Mild hypothermia combined with a scaffold of NgR-silenced neural stem cells/Schwann cells to treat spinal cord injury
Dong Wang, Jinhua Liang, Jianjun Zhang, Shuhong Liu, Wenwen Sun
1 Department of Neurosurgery, the Fourth Center Clinical College of Tianjin Medical University, Tianjin Fourth Central Hospital, Tianjin, China
2 Department of Clinical Detection, Hongqi Hospital of Mudanjiang Medical College, Mudanjiang, Heilongjiang Province, China
3 Department of Epidemiology, Logistics University of People’s Armed Police Force, Tianjin, China
Mild hypothermia combined with a scaffold of NgR-silenced neural stem cells/Schwann cells to treat spinal cord injury
Dong Wang1, Jinhua Liang2, Jianjun Zhang1, Shuhong Liu3, Wenwen Sun1
1 Department of Neurosurgery, the Fourth Center Clinical College of Tianjin Medical University, Tianjin Fourth Central Hospital, Tianjin, China
2 Department of Clinical Detection, Hongqi Hospital of Mudanjiang Medical College, Mudanjiang, Heilongjiang Province, China
3 Department of Epidemiology, Logistics University of People’s Armed Police Force, Tianjin, China
Because the inhibition of Nogo proteins can promote neurite growth and nerve cell differentiation, a cell-scaffold complex seeded with Nogo receptor (NgR)-silenced neural stem cells and Schwann cells may be able to improve the microenvironment for spinal cord injury repair. Previous studies have found that mild hypothermia helps to attenuate secondary damage in the spinal cord and exerts a neuroprotective effect. Here, we constructed a cell-scaffold complex consisting of a poly(D,L-lactide-co-glycolic acid) (PLGA) scaffold seeded with NgR-silenced neural stem cells and Schwann cells, and determined the effects of mild hypothermia combined with the cell-scaffold complexes on the spinal cord hemi-transection injury in the T9segment in rats. Compared with the PLGA group and the NgR-silencing cells + PLGA group, hindlimb motor function and nerve electrophysiological function were clearly improved, pathological changes in the injured spinal cord were attenuated, and the number of surviving cells and nerve fi bers were increased in the group treated with the NgR-silenced cell scaffold + mild hypothermia at 34°C for 6 hours. Furthermore, fewer pathological changes to the injured spinal cord and more surviving cells and nerve fi bers were found after mild hypothermia therapy than in injuries not treated with mild hypothermia. These experimental results indicate that mild hypothermia combined with NgR gene-silenced cells in a PLGA scaffold may be an effective therapy for treating spinal cord injury.
nerve regeneration; spinal cord injury; neural stem cells; Schwann cells; mild hypothermia; cell scaffold; poly(D,L-lactide-co-glycolic acid); neurological function; neural regeneration
Funding: This study was supported by a grant from the Application Basis and Front Technology Projects of Tianjin (Science and Technology Foundation of Tianjin), No. 12JCYBJC18000.
Wang D, Liang JH, Zhang JJ, Liu SH, Sun WW. Mild hypothermia combined with a scaffold of NgR-silenced neural stem cells/Schwann cells to treat spinal cord injury. Neural Regen Res. 2014;9(24):2189-2196.
