pshuttle-Egr-1-hSmac质粒联合X线照射对乳腺癌MDA-MB-435细胞增殖的抑制作用
梁 硕,王志成,李艳博,,郭彩霞,龚守良,林承赫
(1.吉林大学公共卫生学院卫生部放射生物学重点实验室,吉林长春 130021;2.首都医科大学公共卫生与家庭医学学院,北京 100069;3.吉林大学第一医院核医学科,吉林长春 130021)
pshuttle-Egr-1-hSmac质粒联合X线照射对乳腺癌MDA-MB-435细胞增殖的抑制作用
梁 硕1,王志成1,李艳博1,2,郭彩霞2,龚守良1,林承赫3
(1.吉林大学公共卫生学院卫生部放射生物学重点实验室,吉林长春 130021;2.首都医科大学公共卫生与家庭医学学院,北京 100069;3.吉林大学第一医院核医学科,吉林长春 130021)
目的:构建pshuttle-Egr-1-hSmac质粒并转染人乳腺癌MDA-MB-435细胞,观察其抑制肿瘤细胞的辐射增敏作用。方法:转染pshuttle-Egr-1-hSmac质粒的MDA-MB-435细胞经过2 Gy X线照射不同时间(4、8、12、24和48 h)和0.5~5.0 Gy X线照射后24 h收集细胞,采用RT-PCR和Western blotting法检测Smac mRNA及其蛋白表达。将细胞分为对照组、pshuttle质粒组、pshuttle-Egr-1-hSmac质粒组、2 Gy组、pshuttle+2.0 Gy组和pshuttle-Egr-1-hSmac+2.0 Gy组,MTT法检测各组细胞增殖;克隆形成实验检测细胞存活能力;AnnexinⅤ-FITC双染法检测细胞凋亡;PI单染法检测细胞周期。结果:对照组和pshuttle质粒组MDA-MB-435细胞中Smac mRNA无表达,而pshuttle-Egr-1-hSmac质粒组MDA-MB-435细胞Smac m RNA表达水平随时间延长逐渐升高,于24和48 h时表达水平最高;经0.5~5.0 Gy X线照射后24 h MDA-MB-435细胞Smac m RNA表达水平随照射剂量增加而逐渐增加,在2.0和5.0 Gy X线照射后Smac m RNA表达水平最高。pshuttle-Egr-1-hSmac质粒组4、8、12和24 h后Smac蛋白表达水平逐渐升高,24 h后表达水平最高。经过0、0.5、1.0、2.0和5.0 Gy X线照射后24 h Smac蛋白表达水平逐渐升高,尤其以5.0 Gy X线照射时表达水平最高。MTT法检测时程效应,2.0 Gy、pshuttle+2.0 Gy和pshuttle-Egr-1-hSmac+2.0 Gy质粒组24、48和72 h细胞A490值明显低于对照组(P<0.01);剂量效应,pshuttle-Egr-1-hSmac质粒组1.0~5.0 Gy X线照射后, MDA-MB-435细胞A490值明显低于0 Gy X线照射(P<0.05或P<0.01)。pshuttle-Egr-1-hSmac质粒组细胞存活分数明显低于对照组(P<0.01)。pshuttle-Egr-1-hSmac+2.0 Gy组细胞凋亡率明显高于2.0 Gy组(P<0.01), G0/G1期和S期细胞百分率明显低于2.0 Gy照射组(P<0.01),G2/M期细胞百分率明显高于2.0 Gy组(P<0.01)。结论:X线照射能增加pshuttle-Egr-1-hSmac质粒转染的MDA-MB-435细胞有效表达Smac m RNA及蛋白,能抑制细胞存活,且诱导G2/M期阻滞和凋亡增加;Smac基因联合放射治疗可明显增加乳腺癌细胞的放射敏感性。
Smac基因;Egr-1启动子;X射线;基因-放射治疗;细胞凋亡
临床放射治疗(放疗)在乳腺癌治疗中占有重要的地位,但是辐射的副作用不可避免,如何在提高乳腺癌放疗疗效的同时减少副损伤是肿瘤治疗的研究热点[1-3]。辐射敏感启动子Egr-1具有辐射诱导特性,即该启动子在正常条件下不增强下游基因的表达,只有在辐射条件下,下游基因才能在Egr-1介导下表达增强,增强该基因的效应。因此Egr-1可以从时间和空间上调控下游基因的体内表达,提高放疗的敏感性,减轻毒副作用,实现肿瘤综合治疗的目的[4-5]。与Egr-1相连接的基因有很多种类,但是有关Smac基因的研究却未见报道。本研究利用放疗能够杀伤肿瘤细胞和诱导Egr-1启动子的转录作用以及Smac基因能够促进肿瘤细胞凋亡的作用[6-7],将pshuttle-Egr-1-hSmac质粒联合X线照射作用于乳腺癌MDA-MB-435细胞,以降低辐射剂量并能够减轻或避免正常组织损伤,达到抑制肿瘤生长和杀伤肿瘤细胞的目的,为肿瘤治疗开辟新途径。
1 材料与方法
1.1 细胞培养及转染人乳腺癌细胞株MDA-MB-435为吉林大学卫生部放射生物学重点实验室保存,以含10%胎牛血清的DMEM培养液(美国Gibco公司)于37℃、5%CO2培养箱中常规培养,待细胞80%~90%融合可传代。取对数生长期细胞接种于6孔板,6×105个/孔。第2天待细胞达90%~95%,按照Lipofectamine 2000转染试剂盒(美国Invitrogen公司)说明书转染构建正确的质粒。
1.2 细胞照射采用国产X射线深部治疗机进行照射,电压200 k V,电流10 m A,滤板为0.5 mm Cu和1.0 mm Al。照射时靶皮距50 cm,剂量率0.287 Gy·min-1。
