectrometer. Specific surface area, pore volume and pore diameter of the samples were measured by nitrogen adsorption-desorption technique using a Nova 1200e (Quantachrome). The pore volume and pore diameter were estimated by the BJH (Barrett-Joyner-Halenda) method, and the surface area (SBET) was calculated by Brunauer-Emmett-Teller (BET) method. The light absorption properties of the functional materials were studied by UV-VIS diffuse reflectance spectroscopy (DRUV-VIS), performed under ambient conditions using Lambda 950 Perkin Elmer in the wavelength range of 250-600 nm. The blank board was used as the reference. The morphology of the functional materials was determined by Scanning Electron Microscopy (SEM) using an Inspect S PANalytical model and the energy dispersive X-ray analysis detector (EDX).
2.3 Photocatalytic antibacterial method
The preparation of the microbial suspensions of Enterococcus faecalis ATCC 29212 EpowerTM was previously reported [22]. Photocatalytic antibacterial method supposes detection of the bactericidal effect of TiO2-zeolite and Ag-TiO2-zeolite against Enterococcus faecalis colonies. Thus, 10 ml of bacterial suspension of Enterococcus faecalis ATCC 29212 was sampled, in which different doses of functional materials (0.05 g and 0.1 g) were added. The samples were illuminated with a 24 W VIS lamp emitting 390-600 nm light and the distance between the VIS lamp and plate was 20 cm. The illumination times were 60, 120 and 180 min. After irradiation, for assessment of antibacterial effect of functional materials and E. Coli bacteria viability, 1 ml from treated samples was sampled and inseminated in the culture medium pelletaesculin-triazo-compound (produced by Scharlau Chemie S.A., Spain). All the cultivated plates were incubated for 24 h at 44 °C and the cell colonies formed were counted manually. All the antibacterial experiments were realized in a microbiological fume hood and repeated three times for more accuracy.
For evidencing the bactericidal effect of functional materials were followed in two aspects: the irradiation effect against the colonies growth and the effect of natural zeolite (clinoptilolite) against bacterial cultures. In addition, two blank samples were prepared. First blank sample contained only Enterococcus faecalis, without functional materials and the second contained Enterococcus faecalis and natural zeolite (clinoptilolite). In order to study the irradiation effect against the colonies growth, one blank sample was irradiated while the other was kept at dark. Assessment of colony forming units (CFU) growing was evidenced comparatively with the un-irradiated and irradiated blank samples.
3 RESULTS AND DISCUSSION
3.1 Functional materials characterization
Figure 1 described XRD patterns of functional materials based on natural zeolite, TiO2-zeolite and Ag-TiO2-zeolite. The structural and morphological characterization of undoped and Ag-doped TiO2obtained by the fast-hydrothermal method was previously reported [21]. The crystallite size of undoped and Ag-doped TiO2nanocrystals were calculated about 4 nm from XRD spectra and SEM images.
For comparison, the XRD pattern of the natural zeolite in Na form (Na-zeolite) is also shown in Fig. 1. It is revealed that the natural zeolite used is mostly clinoptilolite (2θ=10°, 22.5°, 30°) [11]. The main peak positions of clinoptilolite are unchanged, indicating that the structure of natural zeolite has a good thermal stabilization, after the fast-hydrothermal treatment. In comparison with the XRD pattern of Na-zeolite, the specific peaks corresponding to anatase TiO2form were evidenced at 2θ=25.3°, 37°, 38.6° both for TiO2-zeolite and Ag-TiO2-zeolite.
