Transplantation of placenta-derived mesenchymal stem cell-induced neural stem cells to treat spinal cord injury
Zhi Li, Wei Zhao, Wei Liu, Ye Zhou, Jingqiao Jia, Lifeng Yang
1 Department of Orthopedics, Af fi liated Central Hospital of Shenyang Medical College, Shenyang, Liaoning Province, China
2 Department of Obstetrics, Af fi liated Central Hospital of Shenyang Medical College, Shenyang, Liaoning Province, China
3 Liaoning Province Wellcare Stem Cells Biotechnology Co., Ltd., Benxi, Liaoning Province, China
Transplantation of placenta-derived mesenchymal stem cell-induced neural stem cells to treat spinal cord injury
Zhi Li1, Wei Zhao1, Wei Liu2, Ye Zhou2, Jingqiao Jia3, Lifeng Yang1
1 Department of Orthopedics, Af fi liated Central Hospital of Shenyang Medical College, Shenyang, Liaoning Province, China
2 Department of Obstetrics, Af fi liated Central Hospital of Shenyang Medical College, Shenyang, Liaoning Province, China
3 Liaoning Province Wellcare Stem Cells Biotechnology Co., Ltd., Benxi, Liaoning Province, China
Because of their strong proliferative capacity and multi-potency, placenta-derived mesenchymal stem cells have gained interest as a cell source in the fi eld of nerve damage repair. In the present study, human placenta-derived mesenchymal stem cells were induced to differentiate into neural stem cells, which were then transplanted into the spinal cord after local spinal cord injury in rats. The motor functional recovery and pathological changes in the injured spinal cord were observed for 3 successive weeks. The results showed that human placenta-derived mesenchymal stem cells can differentiate into neuron-like cells and that induced neural stem cells contribute to the restoration of injured spinal cord without causing transplant rejection. Thus, these cells promote the recovery of motor and sensory functions in a rat model of spinal cord injury. Therefore, human placenta-derived mesenchymal stem cells may be useful as seed cells during the repair of spinal cord injury.
nerve regeneration; stem cells; placenta-derived mesenchymal stem cells; spinal cord injury; neural stem cells; nerve-like cells; motor function; sensory function; neural regeneration
Funding: This study was supported by a grant from the Scientific Research Program of Liaoning Provincial Science and Technology Ministry in China, No. 2012225014.
Li Z, Zhao W, Liu W, Zhou Y, Jia JQ, Yang LF. Transplantation of placenta-derived mesenchymal stem cell-induced neural stem cells to treat spinal cord injury. Neural Regen Res. 2014;9(24):2197-2204.
Introduction
Stem cell research has rapidly progressed recently, and mesenchymal stem cells in particular have been widely used as a novel treatment method (Syková et al., 2006; Satija et al., 2009; Patel et al., 2013). The growing evidence has demonstrated that mesenchymal stem cells are pluripotent and provide clear bene fi ts when used in the treatment of various nervous system dysfunctions caused by ischemia and certain degenerative environments (Shin et al., 2004; Shen et al., 2014). In addition, these cells have a variety of advantages such as being widely available, they can be expanded and induced to differentiate in vitro, and long-term survival and integration into nerve tissue (Li et al., 2008; Shao et al., 2009). These features of mesenchymal stem cells suggest their use as a new approach to the treatment of spinal cord injury. Previous studies have reported the use of many different stem cells for the treatment of spinal cord injury, including bone marrow mesenchymal stem cells, neural stem cells, adipose stem cells, umbilical cord mesenchymal stem cells, umbilical cord blood mesenchymal stem cells, embryonic stem cells, and placenta-derived mesenchymal stem cells (PDMSCs). Among these cells, allogeneic and xenogeneic bone marrow mesenchymal stem cells have been frequently studied for the treatment of spinal cord injury. However, bone marrow mesenchymal stem cells are not very prevalent, and their number gradually declines as their proliferation and differentiation capacities decrease with age, which limits their clinical application.
Several studies have demonstrated that PDMSCs and bone marrow mesenchymal stem cells have similar functions and features, as well as similar multi-directional differentiation potentials (Miao et al., 2006; Zhang and Yang, 2010), though PDMSCs have a stronger proliferation ability. The increasing evidence from prior work shows that PDMSCs can be induced to differentiate in vitro into mesodermic cardiocytes, smooth muscle cells, osteoblasts, adipocytes, endodermic pancreatic islet cells, liver cells, ectodermic neurons and astrocytes (Alviano et al., 2007; Wolbank et al., 2007; Portmann-Lanz et al., 2010). These results were consistent with in vivo findings in rat models of myocardial infarction and Parkinson’s disease, mouse models of diabetes mellitus, and primate models of spinal cord injury (Ventura et al., 2007; Wang et al., 2010; Li et al., 2014).