1.3 Smac m RNA和蛋白表达规律将经过脂质体转染空载体pshuttle和pshuttle-Egr-1-hSmac质粒24 h的MDA-MB-435细胞经过2.0 Gy X线照射后不同时间(4、8、12、24和48 h)和不同剂量(0.5、1.0、2.0和5.0 Gy)照射后24 h收集细胞,采用RT-PCR方法和Western blotting法检测Smac m RNA和蛋白表达规律,Smac引物序列上游引物:5′-gctctagaatggcggctctgaagagttggctgt-3′, 含XbaⅠ酶切位点;下游引物:5′-gcggatcctcaa tcctcacgcaggt-3′,含Bam HⅠ酶切位点。
1.4 细胞增殖时程及剂量效应实验MDA-MB-435细胞分为对照组(control)、pshuttle质粒组、pshuttle-Egr-1-hSmac质粒组、2.0 Gy组、pshuttle+2.0 Gy组和pshuttle-Egr-1-hSmac+2.0 Gy组。MDA-MB-435细胞接种于96孔板, 1×104个/孔,每组6复孔。转染质粒24 h后,给予2.0 Gy照射,于照射后0、12、24、48和72 h加入MTT,每孔MTT(5 g·L-1)20μL,继续培养4 h后弃上清,每孔加入150μL DMSO,待充分溶解后酶标仪测定490 nm波长处吸光度(A490)值。以A490值表示细胞增殖情况,A490值降低,细胞生长受抑制。
MDA-MB-435细胞以6×104个/孔接种于96孔细胞培养板,分别设对照组、pshuttle组和pshuttle-Egr-1-hSmac组,经脂质体转染后24 h给予照射,照射剂量分别为0、0.5、1.0、2.0和5.0 Gy,X线照射后24 h加入MTT测量A490值。以A490值表示细胞增殖情况,A490值降低,细胞生长受抑制。
1.5 细胞克隆形成实验对照细胞及转染pshuttle和pshuttle-Egr-1-hSmac质粒24 h后,按照预先设计的细胞密度接种到60 mm无菌塑料培养皿中,各组设3个平行样,接种密度为100个/ 孔(0 Gy)、200个/孔(2 Gy)、600个/孔(4 Gy)、2 000个/孔(6 Gy)、5 000个/孔(8 Gy)和50 000个/孔(10 Gy)。加入5 m L培养液,继续培养14 d,Giemsa染色,空气中干燥,封片,计数克隆数。用未照射组的集落形成率(PE)进行校正,计算细胞存活分数(survival fraction,SF),计算导致细胞63%死亡所需剂量(D0)值。
1.6 细胞周期及凋亡检测分组同1.4。MDAMB-435细胞接种于6孔板,6×105个/孔,细胞转染24 h后给予2.0 Gy照射,24 h后胰酶消化收获细胞,PBS洗2次,0.5 m L PBS重悬细胞,加入AnnexinⅤ/PI或PI避光染色1 h,立即上流式细胞仪进行检测。采用CellQuest软件收取细胞(每份样品收取1×104个细胞),采用ModFit软件分析,结果以细胞凋亡率及各周期细胞百分率表示。
1.7 统计学分析采用SPSS 17.0软件进行统计分析。各组细胞A490值、细胞SF、凋亡率和各细胞周期细胞百分率以±s表示,均数两两比较采用t检验,多组均数比较采用完全随机设计的单因素方差分析。
2 结果
2.1 X线照射后转染pshuttle-Egr-1-hSmac质粒的MDA-MB-435细胞中Smac mRNA表达的规律
2.0 Gy X线照射后,对照组和pshuttle质粒组Smac mRNA在24 h时无表达,而pshuttle-Egr-1-hSmac质粒组MDA-MB-435细胞中Smac m RNA表达水平随时间延长逐渐增高,4、8、12、24和48 h时Smac m RNA表达相对值分别为0.55、0.52、0.81、1.63和1.59,于24和48 h时表达较高,具有一定的时程效应。见图1。
重组质粒转染细胞后24 h进行照射,照射剂量为0.5、1.0、2.0和5.0 Gy,照射后24 h检测Smac m RNA表达。对照组和pshuttle质粒组MDA-MB-435细胞中Smac m RNA无表达。pshuttle-Egr-1-hSmac质粒组MDA-MB-435细胞经0.5、1.0、2.0和5.0 Gy照射后Smac m RNA表达相对值分别为0.31、0.84、1.33和1.29,其中2.0和5.0 Gy照射后Smac m RNA表达较为明显,具有一定的剂量效应。见图2。

图1 2.0 Gy X线照射后不同时间MDA-MB-435细胞中Smac m RNA表达电泳图Fig.1 Electrophoregram of Smac mRNA expressions in MDA-MB-435 cells at different time after 2.0 Gy X-ray irradiationLane 1:Pshuttle group;Lane 2:DL 2000 marker;Lane 3:Control group;Lane 4―8:4,8,12,24,and 48 h in pshuttle-Egr-1-hSmac+2.0 Gy group.