Introduction
Spinal cord injury refers to damage to the spine that results in the complete or partial loss of sensory and/or motor function and affects the quality of life of injured patients. Nerve regeneration and restoration play crucial roles in improving the prognosis and quality of life of patients after spinal cord injury (Kobbe et al., 2009; Ok et al., 2012; Zhou et al., 2014). However, the best method to achieve a physiological recovery of the structure and function of the damaged nerves remains a challenging problem in the fi eld of neurosurgery (Pallini et al., 2005; Meletis et al., 2008; Kobbe et al., 2009; Ok et al., 2012; Batchelor et al., 2013). Recently, stem cell therapy has shown considerable therapeutic potential for treating spinal cord injury. Schwann cells (SCs) are important support cells that surround the nerve cells and play a key role in neuronal regeneration. SCs can secrete various neurotrophic factors, promote neuronal survival and differentiation, and support and guide various nervous processes. The transplantation of SCs was found to promote functional recovery after spinal cord injury, suggesting a new approach to the treatment of spinal cord injury (Nout et al., 2012). When the central nervous system is insulted, the gene expression and protein content of the Nogo protein are signi fi cantly increased, with expression of the Nogo receptor (NgR) increased accordingly, ultimately leading to growth cone collapse and inhibition of neurite extension (Xu et al., 2011; Yan et al., 2011; Antonic et al., 2013; Kim et al., 2013; Bazley et al., 2014). NgR gene silencing using transfection of small interfering RNA (siRNA) can be used to block the inhibitory effect of the Nogo protein, thereby promoting neurite growth after nerve cell differentiation. Recently, mild hypothermia (33–35°C) therapy has been suggested as a potential therapeutic regimenfor brain and spinal cord injuries (Ok et al., 2012). Growing evidence from clinical studies has shown that mild hypothermia effectively reduces the secondary damage to the brain and spinal cord and protects the central nerve from the tissue damage (Batchelor et al., 2013). In the present study, we transplanted a cell-scaffold complex consisting of a poly(D,L-lactide-co-glycolic acid) (PLGA) scaffold seeded with NgR-silenced neural stem cells (NSCs) and SCs into an injured spinal cord in rats to determine the effects of mild hypothermia combined with the NgR-silenced cell scaffolds for the treatment of spinal cord injury.
Materials and Methods
Animals
Ninety-six healthy, adult, female Wistar rats, weighing 250–300 g, were acquired from the Laboratory Animals Room, Chinese Academy of Medical Sciences, China (certi fi cation No. SCXK (Jing) 2006-0008). The animals were housed at a room temperature of 18–26°C and relative humidity of 40–70%. The protocol used in this study was approved by the Scientific Review Committee and the Institutional Review Board of Tianjin Medical University in China. The rats were randomly divided into four groups, each containing 24 rats: the simple PLGA scaffold group, the NSCs + SCs + PLGA group, the NgR-silenced cells (NSCs + SCs) + PLGA group, and the mild hypothermia + NgR-silenced cells (NSCs + SCs) + PLGA group.
Cell culture and NgR silencing
The NSCs and SCs (provided by the First Central Hospital of Tianjin, Tianjin, China) were separated, purified, identi fi ed, and ampli fi ed (Papastefanaki et al., 2007). Next, the obtained cells were transfected with siRNA to silence NgR gene expression (Wang et al., 2010). The expression levels of NgR before and after transfection were determined with reverse transcription (RT)-PCR to verify the ef fi cacy of NgR gene silencing. The total RNA was extracted from cells in each group using the TRIzol method (Takara, Dalian, Liaoning Province, China), and the RNA content was assessed by UV spectrophotometry (Shanghai Haishen Medical Electronic Machine Co., Ltd., Shanghai, China). A volume of 5 µL of total RNA was synthesized into cDNA using the M-MLV reverse transcriptase, and then the 5 µL of reverse transcription product was ampli fi ed by PCR with an NgR upstream primer sequence of 5′-CTG CTG GCA TGG GTG TTA TGG-3′ and downstream primer sequence of 5′-TCT GGC TGG AGG CTG GGA T-3′. The product size was 151 bp. The PCR reaction included 35 cycles of denaturation at 94°C for 1 minute, annealing at 61°C for 45 seconds, and extension at 72°C for 1 minute, with a final extension at 72°C for 7 minutes. Each PCR reaction was replicated three times. The PCR products were separated by electrophoresis on a 2% agarose gel, and the size and brightness of the ampli fi cation products were observed with a UV transmission reflectometer (Shanghai Haishen Medical Electronic Machine Co., Ltd., Shanghai, China). The ampli fi cation bands were analyzed using a gel analysis system, and the gene expression level was calculated as the average optical density value of each band. The ratio of the optical density of each ampli fi cation band to the optical density of the β-actin band was used as the relative mRNA expression level of the ampli fi ed products.