The interest in PDMSCs is currently growing. Li et al. (2013) reported that human PDMSCs loaded on the human amniotic membrane were bene fi cial for the treatment of radial nerve injury. Yang et al. (2013) found that human PDMSCs promoted the healing of tendon grafts in the bonetunnel. In the present study, we transplanted neural stem cells differentiated from human PDMSCs into injured spinal cords of rats and observed the recovery of motor and sensory functions, as well as the pathological changes in the injured spinal cord for 3 successive weeks, to assess this as a novel method for the clinical treatment of spinal cord injury.
Materials and Methods
Experimental animals
A total of 72 Sprague-Dawley rats, half male and half female, aged 10 weeks, weighing 240 ± 10 g, were provided by the Liaoning Changsheng Biotechnology Co., Ltd. (Benxi, Liaoning Province, China; license No. SYXK (Liao) 2010-0001). The experimental animals were cared for under the approval of the Animal Ethics Committee of Shenyang Medical College (Shenyang, Liaoning Province, China). All procedures were performed under pentobarbital sodium anesthesia. Every effort was made to minimize the number of animals used, as well as their pain and suffering.
Experimental reagents
The following reagents were purchased: Mesencult III medium (Stemcell Technologies, Vancouver, Canada); human lymphocyte separation buffer (Tianjin Haoyang Biotech Company, Tianjin, China); PE-labeled anti-human CD29 and CD34, and FITC-labeled anti-human CD44 and CD90 monoclonal antibodies (BD Biosciences, San Jose, CA, USA); recombinant human granulocyte colony-stimulating factor (Filgrastim; Amoytop Biotech Co., Ltd., Xiamen, Fujian Province, China); trypsin, bromide-oxyuridine reagent, rabbit anti-mouse bromide-oxyuridine antibody, lymphocyte separation medium, rabbit anti-mouse GFAP antibody, rabbit anti-mouse NSE antibody SABC kit, and the DAB chromogenic kit (Sigma, St. Louis, MO, USA); and brain-derived neurotrophic factor kit (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China).
Placental specimens
Placental specimens were collected from normal full-term cesarean deliveries in the Department of Obstetrics at the Af fi liated Central Hospital of Shenyang Medical College in China. The maternal age was 23–35 years old and maternal health by physical examination was good. The patients were negative for syphilis, HIV, CMV, HBSAg, and HCV, and had no history of infectious diseases or complications during pregnancy. The participants and their families were informed of the experiment and signed informed consent.
PDMSCs isolation and culture
The placental decidual tissue was harvested under sterile conditions, rinsed with PBS, and cut into 1 × 1 × 1 mm3pieces with scissors. Next, the specimens were digested with 10 mL of 1% collagenase IV in a 37°C water bath for 30 minutes, and the digestion was terminated with DMEM. Then, the cells were triturated by pipetting and filtered with 100-µm mesh screen to obtain a cell suspension. The cell suspension was centrifuged at 1,200 r/min for 5 minutes. After aspirating the supernatant, 10 mL of complete culture medium (low-glucose DMEM containing 10% fetal bovine serum and 1% double antibody) was added. Next, the number of cells was counted and the cell density was adjusted to 3 × 108cells/L. The cells were then incubated in a humidi fi ed incubator with 5% CO2at 37°C for 3 days, at which point the culture medium was replenished and the non-adherent cells were removed. From then on, the medium was changed after every 3–4 days. The cells were passaged for subculture after reaching 80–90% confluence. The PDMSCs were cultured in mesenchymal stem cell culture media in our laboratory, and passage 3 PDMSCs were observed under an inverted microscope.
PDMSCs identi fi cation
Passage 3 PDMSCs were digested with 0.25% trypsin for 3 minutes and prepared into a single cell suspension. The PDMSCs at 3 × 108cells/L were incubated with anti-human, CD90, CD73, CD105, CD14, CD34, and CD45 monoclonal antibodies on ice in the dark for 20 minutes, rinsed with PBS twice, and centrifuged for 5 minutes at 4°C and 1,000 r/min. Next, the supernatant was discarded, and 2 mL of cold PBS was added. The cell surface markers were detected by flow cytometry.