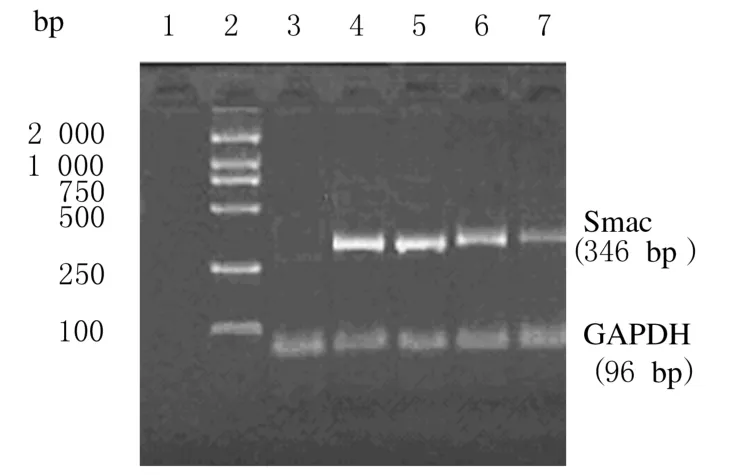
图2 不同剂量X线照射后24 h Smac m RNA表达电泳图Fig.2 Electrophoregram of Smac m RNA expressions 24 h after different doses of X-ray irradiationLane 1:Control group;Lane 2:DL 2000 marker;Lane 3:Pshuttle group;Lane 4-7:5.0,2.0,10,and 0.5 Gy X-ray irradiation in pshuttle-Egr-1-hSmac plasmid group.
2.2 X线照射后转染pshuttle-Egr-1-hSmac质粒的MDA-MB-435细胞中Smac蛋白表达的规律转染pshuttle-Egr-1-hSmac质粒24 h后进行照射,采用Western blotting法检测细胞中Smac蛋白表达。Smac的相对分子质量为25 000,从图3中可见有明显的蛋白条带,与Smac蛋白大小一致,而β-actin的相对分子质量为42 000。MDA-MB-435细胞经2.0 Gy X线照射后,对照组Smac蛋白无表达,pshuttle-Egr-1-hSmac质粒组4、8、12和24 h后蛋白表达逐渐增加,灰度分析后蛋白表达相对值为0.28、0.31、0.44和0.62(图3);不同剂量X线照射后,对照组无Smac蛋白表达,0.5、1.0、2.0和5.0 Gy照射组Smac蛋白表达相对值分别为0.31、0.34、0.68和0.86(图4)。说明质粒pshuttle-Egr-1-hSmac在X射线诱导下可有效表达Smac蛋白,并且以5 Gy照射后24 h表达最为明显。
2.3 重组质粒联合X线照射对MDA-MB-435细胞生长抑制的时程及剂量效应时程效应结果:转染pshuttle和pshuttle-Egr-1-hSmac质粒后MDA-MB-435细胞生长有抑制倾向,但不明显, 2.0 Gy、pshuttle+2.0 Gy和pshuttle-Egr-1-hSmac+2.0 Gy组细胞A490值明显降低,24、48 和72 h时与对照组比较差异有统计学意义(P<0.05或P<0.01),其中pshuttle-Egr-1-hSmac+2.0 Gy组细胞A490值降低最明显,与pshuttle-Egr-1-hSmac组比较差异也有统计学意义(P<0.05或P<0.01)。剂量效应结果:MDA-MB-435细胞A490值随照射剂量增加逐渐降低,对照组和pshuttle组降低不明显;pshuttle-Egr-1-hSmac质粒组MDA-MB-435细胞A490值明显降低,1.0、2.0和5.0 Gy X线照射后,与0 Gy X线照射比较,细胞A490值明显降低(P<0.05或P<0.01)。见表1和2。

图3 2.0 Gy X线照射后不同时间Smac蛋白表达电泳图Fig.3 Electrophoregram of Smac protein expressions at different time after 2.0 Gy X-ray irradiationLane 1―4:4,8,12,and 24 h after 2.0 Gy X-ray irradiation in pshuttle-Egr-1-hSmac+2 Gy group;Lane 5:Control group.