Preparation of the cell-scaffold complex
PLGA (85:15) (Ji’nan New-technology Company, Ji’nan, Shandong Province, China) was modified by treatment with ammonia plasma, and then the block PLGA was cut into 2.5 mm × 1.5 mm × 1.0 mm specimens, vacuum dried, and sterilized by ethylene oxide fumigation. For the simple PLGA scaffold group, 10 µL of L-DMEM medium (Gibco BRL, Gaithersburg, MD, USA) was pipetted onto the PLGA scaffolds. For the NSCs + SCs + PLGA group, 10 µL of NSCs and SCs at a density of 2 × 1010cells/L were seeded onto the PLGA scaffolds. For the NgR-silenced cells + PLGA group and mild hypothermia + NgR-silenced cells + PLGA groups, 10 µL of the NgR-silenced NSCs and SCs at a density of 2 × 1010cells/L were seeded onto the PLGA scaffolds. All cells were incubated at 37°C and 5% CO2in a humidi fi ed incubator. After 4 days of culture, the cell adhesion, proliferation, and differentiation were assessed. The adherent cells were used for the transplantation.
Establishment of the acute spinal cord injury animal model and mild hypothermia intervention
Wistar rats in each group were anesthetized with an intraperitoneal injection of 2.5% ketamine (20 mg/kg) and secured in the prone position. To harvest the T9spinous process, a 2–3 cm-long midline incision was made along the skin and subcutaneous tissue of the back, stripping and retracting the paraspinal muscles. The T8and T9spinous processes and lamina were exposed and clamped with a laminectomy forceps to expose and cut the dura mater. The right side of the spinal cord was hemi-resected. Next, hindlimb paralysis after the injury was veri fi ed using previously published criteria (Pearse et al., 2007). Finally, the wound was sutured closed. In the NgR-silenced cells + PLGA group and the NSCs + SCs + PLGA group, the injured spinal tissue was exposed 6 hours after injury and transplanted with the corresponding cell-PLGA complexes. In the simple PLGA scaffold group, the injured spinal tissue was exposed 6 hours after injury and transplanted with the PLGA/DMEM culture medium. The incisions were then sutured closed. All rats were treated with daily abdominal massages, twice artificial urinations, and paraplegia care after transplantation. The rectal temperature in the rats was continuously monitored using HP-V26 temperature measuring instruments (HP-V26, HP, Palo Alto, CA, USA). In the mild hypothermia + NgR-silenced cells + PLGA group, the rats were placed on a cold blanket to maintain their rectal temperatures at 34 ± 0.5°C for 6 hours after cell trans-plantation.
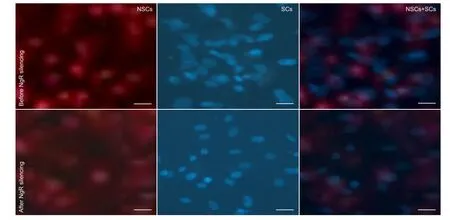
Figure 2 Adhesion and growth of neural stem cells (NSCs) and Schwann cells (SCs) before and after NgR silencing at 4 days after culture ( fl uorescence microscopy, scale bars: 0.5 μm).
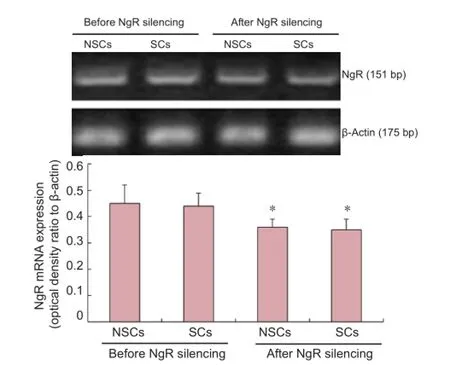
Figure 1 NgR silencing by siRNA transfection in neural stem cells (NSCs) and Schwann cells (SCs).
Evaluation of lower limb motor function
All rats were assessed using an inclined plate test and to determine the modi fi ed Tarlov score for lower extremity motor function before injury and at 1, 2, 4, 6, and 8 weeks after injury. For the inclined plate tests, the rats were placed in a modi fi ed Rivilin inclined plate, with the heads to the right.