PDMSCs differentiation into neural stem cells and transfection
When the passage 3 PDMSCs reached 30% con fl uence, the culture medium was discarded, and DMEM/F12 medium supplemented with condensed gel (1:2,000 dilution) was added. The cells were then incubated in a humidified incubator with 5% CO2at 37°C for 20 minutes, and green fl uorescent protein (GFP)-recombinant lentiviral vector was introduced into the human PDMSCs at various MOI values (0, 1, 5, 25, 50, and 100). The transfected PDMSCs were cultured using standard techniques for 72 hours, and were then microscopically observed. Three high-power fields of view were randomly selected to measure the cell number. The transfection rate was roughly estimated to be 90.3%.
After the PDMSCs were stained with the GFP-lentiviral vector and induced to differentiate into neural stem cells, the neural stem cells were differentiated into neuron-like cells. Brie fl y, a T175 culture fl ask of PDMSCs with 2 × 106and 4 × 105cells were seeded into four T75 flasks. On day 2, the cells were transfected with GFP-lentivirus and the culture medium was changed 8 hours later. Twenty-four hours after the medium change, the cells were transferred to 24-well plates with 2.7 × 105cells per well, neural stem cell induction medium (1.5 mL) was added, and the medium was changed every other day. Three days after the medium change, the cells in the 24-well plates were transferred to T75 fl asks with three wells per fl ask, and 10 mL of neural stem cell induction medium was added. Three days after the transfer, each fl ask was infused with 10 mL of media. Six days after the transfer, the cell suspension was centrifuged and freezing medium (9 parts neural stem cell induction medium to 1 part DMSO) was added. There were 1.1 × 106cells per vial, 100 µL of cell suspension (1.1 × 105) was added to each vial, and they wereincubated with 10 mL of neuron-like cell induction medium overnight and then observed under the microscope.
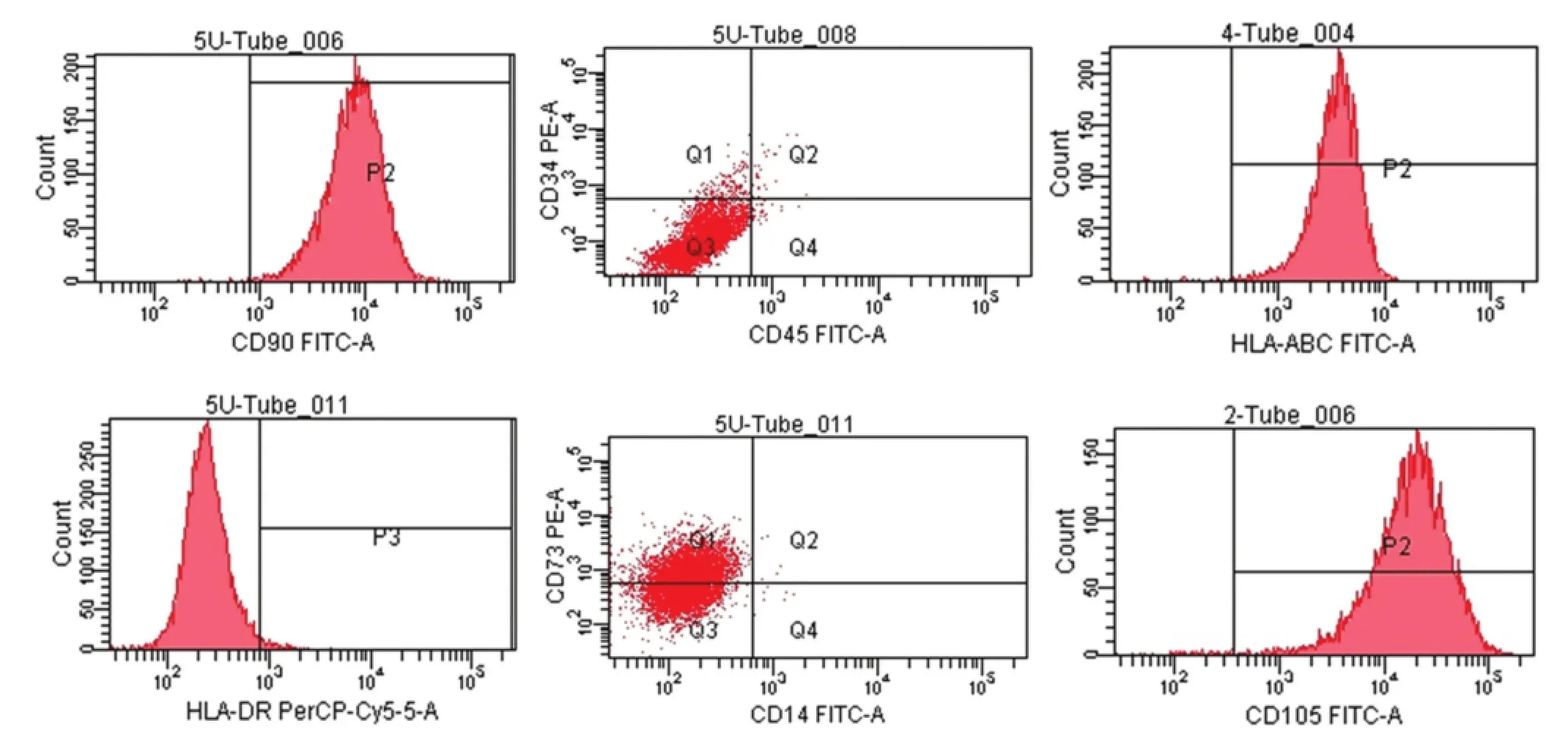
Figure 2 Identi fi cation of human placenta-derived mesenchymal stem cells.