图4 不同剂量X线照射后24 h Smac蛋白表达电泳图Fig.4 Electrophoregram of Smac protein expressions after different doses of X-ray irradiation for 24 hLane 1-4:0.5,1.0,2.0,and 5.0 Gy X-ray irradiation in pshuttle-Egr-1-hSmac plasmid group;Lane 5:Control group.
表1 2.0 Gy X线照射后不同时间各组细胞A490值Tab.1 A490values of cells after 2 Gy irradiation in various groups(n=6±s)
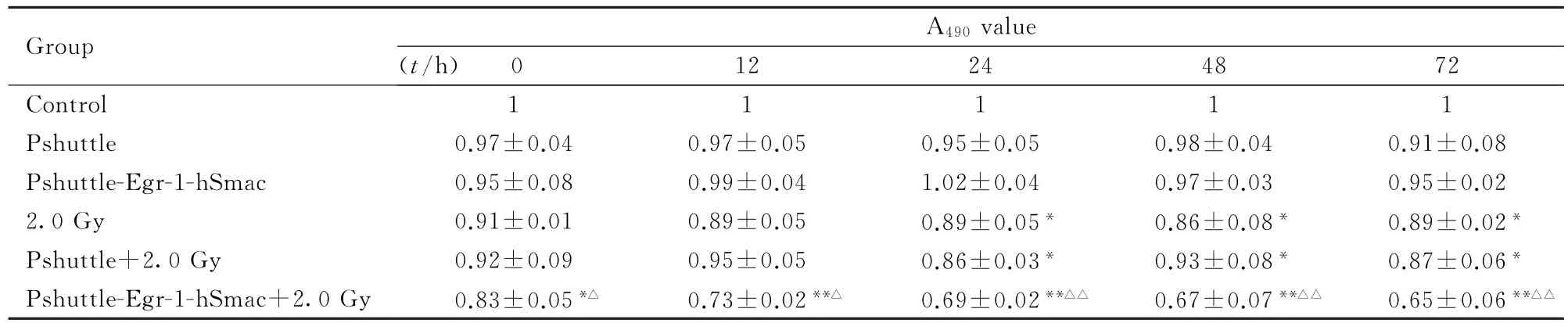
表1 2.0 Gy X线照射后不同时间各组细胞A490值Tab.1 A490values of cells after 2 Gy irradiation in various groups(n=6±s)
∗P<0.05,∗∗P<0.01 vs control group;△P<0.05,△△P<0.01 vs pshuttle-Egr1-hSmac group.
Group A490value (t/h) 0 12 24 48 72 Control 1 1 1 1 1 Pshuttle 0.97±0.04 0.97±0.05 0.95±0.05 0.98±0.04 0.91±0.08 Pshuttle-Egr-1-hSmac 0.95±0.08 0.99±0.04 1.02±0.04 0.97±0.03 0.95±0.02 2.0 Gy 0.91±0.01 0.89±0.05 0.89±0.05∗0.86±0.08∗0.89±0.02∗Pshuttle+2.0 Gy 0.92±0.09 0.95±0.05 0.86±0.03∗0.93±0.08∗0.87±0.06∗Pshuttle-Egr-1-hSmac+2.0 Gy 0.83±0.05∗△0.73±0.02∗∗△0.69±0.02∗∗△△0.67±0.07∗∗△△0.65±0.06∗∗△△
表2 不同剂量X线照射后各组细胞A490值Tab.2 A490values of cells after different doses X-ray irradiation in various groups(n=6,±s)

表2 不同剂量X线照射后各组细胞A490值Tab.2 A490values of cells after different doses X-ray irradiation in various groups(n=6,±s)
∗P<0.05,∗∗P<0.01 vs control group.