The angle of the incline was gradually increased from the horizontal position (0°), and the maximum angle at which the rats could remain on the plate for 5 seconds without falling was recorded. The fi nal results were obtained by averaging the maximum angle from three measurements (Xiao et al., 2012). The Basso, Beattie, and Bresnahan (BBB) scale was used to evaluate motor function (Pallini et al., 2005; Meletis et al., 2008). The rats in each group were graded by blinded observers at 1, 2, 4, 6, and 8 weeks after injury, the motor function was assessed six times with the BBB scale, and the mean score at each time point was calculated.
Hematoxylin-eosin and immuno fl uorescence staining
Six rats in each group were randomly selected at 4 weeks after injury and euthanized under anesthesia. The injured T9spinal tissue was harvested and prepared for histological assessment of the degree of recovery. The spinal tissues were fi xed in 40 g/L paraformaldehyde, frozen, and cut into 5-µm-thick sections for hematoxylin-eosin and immuno fl uorescence staining. The sections were stained with hematoxylin and then immersed in acid followed by ammonia. The sections were then washed with running water, immersed in distilled water, dehydrated through a graded alcohol series, stained with eosin, and then observed under a light microscope. Other sections were stained with PKH-26 (Sigma, St. Louis, MO, USA) to detect the presence and distribution of NSCs after transplantation. The number of PKH-26-positive cells in each section was calculated from ten random fi elds of view at high magni fi cation (200×) (Olympus, Tokyo, Japan), and averaged for each group.
Transmission electron microscope observation
Two rats in each group were randomly selected at 8 weeks after surgery and perfused with 2.5% glutaraldehyde. Sections of the injured spinal tissue were further fixed with glutaraldehyde overnight. Two 1 mm spinal segments were harvested from the proximal and distal ends of the left hemisphere. The obtained segments were fi xed with osmium tetroxide at 4°C for 2 hours, rinsed, dehydrated with a gradient of acetone, stained with uranyl acetate at 4°C for 4 hours, embedded in 618 epoxy, and observed under a transmission electron microscope (Shanghai Yanyou Instrument Co., Ltd., Shanghai, China).
Electrophysiology detection
Six rats in each group were randomly selected at 8 weeks after surgery, and their somatosensory evoked potentials and motor evoked potentials were detected using the KEYPOINT 4 method (Meletis et al., 2008). The rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate and placed on a horizontal plane. Stimulating electrodes (Shanghai Yanyou Instrument Co., Ltd., Shanghai, China) were placed on the hindlimbs, while recording electrodes were used to monitor the hindlimb cortical sensory area (Hiraizumi et al., 1996), which is at the intersection between the coronal suture and the sagittal suture healing line. A reference electrode was placed 0.5 cm posterior to the recording electrodes. A direct-current squarewave electrical stimulation was applied to the rat hindlimb at 5–15 mA current, 0.2 ms width, and 3 Hz frequency, which was superimposed 50–60 times. The somatosensory evoked potential latency and amplitude were recorded to determine the nerve electrophysiological recovery. For the motor evoked potentials test, the rats were first anesthetized, and then stimulating electrodes were inserted into the cerebral motor cortex 2 mm anterior to the coronal suture and 2 mm lateral to the sagittal suture. A stimulus with an intensity of 40 mA, pulse width of 0.1 ms, and stimulation frequency of 1 Hz was superimposed 300–500 times at a scanning speed of 5 ms/div and sensitivity of 5 µV/div. The motor evoked potentials latency and amplitude were observed and recorded.
Statistical analysis
The statistical analysis was performed by the second author using SPSS 17.0 software (SPSS, Chicago, IL, USA). The data are expressed as the mean ± SD. Repeated measures analysis of variance and Student-Newman-Keuls tests were performed. P-values less than 0.05 were considered to be statistically signi fi cant.
Results
NgR expression in the NgR-silenced NSCs and SCsviasiRNA transfection
RT-PCR detection showed that, after the NgR gene was silenced by siRNA transfection for 48 hours, the expression of the NgR gene in both NSCs and SCs was significantly down-regulated compared with the corresponding control groups (P < 0.05;Figure 1).