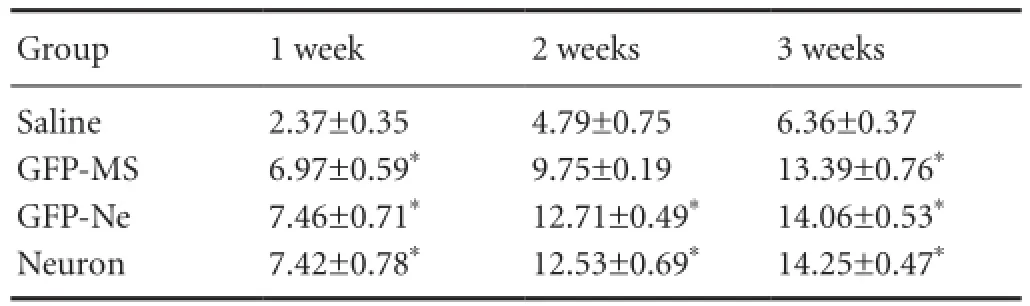
Table 1 Comparison of Basso, Beattie, and Bresnahan scale scores at different time points after injury
Spinal cord injury model and experimental groups
Sprague-Dawley rats were fasted for 12 hours before surgery, weighed, and anaesthetized with 0.01 mL/g of 2% sodium pentobarbital through an intraperitoneal injection. The rats were secured in the prone position on the operating table under anesthesia. After disinfecting the skin, a T10-centered longitudinal incision was made through the back skin and subcutaneous tissue, and the paraspinal muscles were stripped on both sides. The T10spinous process and vertebral plate were exposed and resected to expose the spinal dura mater. Using the NYU impactor method, the spinal cord injury was produced by dropping a 10-g weight from a height of 25 mm. The spinal cord transection injury model was veri fi ed by the appearance of dragging hindlimbs and dorsum. Seventy-two rats were randomly divided into four groups, each containing 18 rats: a saline control group (Saline group), a GFP-labeled PDMSCs transplantation group (GFP-MS group), a GFP-labeled PDMSCs-differentiated neural stem cell transplantation group (GFP-Ne group), and a PDMSCs-differentiated neural stem cells transplantation group (Neuron group). Immediately after creating the spinal cord transection injury, 0.2 mL of cell suspension at 1 × 106cells/mL was injected into the injured spinal cord using a micro syringe, while the saline group was injected with 0.2-mL saline. After the injection, the skin incisions were dressed with a gelatin sponge, sutured, and disinfected with iodine. After resuscitation from anesthesia, the rats were housed in cages.
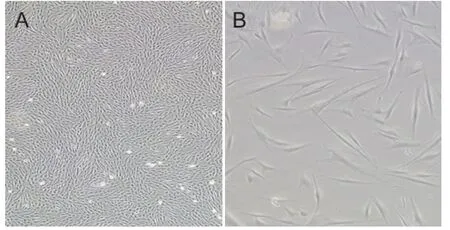
Figure 1 The morphology of passage 3 human placenta-derived mesenchymal stem cells was highly homogeneous.
Outcome measures
Hindlimb functional recovery
The rat hindlimb functions were evaluated using the Basso, Beattie, and Bresnahan (BBB) scale by two investigators who were experienced in BBB rating and were blinded withrespect to the treatment. The evaluation of BBB score was performed at 3 days before the surgery and each week for 3 weeks afterwards.