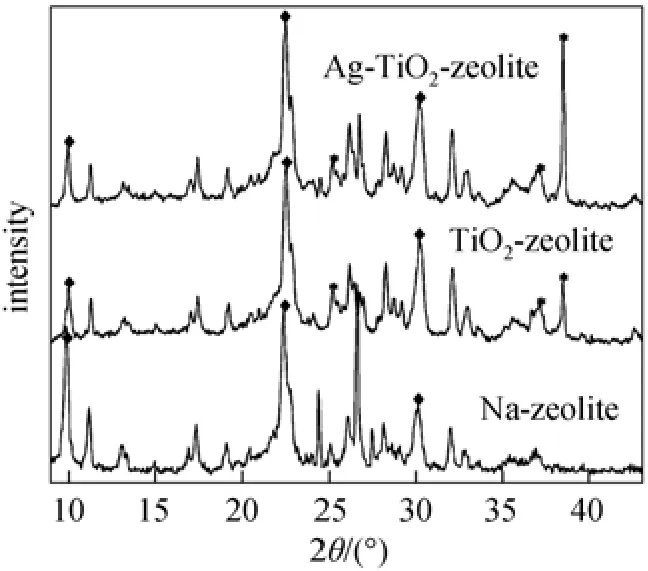
Figure 1 XRD patterns of functional materials◆ clinoptilolite; * anatase TiO2
DRUV-VIS patterns are examined to determine the absorption edge quantification and absorption wavelength range correlated with band gap energy. Fig. 2 presents an intense absorption maximum at about 250 nm, which can be assigned to isolated titanium with tetrahedral coordination. For the TiO2-zeolite the absorption band intensity is higher in the UV domain, because the anatase form of undoped TiO2with the band gap energy about 3.2 eV adsorbs strongly in this domain [23]. In case of Ag-TiO2-zeolite the absorption edge is slightly shifted to the visible range.
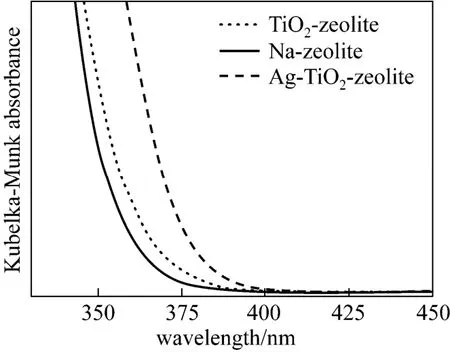
Figure 2 DRUV-VIS spectra of functional materials
FT-IR results obtained for the functional materials in comparison with Na-zeolite are shown in Fig. 3. These results confirm the formation of functional materials as evidenced by distinct absorbance bands near 3540 and 3360 cm−1(OH stretching), 1630-1640 cm−1(Lewis sites), 1100 cm−1(asymmetric vibrations of SiOSi and SiOAl), 560 cm−1(TiO2in anatase and five-membered rings) [24]. The presence of doped TiO2into functional materials seems to affect the symmetric stretching modes of water coordinated to the magnesium at the edges of channels.
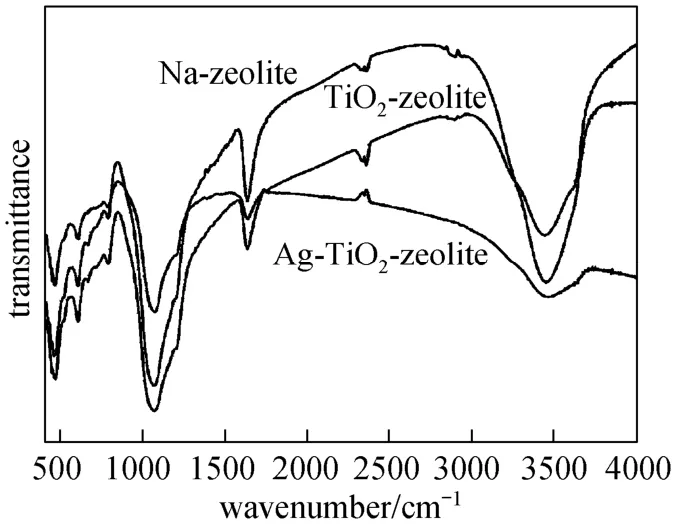
Figure 3 FT-IR spectra of Na-zeolite, TiO2-zeolite and Ag-TiO2-zeolite for the wavelength range 500-4000 cm−1
Table 1 listed the specific surface areas (SBET) and the pore volume (VP) of Na-zeolite, TiO2-zeolite and Ag-TiO2-zeolite functional materials. It can be found that Na-zeolite has smaller values than other functional materials. Ag-doped TiO2has the highest values, due to the contribution of the coating on zeolite surface.