Group A490value (D/Gy)0 0.5 1.0 2.0 5.0 Control 1 1 1 1 1 Pshuttle 1.01±0.04 1.02±0.05 1.02±0.03 1.01±0.02 0.99±0.07 Pshuttle-Egr-1-hSmac 1.01±0.02 0.94±0.05 0.91±0.02∗0.87±0.02∗0.71±0.02∗∗
2.4 各组细胞剂量存活曲线与对照组比较, pshuttle组细胞SF未见明显变化,而pshuttle-Egr-1-hSmac质粒组细胞SF明显降低(P<0.01,见表3)。将各组细胞的SF进行直线相关与回归分析得出如下方程:Y=-9.9316X+102.45, R2=0.9661(对照组);Y=-9.6487X+100.62,R2=0.9559(pshuttle组);Y=-9.2199X+80.614,R2=0.9126(pshuttle-Egr-1-hSmac组)。进而计算得出各组细胞的D0值为3.31、3.29和2.70,对照组与pshuttle质粒组细胞D0值相近,而pshuttle-Egr-1-hSmac质粒组细胞D0值明显降低,说明该组细胞放射敏感性高。
表3 各组MDA-MB-435细胞SFTab.3 SF of MDA-MB-435 cells in various groups(n=3,±s,η/%)

表3 各组MDA-MB-435细胞SFTab.3 SF of MDA-MB-435 cells in various groups(n=3,±s,η/%)
∗P<0.01 compared with control group.
Group SF (D/Gy)0 2 4 6 8 10 Control 99.00±1.11 85.20±3.32 70.30±4.07 40.20±1.88 12.30±1.44 9.76±0.89 Pshuttle 95.10±2.32 84.90±5.98 72.30±2.55 39.00±5.14 13.10±1.61 9.78±1.11 Pshuttle-Egr-1-hSmac 90.20±1.29∗66.10±3.15∗30.20±1.50∗11.10±0.36∗4.02±0.47∗0.61±0.08∗
2.5 重组质粒联合X线照射作用下MDA-MB-435细胞凋亡率MDA-MB-435细胞转染pshuttle-Egr-1-hSmac质粒后,细胞凋亡率未见明显增加;与对照组比较,2.0 Gy、pshuttle+2.0 Gy和pshuttle-Egr-1-hSmac+2.0 Gy组细胞凋亡率明显升高(P<0.05或P<0.01),其中以pshuttle-Egr-1-hSmac+2.0 Gy组升高最为明显;与2.0 Gy组比较,pshuttle-Egr-1-hSmac+2.0 Gy组细胞凋亡率明显升高(P<0.01)。见表4。
表4 各组MDA-MB-435细胞凋亡率Tab.4 Apoptotic rates of MDA-MB-435 cells in various groups(n=4,±s,η/%)
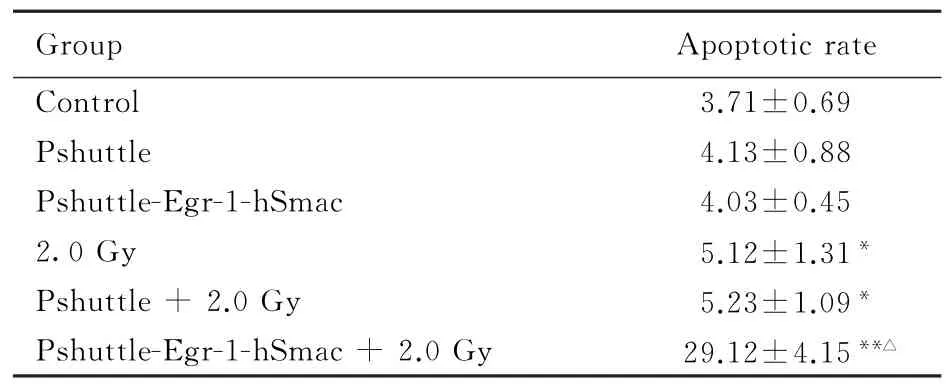
表4 各组MDA-MB-435细胞凋亡率Tab.4 Apoptotic rates of MDA-MB-435 cells in various groups(n=4,±s,η/%)
∗P<0.05,∗∗P<0.01 vs control group;△P<0.01 vs 2.0 Gy group.