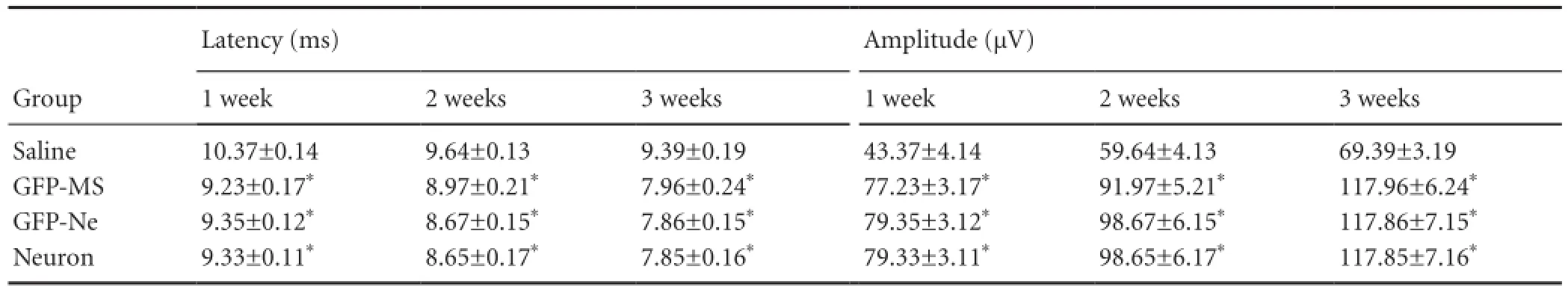
Table 2 Comparison of the somatosensory evoked potential in the rat hindlimbs at different time points after surgery
Table 3 Comparison of the motor evoked potential in the rat hindlimbs at different time points after surgery
Neuroelectrophysiological test
At 1, 2, and 3 weeks after the surgery, the somatosensory evoked potentials and motor evoked potential in the hindlimbs and muscle groups were determined for all rats.
Histopathological detection
At 1, 2, and 3 weeks after the surgery, specimens from six rats in each group were harvested at each time point. The injured spinal cord specimens were stained with hematoxylin and eosin and were microscopically observed.
Statistical analysis
The data were statistically analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). The differences among different specimens were assessed using an analysis of variance with a randomized block experimental design. P-values less than 0.05 were considered to be statistically signi fi cant. The data are presented as the mean ± SD.
Results
Identi fi cation of PDMSCs
By inverted microscopy, the mesenchymal cells showed a typical morphology, with a spindle-type shape and strong refraction (Figure 1). Flow cytometry analysis indicated that the cells were positive for surface markers CD90, CD105, and CD73 and negative for CD34, CD14, and CD45 (Figure 2). These results demonstrate that the extracted and cultured cells were mesenchymal stem cells.
PDMSCs differentiated into neural stem cells and were successfully transfected
The GFP-lentivirus transfected cells are shown inFigure 3A,B. After incubating overnight in neural stem cell induction medium in the 24-well plates, adherent small cell clusters were visible (Figure 3C). After 1 day, single cells remained suspended in solution; after 5 days of culture in the 24-well plates, the cells began to adhere and covered the bottom, but adherent cell clusters were still visible (Figure 3D). Two days after being transferred to the fl asks, cells were found in suspension and in a small number of cell clusters (Figure 3E); after 6 days, the cells in suspension increased in size (Figure 3F); and after neuron-like cell induction medium was added, the cells began to adhere and synapse-like structures formed (Figure 3G).
Behavioral test results
The BBB score of normal rat hindlimbs before spinal cord injury was 23 points. After injury, the rats were observed dragging their hindlimbs and with their foot dorsum touching the ground. The BBB scores gradually increased after the cell transplantation, and reached 13 points after 3 weeks. At 3 weeks after spinal cord injury, the BBB score in all three cell transplantation groups was significantly higher than that in the saline group (P < 0.05). There were no signi fi cant differences among the three cell transplantation groups (P >0.05;Table 1).
Neuroelectrophysiological results
Latency
The latency of the somatosensory evoked potential and motor evoked potential in the rat hindlimbs was increased afterspinal cord transection injury. The evoked potential latencies gradually decreased in rats after cell transplantation. Three weeks after the injury, the evoked potential latencies in the three cell transplantation groups were significantly shorter than that in the saline group (P < 0.05). There were no signi fi cant differences among the three cell transplantation groups (P > 0.05;Tables 2, 3).
Amplitude
The amplitude of the somatosensory evoked potential and motor evoked potential in the rat hindlimbs was decreased after spinal cord transection injury. The evoked potential amplitudes gradually increased in rats after cell transplantation. Three weeks after injury, the evoked potential amplitudes in the three cell transplantation groups were signi fi cantly higher than that in the saline group (P < 0.05). There were no significant differences among the three cell transplantation groups (P > 0.05;Tables 2, 3).