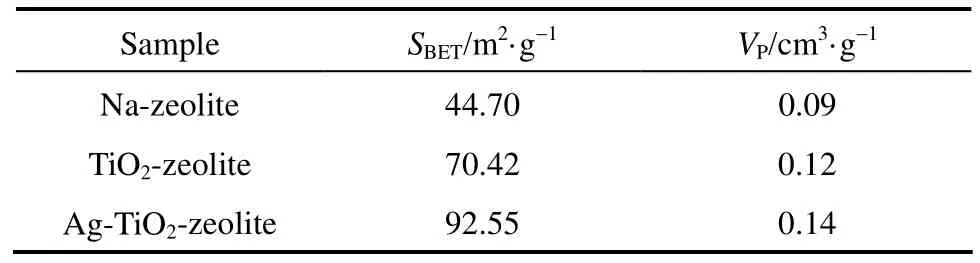
Table 1 Specific surface area and pore structure of Na-zeolite, TiO2-zeolite and Ag-TiO2-zeolite
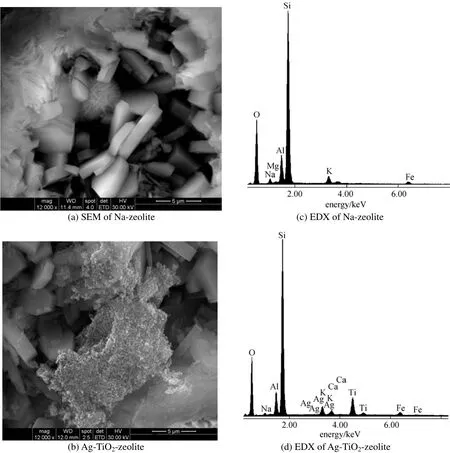
Figure 4 SEM morphology for Na-zeolite (a) and Ag-TiO2-zeolite (b); EDX spectra for elemental analysis of Na-zeolite (c) and Ag-TiO2-zeolite (d)
The SEM images [Figs. 4 (a) and 4 (b)] present the lamellar texture of clinoptilolite, according to the literature data [25]. The TiO2particles are distributed randomized and form cluster agglomerates on the surface and in site of clinoptilolite channels. At 12000× magnification it is obvious that the TiO2nanocrystals (spherical) are non-uniformly distributed on the zeolite surface, forming porous surface. EDX microprobe provided a semiquantitative elemental analysis of the surface, indicating that Ti and Ag were present on the clinoptilolite surface. Also, the natural zeolite contains the major elements such as Na, Si, Al, Ca, K, Mg as can be seen from the EDX spectra [Figs. 4 (c) and 4 (d)].
3.2 Antibacterial activity against Enterococcus faecalis species
CFU value in the blank samples [plate with Enterococcus faecalis and plate with Enterococcus faecalis and natural zeolite (clinoptilolite) un-irradiated/ irradiated] was about 34 colonies. Thus, it was evidenced that only the irradiation did not produce death of the bacterial cells and there are no change of the CFU colonies against the un-irradiated blank samples. Also, the natural zeolite (clinoptilolite) did not present bactericidal effect against the Enterococcus faecalis colonies.
In Figs. 5 (a) and 5 (b) are presented the bactericidal effect of the TiO2-zeolite against Enterococcus faecalis. Blank sample is based only on the Enterococcus faecalis colonies. It can be observed that for using dose of 0.05 g material the bactericidal efficiency was about 53% after irradiation time of 180 min, and CFU values was 18 colonies [Fig. 5 (b)]. By increasing the material dose to 0.1 g, it was observed that after 60 min the CFU colonies were decreased, and the bactericidal efficiency was about 76% after irradiation time of 180 min.