Group Apoptotic rate Control 3.71±0.69 Pshuttle 4.13±0.88 Pshuttle-Egr-1-hSmac 4.03±0.45 2.0 Gy 5.12±1.31∗Pshuttle+2.0 Gy 5.23±1.09∗Pshuttle-Egr-1-hSmac+2.0 Gy 29.12±4.15∗∗△
2.6 重组质粒联合X线照射作用下MDA-MB-435细胞周期的细胞百分率对照组、pshuttle组和pshuttle-Egr-1-hSmac组各期MDA-MB-435细胞百分率基本一致;2.0 Gy X线照射后,2.0 Gy组、pshuttle+2.0 Gy组和pshuttle-Egr-1-hSmac+2.0 Gy组G0/G1和S期细胞百分率明显降低(P<0.01),而G2/M期细胞百分率明显升高(P<0.01),其中pshuttle-Egr-1-hSmac+2.0 Gy组细胞百分率与对照组、2.0 Gy组和pshuttle-Egr-1-hSmac组比较差异均有统计学意义(P<0.01)。见表5。
3 讨论
放疗是乳腺癌治疗的一个重要手段,但是放疗所致邻近部位的放射损伤或者患者的辐射耐受严重影响和限制其疗效和广泛应用,如何增强乳腺癌细胞对射线的敏感性对提高乳腺癌预后非常重要[8-9]。辐射敏感启动子Egr-1具有辐射诱导特性,该启动子在正常条件下不增强下游基因的表达,只有在照射条件下,下游基因在Egr-1介导下表达增强,从而增强该基因的效应[10-11]。Egr-1可以从时间和空间上调控下游基因的体内表达,提高放疗的敏感性。Smac是2000年发现的一种存在于线粒体并且调节细胞凋亡的蛋白质,主要通过抑制凋亡抑制蛋白(inhibitor of apoptosis proteins,IAPs)的活性而发挥促凋亡作用,是一种线粒体依赖性凋亡途径[12-15]。
表5 各组MDA-MB-435细胞周期细胞百分率Tab.5 Percentages of MDA-MB-435 cells at each cell cycle in various groups(n=4,±s,η/%)

表5 各组MDA-MB-435细胞周期细胞百分率Tab.5 Percentages of MDA-MB-435 cells at each cell cycle in various groups(n=4,±s,η/%)
∗P<0.05,∗∗P<0.01 vs control group;△P<0.05,△△P<0.01 vs pshuttle-Egr-1-hSmac group;#P<0.05,##P<0.01 vs 2.0 Gy group.
Group Percentage of MDA-MB-435 cells G0/G1S G2/M Control 61.28±1.64 31.56±1.11 8.16±0.64 Pshuttle 62.95±1.8 26.68±1.59 10.37±0.85 Pshuttle-Egr-1-hSmac 60.82±1.22 31.93±1.01 8.25±2.19 2.0 Gy 48.42±1.56∗28.30±1.74∗23.28±1.43∗∗Pshuttle+2.0 Gy 49.78±1.78∗24.83±2.67∗30.39±2.26∗∗Pshuttle-Egr-1-hSmac+2.0 Gy 36.92±1.83∗∗△△#22.18±0.85∗△#40.9±1.43∗∗△#
本研究利用已构建成功的pshuttle-Egr-1-hSmac质粒转染人乳腺癌MDA-MB-435细胞,结果显示:2.0 Gy X线照射后24 h,对照组和pshuttle质粒组均无Smac m RNA表达,而转染pshuttle-Egr-1-hSmac质粒的细胞经过2.0 Gy照射后,Smac mRNA表达水平随着时间的延长逐渐升高,于照射后24和48 h表达较高,具有一定的时程效应关系;pshuttle-Egr-1-hSmac质粒组转染后进行0.5~5.0 Gy X线照射,细胞中Smac mRNA表达具有一定的量效关系,在2.0和5.0 Gy照射后表达较为明显;蛋白检测结果显示: pshuttle组MDA-MB-435细胞中无Smac蛋白表达,经0.5、1.0、2.0和5.0 Gy照射后表达逐渐增多,5.0 Gy照射时其蛋白表达最为明显, 2.0 Gy照射后4 h开始即有表达且逐渐增多,照射后24 h时达到最高。本研究结果提示:在X线照射后,从Smac m RNA和蛋白水平上看,Egr-1启动子发挥了辐射诱导增强的作用,具有一定的时程效应和剂量效应关系,而空载体和正常对照则无表达。
本研究中MTT检测结果显示:MDA-MB-435细胞在转染pshuttle和pshuttle-Egr-1-hSmac质粒后0~72 h,细胞增殖能力稍有降低,但不明显;而各组细胞经过2.0 Gy X射线照射后,细胞增殖能力明显降低,尤其以转染了pshuttle-Egr-1-hSmac质粒的细胞增殖能力降低的更明显;0~5.0 Gy X射线照射后,对照组MDA-MB-435细胞增殖能力降低,但差异不明显;转染pshuttle质粒后进行相应剂量照射,转染了pshuttle-Egr-1-hSmac质粒则明显抑制细胞增殖。上述结果表明:转染重组质粒对MDA-MB-435细胞生长影响不明显,而X射线照射能抑制MDA-MB-435细胞生长,在转染后进行照射则可加强其抑制效果,可能与辐射诱导Smac表达有关。本研究结果显示:转染空载体pshuttle后,MDA-MB-435细胞SF未见明显改变,即转染空载体未对细胞产生明显影响,而转染pshuttle-Egr-1-hSmac质粒的细胞SF明显下降,说明MDA-MB-435细胞存活能力明显降低,辐射对其具有明显影响。本研究结果显示:对照组和pshuttle质粒组D0值基本相同,说明放射敏感性无明显差别;而pshuttle-Egr-1-hSmac质粒组的D0值降低,说明细胞放射敏感性增高,提示转染pshuttle-Egr-1-hSmac质粒能增强MDA-MB-435细胞的放射敏感性;电离辐射可以抑制乳腺癌细胞的生长,转染pshttle-Egr-1-hSmac质粒后进行相应电离辐射细胞生长抑制更明显,可能与电离辐射诱导Smac表达有关。