NSCs and SCs seeded in the cell-scaffold complexes
After 4 days of cell culture, a large number of NSCs and SCs had adhered to the PLGA scaffold, as observed by fluorescence microscopy. The cells grew along the PLGA scaffold, and only a very small number of randomly arranged cells were found outside of the complexes. Compared with the non-transfected group, more cells were adhered and growing after NgR gene silencing (Figure 2).
Mild hypothermia combined with transplantation of the NgR-silenced cell scaffold improved hindlimb motor function in rats after spinal cord injury
The inclined plate test angle and modified BBB scores of rats in the NSCs + SCs + PLGA group, NgR-silenced cells + PLGA group, and mild hypothermia + NgR-silenced cells + PLGA group were signi fi cantly higher than those in the simple PLGA scaffold group (P < 0.05 or P < 0.01). The motor function of rats in the NgR-silenced cells + PLGA group and mild hypothermia + NgR-silenced cells + PLGA group was better than that in the NSCs + SCs + PLGA group (P < 0.05;Figure 3).
Mild hypothermia combined with transplantation of the NgR-silenced cell scaffolds improved the nerve electrophysiological function in rats after spinal cord injury
The somatosensory evoked potentials and motor evoked potentials waveforms disappeared completely in each group after the spinal cord injury. At 8 weeks after transplantation, the lag phase of the somatosensory evoked potentials and motor evoked potentials was shortened and the amplitude was increased in the NSCs + SCs + PLGA group, NgR-silenced cells + PLGA group, and mild hypothermia + NgR-silenced cells + PLGA group (P < 0.05 or P < 0.01) compared with the simple PLGA scaffold group. Furthermore, the NgR-silenced cells + PLGA group and mild hypothermia + NgR-silenced cells + PLGA group had shorter lag phases and higher amplitudes of somatosensory evoked potentials and motor evoked potentials than the NSCs + SCs + PLGA group (P < 0.05). The lag phase and amplitude of the somatosensory evoked potentials and motor evoked potentials were not different between the NgR-silenced cells + PLGA group and the mild hypothermia + NgR-silenced cells + PLGA group (P > 0.05;Figure 4).
Mild hypothermia combined with transplantation of the NgR-silenced cell scaffolds improved the pathological changes found in rats after spinal cord injury
Spinal cord tissue rupture was visible by hematoxylin-eosin staining in the simple PLGA scaffold group, as well as scar connection, structural disorder, and apparent porosis. In theNSCs + SCs + PLGA group and NgR-silenced cells + PLGA group, normal nerve cell-like morphological changes were observed, along with tissue porosis. Standard nerve cell-like morphological changes were also found in the mild hypothermia + NgR-silenced cells + PLGA group, but the porosis was absent (Figure 5).
Immunofluorescence staining showed that the number of PKH-26-positive cells (surviving cells) in the spinal cord sections was highest in the mild hypothermia + NgR-silenced cells + PLGA group, next highest in the NgR-silenced cells + PLGA group, lower in the NSCs + SCs + PLGA group, and lowest in the simple PLGA scaffold group (P < 0.05;Figure 5).
Under transmission electron microscopy, glial scars were found in the simple PLGA scaffold group, and macrophages had phagocytized the degenerated and necrotic myelinated nerve fibers. In the mild hypothermia + NgR-silenced cells + PLGA group, a large number of myelinated nerve fibers and non-myelinated nerve fi bers, as well as many axons, were found. In addition, the sheath myelin was intact in the regenerated axons. In the NSCs + SCs + PLGA group and NgR-silenced cells + PLGA group, the number of myelinated nerve fibers and non-myelinated nerve fibers was higher than in the simple PLGA scaffold group, but lower than in the mild hypothermia + NgR-silenced cells + PLGA group (Figure 5).
Discussion
Injured spinal cord nerve cannot regenerate or repair, which is a major problem in the medical field. The accumulating evidence has highlighted the changes in the microenvironment after spinal cord injury and the effects of mild hypothermia, as well as nerve cell scaffolds, for the repair of spinal cord injury and nerve cell regeneration (Teng et al., 2002; Du et al., 2014; Peterson and Anderson, 2014).