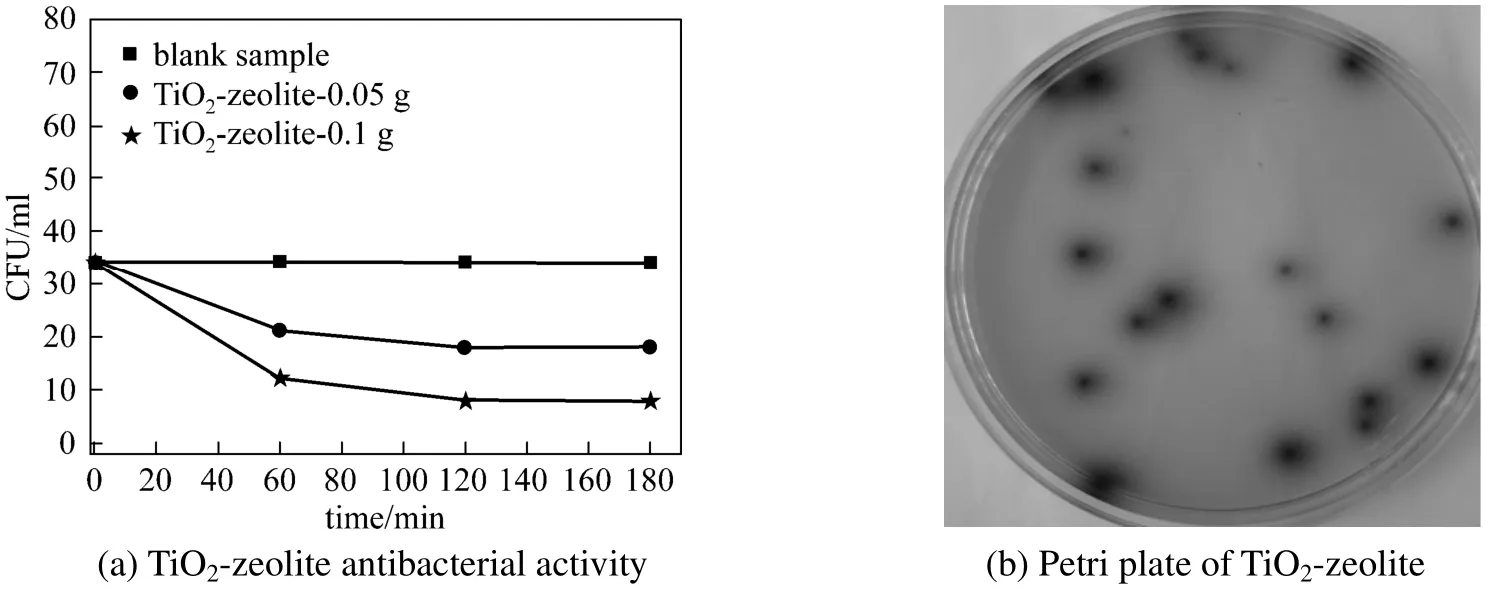
Figure 5 Antibacterial activity of TiO2-zeolite against Enterococcus faecalis species (a); Petri plate of TiO2-zeolite (0.05 g) after 60 min of irradiation (b)
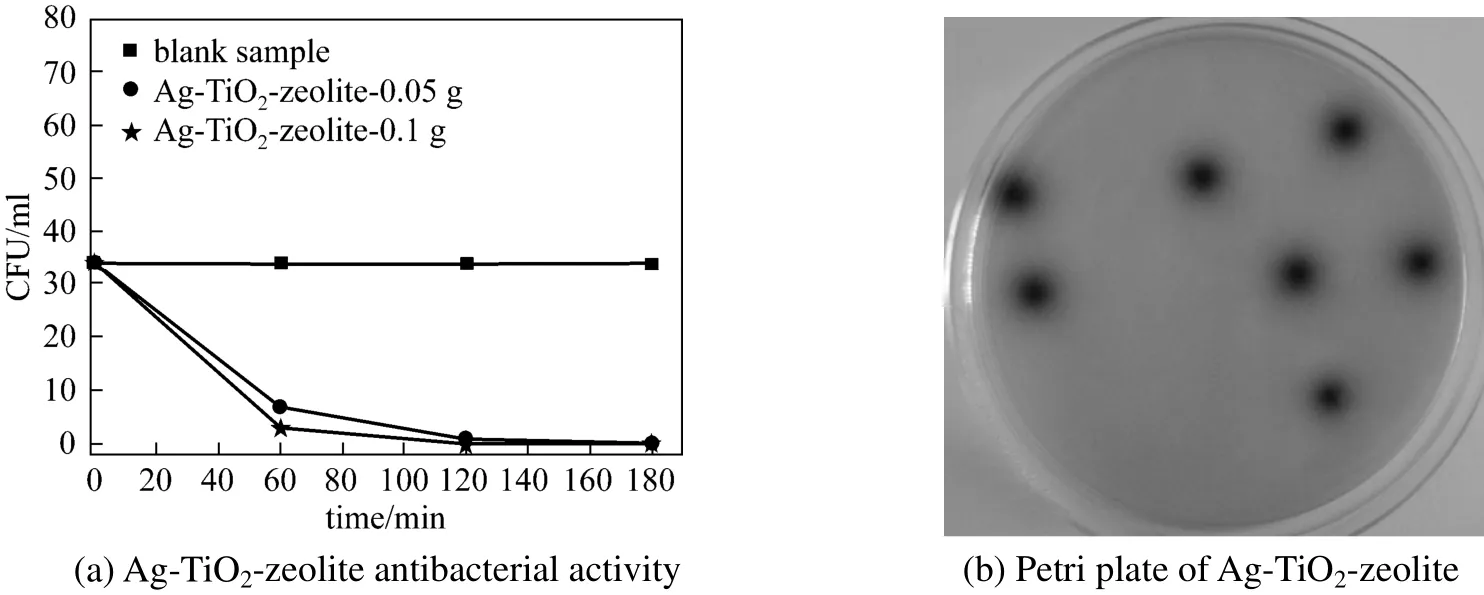
Figure 6 Antibacterial activity of Ag-TiO2-zeolite against Enterococcus faecalis species (a); Petri plate of Ag-TiO2-zeolite (0.05 g) after 60 min of irradiation (b)
Figures 6 (a) and 6 (b) presents the bactericidal effect of the Ag-TiO2-zeolite against the Enterococcus faecalis colonies. At a material dose of 0.05 g the bacteria viability decreases progressively with the irradiation time, such after 180 min are obtained sterile samples. By increasing the material dose at 0.1 g is sufficient a lower illumination time (120 min) for obtaining sterile plates, the bactericidal efficiency being 100% [Fig. 6 (b)].
The Ag-TiO2-zeolite have the higher disinfection efficiency than that of TiO2-zeolite due to silver species acting as photogenerated electrons trapping sites prevent the electron-hole pairs recombine rapidly after photo-excitation leading to enhancement of bactericidal effect. Based on the DRUV-VIS spectra it can be observed that the absorption edge of Ag-TiO2-zeolite slightly shifted to the visible range in comparison with TiO2-zeolite. Also, the higher specific surface area of the Ag-TiO2-zeolite (92.55 m2·g−1) should improve its bactericidal activity. This mechanism produces more hydroxyl radicals for disinfection. Moreover, silver itself is a well antibacterial agent.
Based on the literature data, the mechanisms of Ag-doped TiO2are the interaction of respiratory enzymes damaged by silver or silver oxide and cell membrane damaged by reactive oxygen species (ROS) when cells contact to materials surfaces [26].
4 CONCLUSIONS
Functional materials based on natural zeolite (clinoptilolite) and undoped and Ag-doped TiO2nanocrystals were successfully obtained by solid state reaction in fast-hydrothermal conditions. Structural and morphological characterizations of TiO2-zeolite and Ag-TiO2-zeolite materials confirm that natural zeolite has a good thermal stabilization after the fast-hydrothermal treatment, TiO2particles being distributed randomized with cluster agglomerate groups on the surface and in site of clinoptilolite channels. Based on assessment of the antibacterial activity against Enterococcus faecalis it was observed that bactericidal performance is dependent on materials dose and irradiation time. The lowest value of bactericidal performance about 50% was obtained for TiO2-zeolite, and the highest efficiency, sterile plates, was obtained for Ag-TiO2-zeolite after 180 min of irradiation.