Histopathological changes
After the spinal cord transection injury, the nerve cells were scattered and became necrotic, showing large nuclei (Figure 4A, B). One week after cell transplantation, the stem cells were found distributed throughout the injury site in the GFP-MS group under fluorescence microscopy (Figure 4C, D). A portion of the stem cells had scattered around the injured spinal cord in the neuron group (Figure 4E, F), and the GFP-labeled neural stem cells were aggregated at the nerve stump in the GFP-Ne group (Figure 4G, H). These results suggest that the neural stem cells migrated under chemotaxis.
The histopathological changes in the injured spinal cord at 2 weeks after cell transplantation are shown inFigure 5. In the PDMSC-induced neural stem cell transplantation group (neuron group), the neural stem cells appeared to display chemotaxis towards nerves and blood vessels.
Three weeks after cell transplantation, spinal nerve tissues were found sparsely distributed in the saline group (Figure 6A, B). The GFP-labeled mesenchymal stem cells grew into the spinal cord, and the GFP signal faded in the GFP-MS group, as observed under fluorescence microscope (Figure 6C, D). The spinal nerve cells were arranged tightly in the neuron group and GFP-Ne group, as observed under fl uorescence microscope (Figure 6E–H).
Discussion
With the progression of stem cell research, stem cell transplantation has been suggested as a potential method for restoring injured spinal cord. Various types of stem cells have been applied in different animal models of spinal cord injury, as well as in clinical trials. Theoretically, embryonic stem cells isolated from the inner layer of blastocysts are the ideal cells for transplantation because of the trans-blastoderm differentiation and their pluripotent potential. However, the application of such cells is limited by ethical problems and their teratogenicity or tumorigenicity. Stem cells in the central nervous system display a strong ability to differentiate, but the number of stem cells declines during the process of neural development and sub-cultured cells are prone to aging, limiting their clinical application. Inducing pluripotent stem cells requires specialized skills and not very many cells can be obtained currently. Schwann cells and olfactory ensheathing cells are not suitable for clinical application because of their limited ampli fi cation potential, although their effects after cell transplantation are good. Adipose stem cells and bone marrow stem cells exist in limited numbers and are dif fi cult to obtain. The umbilical cord is too small to harvest enough umbilical cord-derived mesenchymal stem cells for use. The placenta, however, is readily available, contains a large number of cells that can be isolated, is relatively low cost, and does not present ethical issues (Parolini et al., 2008; Kadam et al., 2010; Lu et al., 2011). PDMSCs have a strong ability to proliferate and a low immunogenicity, which contribute to their strengths as a novel seed cell for the treatment of spinal cord injury.
In the present study, the obtained PDMSCs were positive for CD90, CD44, and CD105 surface markers, and did not express CD34 or CD45, as detected by fl ow cytometry analysis. These results showed that the cultured cells displayed stem cell surface markers indicating that they were in fact PDMSCs. Prior experiments found that human PDMSCs can differentiate into chondrocytes (Li et al., 2014). The results presented here demonstrate that PDMSCs can be induced to differentiate into neural stem cells, and further induced into neuron-like cells, forming synapse-like structures. Additional studies are needed to determine whether these cells have similar functions as nerve cells.
Previous studies showed that human PDMSCs are pluripotent. Therefore, we transplanted the PDMSC-induced neural stem cells into a rat model of spinal cord injury. The results showed that after transplantation of the PDMSCs or PDSMC-induced neural stem cells, the motor functions of the rats with spinal cord injury were signi fi cantly improved. The BBB score after injury of 2 points increased to 13 points at 3 weeks after cell transplantation, whereas the BBB score in the saline control group was only 6 points at that time. This evidence indicated that transplantation of PDMSCs or PDMSC-induced neural stem cells can improve the motor dysfunction in the rat model of spinal cord injury. In addition, neuroelectrophysiological tests revealed that the latencies of the somatosensory evoked potentials and motor evoked potentials were decreased, and the amplitudes were increased, in the rat model of spinal cord injury at 3 weeks after cell transplantation. This evidence indicated that transplantation of PDMSCs or PDMSC-induced neural stem cells improved the hindlimb sensory and motor dysfunctions caused by the spinal cord injury, which is consistent with the BBB scores.
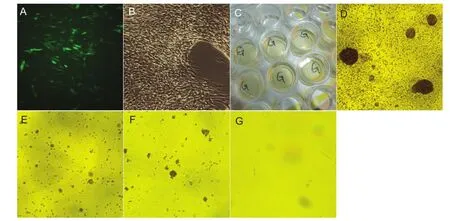
Figure 3 Human placenta-derived mesenchymal stem cells were differentiated into neural stem cells and then transfected.