本研究中凋亡检测结果显示:MDA-MB-435细胞转染pshuttle-Egr-1-hSmac质粒后,凋亡率未增加;2.0 Gy照射后,与对照组比较,pshuttle-Egr-1-hSmac组细胞凋亡率明显升高,与其他组比较差异均有统计学意义,说明基因联合放疗对乳腺癌MDA-MB-435细胞的促凋亡作用具有良好的效果,能有效地诱导细胞凋亡、杀伤细胞。Smac基因是线粒体内释放的凋亡促进蛋白,而电离辐射也可以诱导细胞凋亡,二者的联合应用势必增加细胞凋亡率。本研究结果显示:转染pshuttle和pshuttle-Egr-1-hSmac后MDA-MB-435细胞周期无明显变化,2.0 Gy、pshuttle+2.0 Gy和pshuttle-Egr-1-hSmac+2.0 Gy组MDA-MB-435细胞G0/G1和S期细胞百分率明显下降,G2/M期细胞百分率明显升高,其中pshuttle-Egr-1-hSmac +2.0 Gy组变化最为明显,导致MDA-MB-435细胞G2/M期阻滞。上述结果可能与电离辐射的作用有密切关联,也是辐射诱导细胞凋亡的主要机制之一。
综上所述,pshuttle-Egr-1-hSmac联合X线照射能有效地抑制MDA-MB-435细胞的生长,提高细胞放射敏感性,影响细胞周期进程,导致MDA-MB-435细胞发生G2/M期阻滞,促进细胞凋亡,从而达到更好的杀伤肿瘤的作用。本研究为提高基因-放疗效果开辟了新途径,为基因-放疗的临床应用提供了实验依据。
[1]Cammarota F,Giugliano FM,Iadanza L,et al. Hypofractionated breast cancer radiotherapy.Helical tomotherapy in supine position or classic 3D-conformal radiotherapy in prone position:Which is better?[J].Anticancer Res,2014,34(3):1233-1238.
[2]Auer J,Keller U,Schmidt M,et al.Individual radiosensitivity in a breast cancer collective is changed with the patients’age[J].Radiol Oncol,2014,48(1):80-86.
[3]Belkacemi Y,Khodari W,Grelier N,et al.Breast cancer radiotherapy:current changes[J].Rev Prat,2013, 63(10):1402-1403.
[4]Woo SM,Min KJ,Kim S,et al.Silibinin induces apoptosis of HT29 colon carcinoma cells through early growth response-1(EGR-1)-mediated non-steroidal antiinflammatory drug-activated gene-1(NAG-1) up-regulation[J].Chem Biol Interact,2014,211(4):36-43.
[5]Li YB,Guo CX,Wang ZC,et al.Radiosensitization of breast cancer cells by TRAIL-endostatin-targeting gene therapy[J].Neoplasma,2013,60(6):613-619.
[6]Monteiro JP,Oliveira PJ,Jurado AS.Mitochondrial membrane lipid remodeling in pathophysiology:a new target for diet and therapeutic interventions[J].Prog Lipid Res, 2013,52(4):513-528.
[7]Wang K,Lin B.Inhibitor of apoptosis proteins(IAPs)as regulatory factors of hepatic apoptosis[J].Cell Signal,2013, 25(10):1970-1980.
[8]Badakhshi H,Gruen A,Sehouli J,et al.The impact of patient compliance with adjuvant radiotherapy:a comprehensive cohort study[J].Cancer Med,2013,2(5): 712-717.
[9]Blitzblau RC,Horton JK.Radiotherapy after mastectomy[J].Surg Oncol Clin N Am,2013,22(3): 563-577.
[10]Hu Y,Ouyang W,Wu F,et al.Enhanced radiosensitivity of SW480 cells via TRAIL up-regulation mediated by Egr-1 promoter[J].Oncol Rep,2009,22(4):765-771.
[11]Zhou Y,Song X,Jia R,et al.Radiation-inducible human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)gene therapy:a novel treatment for radioresistant uveal melanoma[J].Pigment Cell Melanoma Res,2010, 23(5):661-674.
[12]Moon D,McCormack D,McDonald D,et al.Pterostilbene induces mitochondrially derived apoptosis in breast cancer cells in vitro[J].J Surg Res,2013,180(2):8-15.
[13]Hsiao WT,Tsai MD,Jow GM,et al.Involvement of Smac,p53,and caspase pathways in induction of apoptosis by gossypol in human retinoblastoma cells[J].Mol Vis, 2012,18(20):2033-2042.