REFERENCES
1 Cabelli, V., Dufour, A.P., McCabe, L.J., Levin, M.A., “Swimming-associated gastroenteritis and water quality”, Am. J. Epidemiol., 115, 606-616 (1982).
2 Dufour, A.P., “Health effects criteria for fresh recreational waters”, Office of Research and Development, US Environmental ProtectionAgency, EPA-600/1-84-004, Washington DC (1984).
3 Dufour, A.P., Ballantine, P., “Ambient water quality criteria for bacteria. Bacteriological ambient water quality criteria for marine and freshwater recreational waters”, Office of Water Regulations and Standards, Criteria and Standards Division, US Environmental Protection Agency, EPA 440/5-84-002, Washington DC (1986).
4 Malato, S., Fernández-Ibáñez, P., Maldonado, M.I., Blanco, J., Gernjak, W., “Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends”, Cataly. Today, 147, 1-59 (2009).
5 Cao, Y.A., Yang, W.S., Zhang, W.F., Liu, G.Z., Yue, P.L., “Improved photocatalytic activity of Sn4+doped TiO2nanoparticulate films prepared by plasma-enhanced chemical vapor deposition”, New. J. Chem., 28, 218-222 (2004).
6 Shon, H.K., Phuntsho, S., Vigneswaran, S., “Effect of photocatalysis on the membrane hybrid system for wastewater treatment”, Desalination, 225, 235-248 (2008).
7 Karunakaran, C., Abiramasundari, G., Gomathisankar, P., Manikandan, G., Anandi, V., “Preparation and characterization of ZnO-TiO2nanocomposite for photocatalytic disinfection of bacteria and detoxification of cyanide under visible light”, Mater. Res. Bull., 46, 1586-1592 (2011).
8 Li, F., Sun, S., Jiang, Y., Xia, M., Sun, M., Xue, B., “Photodegradation of an azo dye using immobilized nanoparticles of TiO2supported by natural porous mineral”, J. Hazard. Mater., 152, 1037-1044 (2008).
9 Yang, Q., Liao, Y., Mao, L., “Kinetics of photocatalytic degradation of gaseous organic compounds on modified TiO2/AC composite photocatalyst”, Chin. J. Chem. Eng., 20, 572-576 (2012).
10 Panpa, W., Sujaridworakun, P., Jinawath, S., “Photocatalytic activity of TiO2/ZSM-5 composites in the presence ofion”, Appl. Catal. B Environ., 80, 271-276 (2008).
11 Lazau, C., Ratiu, C., Orha, C., Pode, R., Manea, F., “Photocatalytic activity of undoped and Ag-doped TiO2-supported zeolite for humic acid degradation and mineralization”, Mater. Res. Bull., 46, 1916-1921 (2011).
12 Fujishima, A., Rao, T.N., Tryk, A.D., “Titanium dioxide photocatalysis”, J. Photochem. Photobiol. C Photochem. Rev., 1, 1-21 (2000).
13 Torimoto, T., Okawa, Y., Takeda, N., Yoneyama, H., “Effect of activated carbon content in TiO2-loaded activated carbon on photodegradation behaviors of dichloromethane”, J. Photochem. Photobiol. A Chem., 103, 153-157 (1997).
14 Shankar, M.V., Anandan, S., Venkatachalam, N., Arabindoo, B., Murugesan, V., “Fine route for an efficient removal of 2,4-dichlorophenoxyacetic acid (2,4-D) by zeolite-supported TiO2”, Chemosphere, 63, 1014-102 (2006).
15 Yoon, S.J., Lee, Y.H., Cho, W.J., Koh, I.O., Yoon, M., “Synthesis of TiO2-entrapped EFAL-removed Y-zeolites: Novel photocatalyst for decomposition of 2-methylisoborneol”, Catal. Commun., 8, 1851-1856 (2007).
16 Sato, T., Koizumi, Y., Taya, M., “Photocatalytic deactivation of airborne microbial cells on TiO2-loaded plate”, Biochem. Eng. J., 14, 149-152 (2003).