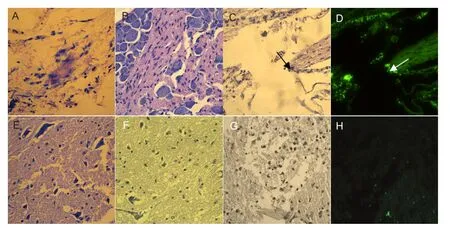
Figure 4 Histopathological changes in the injured rspinal cord at 1 week after cell transplantation (hematoxylin-eosin staining, × 40).
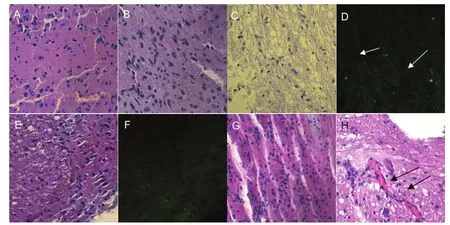
Figure 5 Histopathological changes in the injured spinal cord at 2 weeks after cell transplantation (hematoxylin-eosin staining, × 40).
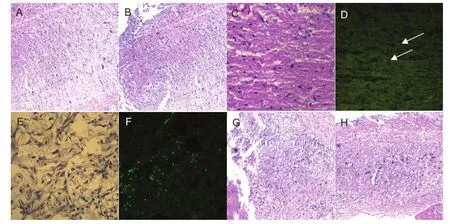
Figure 6 Histopathological changes in the injured spinal cord at 3 weeks after cell transplantation (hematoxylin-eosin staining, × 40).
In the present study, the histopathological staining showed that the transplanted PDMSCs and PDMSC-induced neural stem cells restored the injured spinal cord and did not cause rejection. One and two weeks after cell transplantation, the GFP-labeled human PDMSCs were scattered throughout the spinal cord injury site in the GFP-MS group, as observed under fluorescence microscopy. One week after cell transplantation, the GFP-labeled neural stem cells had aggregated around the nerve stumps and blood vessels in the GFPNe group, suggesting that the neural stem cells displayed chemotaxis. However, the BBB score in the GFP-Ne group was higher, the evoked potential latencies were shorter, and the amplitude was higher than those of the GFP-MS group,though these differences were not signi fi cant. This evidence suggested that transplantation of the PDMSC-induced neural stem cells may have achieved better results during the early transplantation period. Three weeks after cell transplantation, the GFP-Ne group had no advantage in BBB score or evoked potentials, and the repair of the spinal cord injury in the two groups were similar, with spinal nerve cells arranged tightly by histopathology. The mechanisms underlying this repair need further study. GFP-lentiviral vectors are commonly used to label stem cells. A large number of studies have demonstrated that GFP labeling does not affect the proliferation or differentiation of stem cells (Alimperti et al., 2012; Grinev et al., 2012; Nakamura et al., 2012; Chen et al., 2013), which is also supported by our fi ndings reported here.
In summary, human PDMSCs were successfully isolated and cultured in this study. These cells showed pluripotent differentiation ability and can be induced to differentiate into neural stem cells, which help to repair spinal cord injuries without causing rejection after transplantation. Human PDMSCs may be used as seed cells in a stem cell bank, providing a novel seed cell for clinical cell transplantation to treat spinal cord injury.
Author contributions:Li Z and Yang LF designed the study and were responsible for the study. Li Z and Jia JQ implemented the study. Zhao W, Liu W and Zhou Y evaluated the study. Li Z drafted the manuscript. Yang LF revised the manuscript. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Alimperti S, Lei P, Tian J, Andreadis ST (2012) A novel lentivirus for quantitative assessment of gene knockdown in stem cell differentiation. Gene Ther 19:1123-1132.
Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, Lanzoni G, Cantoni S, Cavallini C, Bianchi F, Tazzari PL, Pasquinelli G, Foroni L, Ventura C, Grossi A, Bagnara GP (2007) Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol 7:11.
Chen CH, Yu FY (2013) Lentiviral vectors containing enhanced-green fl uorescent protein gene labeling rabbit synovial mesenchymal stem cells. Zhonghua Linchuang Yishi Zazhi: Dianzi Ban 7:11476-11480.
Grinev VV, Severin IN, Posrednik DV, Kosmacheva SM, Potapnev MP (2012) Highly ef fi cient transfer and stable expression of two genes upon lentivirus transduction of mesenchymal stem cells from human bone marrow. Genetika 48:389-400.
Kadam S, Muthyala S, Nair P, Bhonde R (2010) Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud 7:168-182.
Li RP, Ji FQ, Sun HM, Wang DN, Zeng XB, Zhao CL, Yang H (2008) The function of amnion in the differentiation of human umbilical cord blood cells into dopaminergic neurons. Jiepou Xuebao 39:790-794.