[14]Allensworth JL,Sauer SJ,Lyerly HK,et al.Smac mimetic Birinapant induces apoptosis and enhances TRAIL potency in inflammatory breast cancer cells in an IAP-dependent and TNF-α-independent mechanism[J].Breast Cancer Res Treat, 2013,137(2):359-371.
[15]Yang D,Zhao Y,Li AY,et al.Smac-mimetic compound SM-164 induces radiosensitization in breast cancer cells through activation of caspases and induction of apoptosis[J].Breast Cancer Res Treat,2012,133(1):189-199.
Inhibitory effect of pshuttle-Egr-1-hSmac plasmid combined with X-ray irradiation on proliferation of breast cancer MDA-MB-435 cells
LIANG Shuo1,WANG Zhi-cheng1,LI Yan-bo1,2,GUO Cai-xia2,GONG Shou-liang1,LIN Cheng-he3
(1.Key Laboratory of Radiobiology,Ministry of Health,School of Public Health,Changchun 130021, China;2.School of Public Health and Family Medicine,Capital Medical University,Beijing 100069, China;3.Department of Nuclear Medicine,First Hospital,Jilin University,Changchun 130021,China)
ObjectiveTo construct the pshuttle-Egr-1-hSmac plasmid and transfect human breast cancer MDA-MB-435 cells,and to observe its radiotherapy enhancing effect on tumor cells.MethodsThe empty vector pshuttle and pshuttle-Egr-1-hSmac plasmid were transfected into MDA-MB-435 cells by liposomal.At different time(4,8,12,24 and 48 h)after irradiation with 2.0 Gy X-ray and 24 h after irradiation with 0.5―5.0 Gy,the total RNA and protein were collected and extracted from these cells to analyze the Smac m RNA and protein expression levels with RT-PCR and Western blotting methods.The cells were divided into control,pshuttle, pshuttle-Egr-1-hSmac,2.0 Gy irradiation group,pshuttle+2.0 Gy irradiation and pshuttle-Egr-1-hSmac+2.0 Gy irradiation groups.MTT method was used to evaluate cell proliferation,and the cell survival ability was measured with clone formation assay;AnnexinⅤ/PI double staining and PI single staining were used to examine the apoptosis and cell cycle of MDA-MB-435 cells.ResultsThere was no Smac m RNA expression in MDA-MB-435 cells in control and pshuttle groups,but the Smac mRNA expression levels in MDA-MB-435 cells in pshuttle-Egr-1-hSmac plasmid group were gradually increased with the time prolongation,and reached the maximum at 24 and 48 h;the Smac m RNA expression levels in MDA-MB-435 cells were increased gradually 24 h after irradiation of 0.5―5.0 Gy X-ray with the increasing of irradiation doses,and reached the maximum after 2.0 and 5.0 Gy irradiation.The Smac protein expression levels in pshuttle-Egr-1-hSmac plasmid group were increased gradually with the time prolongation,and reached the maximum at 24 h.The Smac protein expression lervels were increased 24 h afer irradiation of 0,0.5,1.0,2.0 and 5.0 Gy X-ray,especially in 5.0 Gy group.The MTT results showed that the A490values in 2.0 Gy,pshuttle+2.0 Gy and pshuttle-Egr-1-hSmac groups 24, 48,and 72 h after irradiation were lower than those in control group(P<0.01);the A490values of MDA-MB-435 cells in pshuttle-Egr-1-hSmac group after 1.0-5.0 Gy X-ray irradiation were lower than those in 0 Gy group(P<0.05 or P<0.01);the survival fraction(SF)in pshuttle-Egr-1-hSmac group was lower than those in control group (P<0.01).The percentages of the cells at G0/G1and S phase in pshuttle-Egr-1-hSmac group were lower than those in 2.0 Gy group(P<0.01),the percentage of the cells at G2/M phase was higher than that in 2.0 Gy group(P<0.01);the apoptotic rate of the cells in pshuttle-Egr-1-hSmac group was higher than that in 2.0 Gy group(P<0.01).ConclusionX-ray irradiation can significantly increase the Smac mRNA and protein expression levels in MDA-MB-435 cells transfected with pshuttle-Egr-1-hSmac plasmid,inhibit the cell survival rate,and induce G2/M arrest and apoptotic increasing;Smac gene combined with radiotherapy could significantly increase the radiosensitivity of breast cancer cells.
Smac gene;Egr-1 promoter;X-ray;gene-radiotherapy;apoptosis
R737.9
A
2014-01-10
国家自然科学基金资助课题(30870747);吉林大学基本科研项目资助课题(2012)
梁 硕(1973-),男,吉林省长春市人,讲师,医学博士,主要从事肿瘤基因-放射治疗方面的研究。
林承赫(Tel:0431-88782717,E-mail:linchh1967@163.com)