17 Rincon, A.G., Pulgarin, C., Adler, N., Peringer, P., “Interaction between E. coli inactivation and DBP-precursors-dihydroxybenzene isomers-in the photocatalytic process of drinking-water disinfection with TiO2”, J. Photochem. Photobiol. A, 39, 233-241 (2011).
18 Madrid, P.A., Morales, R.S., Cordoba-Fierro, L., Nevarez-Moorillo, G.V., Miki-Yoshida, M., Orrantia-Borunda, E., Solis, F.J., “TEM evidence of ultrastructural alteration on Pseudomonas aeruginosa by photocatalytic TiO2thin films”, J. Photochem. Photobiol. B Biol., 70, 45-50 (2003).
19 Durgakumaria, V., Subrahmanyama, M., Subba Raoa, K.V., Ratnamalaa, A., Noorjahana, M., Tanakab, K., “An easy and efficient use of TiO2supported HZSM-5 and TiO2+HZSM-5 zeolite combinate in the photodegradation of aqueous phenol and p-chlorophenol”, Appl. Catal. A Gen., 234, 155-165 (2002).
20 Lachheb, H., Puzenat, E., Houas, A., Ksibi, M., Elaloui, E., Guillard, C., Hermann, J.M., “Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania”, Appl. Catal. B Environ., 39, 75-90 (2002).
21 Lazau, C., Sfirloaga, P., Orha, C., Ratiu, C., Grozescu, I., “Development of a novel fast-hydrothermal method for synthesis of Ag-doped TiO2nanocrystals”, Mater. Lett., 65, 337-339 (2011).
22 Dabici, A., Sfirloaga, P., Lazau, C., Bandas (Ratiu), C., Misca, C., Vaszilcsin, N., “Effect of natural zeolite functionalized with TiO2for enterococcus faecalis removal from water”, Digest J. Nanomater. Biostructures, 6, 1325-1332 (2011).
23 Natori, H., Kobayashi, K., Takahashi, M., “Preparation and photocatalytic property of phosphorus-doped TiO2particles”, J. Oleo Sci., 58, 389-394 (2009).
24 Wu, L.P., Li, X.J., Yuan, Z.H., Chen, Y., “The fabrication of TiO2-supported zeolite with core/shell heterostructure for ethanol dehydration to ethylene”, Catal. Commun., 11, 67-70 (2009).
25 Li, F., Jiang, Y., Yu, L., Yang, Z., Ho, T., Sun, S., “Surface effect of natural zeolite (clinoptilolite) on the photocatalytic activity of TiO2”, Appl. Surf. Sci., 252, 1410-1416 (2005).
26 Ubonchonlakate, K., Sikong, L., Saito, F., “Photocatalytic disinfection of P. aeruginosa bacterial Ag-doped TiO2film”, Procedia Eng., 32, 656-662 (2012).
10.1016/S1004-9541(14)60031-8
2012-10-04, accepted 2013-06-28.
* Supported by the Sectoral Operational Programme Human Resources Development (SOP HRD) Financed from the European Social Fund and by the Romanian Government under the Contract Number POSDRU/89/1.5/S/63700.
** To whom correspondence should be addressed. E-mail: carmen.lazau@gmail.com
 Chinese Journal of Chemical Engineering2014年1期
Chinese Journal of Chemical Engineering2014年1期
- Chinese Journal of Chemical Engineering的其它文章
- Steam Reforming of Dimethyl Ether by Gliding Arc Gas Discharge Plasma for Hydrogen Production*
- Interaction Analysis and Decomposition Principle for Control Structure Design of Large-scale Systems*
- Influence of Design Margin on Operation Optimization and Control Performance of Chemical Processes*
- Enhancing Structural Stability and Pervaporation Performance of Composite Membranes by Coating Gelatin onto Hydrophilically Modified Support Layer*
- Measurement and Modeling for the Solubility of Hydrogen Sulfide in Primene JM-T*
- A Group Contribution Method for the Correlation of Static Dielectric Constant of Ionic Liquids*