Li Z, Qin H, Feng Z, Liu W, Zhou Y, Yang L, Zhao W, Li Y (2013) Human umbilical cord mesenchymal stem cell-loaded amniotic membrane for the repair of radial nerve injury. Neural Regen Res 8:3441-3448.
Li Z, Zhao W, Liu W, Zhou Y, Jia JQ, Yang LF (2014) Chondrogenic differentiation of placenta-derived mesenchymal stem cells in vitro. Zhongguo Zuzhi Gongcheng Yanjiu 18:5203-5208.
Lu GH, Zhang SZ, Chen Q, Wang XF, Lu FF, Liu J, Li M, Li ZY (2011) Isolation and multipotent differentiation of human decidua basalis-derived mesenchymal stem cells. Nanfang Yike Daxue Xuebao 31262-31265.
Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X (2006) Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol Int 30:681-687.
Nakamura T, Sekiya I, Muneta T, Hatsushika D, Horie M, Tsuji K, Kawarasaki T, Watanabe A, Hishikawa S, Fujimoto Y, Tanaka H, Kobayashi E (2012) Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy 14:327-328.
Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, et al (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the fi rst international Workshop on Placenta Derived Stem Cells. Stem Cells 26:300-311.
Patel DM, Shah J, Srivastava AS (2013) Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int 2013:496218.
Portmann-Lanz CB, Schoeberlein A, Portmann R, Mohr S, Rollini P, Sager R, Surbek DV (2010) Turning placenta into brain: placental mesenchymal stem cells differentiate into neurons and oligodendrocytes. Am J Obstet Gynecol 202:294.e1-294.e11.
Satija NK, Singh VK, Verma YK, Gupta P, Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP, Gurudutta GU (2009) Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med 13:4385-4402.
Shao M, Sun G, An HC (2009) Differentiation of in vitro cultured bone marrow mesenchymal stem cells into neurocytes and differential expression of protein. Zhongguo Zuzhi Gongcheng Yanjiu Yu Linchuang Kangfu 13:197-200.
Shen J, Nair A, Saxena R, Zhang CC, Borrelli J Jr, Tang L (2014) Tissue engineering bone using autologous progenitor cells in the peritoneum. PLoS One 9:e93514.
Shin M, Yoshimoto H, Vacanti JP (2004) In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng 10:33-41.
Syková E, Jendelová P, Urdzíková L, Lesný P, Hejcl A (2006) Bone marrow stem cells and polymer hydrogels--two strategies for spinal cord injury repair. Cell Mol Neurobiol 26:1113-1129.
Ventura C, Cantoni S, Bianchi F, Lionetti V, Cavallini C, Scarlata I, Foroni L, Maioli M, Bonsi L, Alviano F, Fossati V, Bagnara GP, Pasquinelli G, Recchia FA, Perbellini A (2007) Hyaluronan mixed esters of butyric and retinoic acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J Biol Chem 282:14243-14252.
Wang K, Xu JM, Hua YM, Wang JL (2011) Salidroside induces human placenta mesenchymal stem cells to differentiate towards hepatocytes like cell in vitro. Jiangsu Yiyao 37:1765-1767.
Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, Redl H, Gabriel C (2007) Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng 13:1173-1183.
Yang LF, Liu W, Zhou Y, Feng ZS, Zhang L, Zhao W, Li Z (2013). Human placenta-derived mesenchymal stem cell transplantation promotes tendon graft healing in a bone tunnel. Zhongguo Zuzhi Gongcheng Yanjiu 17:8539-8544.
Zhang HY, Yang NL (2010) Biological characteristics of placenta-derived mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu 14:7535-7538.
Copyedited by McCarty W, Norman C, Zhang N, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.147953
Lifeng Yang, Department of Orthopedics, Affiliated Central Hospital of Shenyang Medical College, Shenyang 110024, Liaoning Province, China, lz8534@sina.com.
http://www.nrronline.org/
Accepted: 2014-11-09
- 中国神经再生研究(英文版)的其它文章
- Hydrogen sul fi de controls peripheral nerve degeneration and regeneration: a novel therapeutic strategy for peripheral demyelinating disorders or nerve degenerative diseases
- Activities of nicotinic acetylcholine receptors modulate neurotransmission and synaptic architecture
- A novel arti fi cial nerve graft for repairing longdistance sciatic nerve defects: a self-assembling peptide nano fi ber scaffold-containing poly(lactic-co-glycolic acid) conduit
- The effects of claudin 14 during early Wallerian degeneration after sciatic nerve injury
- Transplantation of human amniotic epithelial cells repairs brachial plexus injury: pathological and biomechanical analyses
- Long-term treatment with PP2 after spinal cord injury resulted in functional locomotor recovery and increased spared tissue