Transplanted NSCs can differentiate into astrocytes because of a lack of external induction factors, leading to the formation of a large number of glial scars and a reduction in the number of differentiated neurons. In addition, axon growth within the neurons is dif fi cult to control (Ban et al., 2009; Peterson and Anderson, 2014). Therefore, biological scaffolds that better mimic the normal microenvironment have been developed applied in scienti fi c research (Teng et al., 2002; Saberi et al., 2008; Ban et al., 2009; Chi et al., 2010; Choi et al., 2012; Batchelor et al., 2013). In the present study, we used PLGA, a tissue engineering scaffold, for nerve repair. The PLGA copolymers provide the three-dimensional environment that is required for the growth of nerve cells, thereby promoting nerve regeneration. In addition, the cell axons are able to closely adhere to the PLGA scaffold. The ideal tissue engineering scaffold material should be easily inoculated with in vitro cultured and expanded SCs to facilitate the formation of a peripheral nerve bridging graft with NSCs (Saberi et al., 2008; Chi et al., 2010; Peng et al., 2010), which can then migrate from the graft towards the damaged area, and be able to guide the differentiation of NSCs (Ban et al., 2009; Xu et al., 2011; Karimi-Abdolrezaee and Eftekharpour, 2012; Akbary and Arora, 2014).
Several previous studies (Ban et al., 2009; Antonic et al., 2013; Batchelor et al., 2013) have reported that the important aspects of the microenvironment after spinal cord injury include the nerve cells and their genes, proteins, mitochondria, and expression of cytokines, and changes in the local environment are accompanied by changes in the regeneration capacity of injured nerve axons. Treatment with mild hypothermia was reported to effectively reduce secondary nerve damage and prevent severe brain trauma and spinal cord injury (Young, 2002; Pallini et al., 2005; Dididze et al., 2013; Hou et al., 2013; Zaminy et al., 2013; Zhang and He, 2014), protect the mitochondrial function of nerve cells, enhance the expression of nerve cell nutrition factors, and improve the microcirculation in the areas of spinal cord damage. By altering the microenvironment of the spinal nerve cells, mild hypothermia promotes the survival, division, proliferation, differentiation, and migration of nerve cells; accelerates the recovery of spinal cord conduction function; and prolongs the proliferation and survival of NSCs and SCs. Additionally, hypothermia contributes to vascular proliferation, increases the microcirculation to the spinal nerve tissue, and increase the long-term survival of transplanted cells (Young, 2002; Pallini et al., 2005; Lepore et al., 2006; Saberi et al., 2008; Ok et al., 2012; Hou et al., 2013; Zhang and He, 2014).
In the present study, we transplanted cell-scaffold complexes into the spinal cord of rats and treatment them with mild hypothermia to determine the effects of the treatment on spinal cord injury. The results of this study showed that transplantation of gene-modi fi ed cell-scaffold complexes and treatment with mild hypothermia significantly improved the repair of spinal cord injury compared with treatment with a simple cell-scaffold complex, both in terms of the histology and nerve functions. The hematoxylin-eosin staining of the spinal cord sections showed normal nerve cell-like morphological changes in the mild hypothermia + NgR-silenced cells + PLGA group, and the cavity created by the injury was absent. In addition, the tissue restoration overall was better than in the simple PLGA scaffold group, the NSCs + SCs + PLGA group, and the NgR-silenced cells + PLGA group. The immuno fl uorescence staining of the spinal cord tissue showed that the number of PKH-26-positive cells was higher in the mild hypothermia + NgR-silenced cells + PLGA group than that in the simple PLGA scaffold group, NSCs + SCs + PLGA group, and NgR -silenced cells + PLGA group. This result suggests that the transplanted cells survived better in the mild hypothermia + NgR-silenced cells + PLGA group. By transmission electron microscopy, a large number of myelinated nerve fi bers and non-myelinated nerve fibers were found in the mild hypothermia + NgR-silenced cells + PLGA group, and the number of axons was significantly higher than that in the simple PLGA scaffold group, NSCs + SCs + PLGA group, and NgR-silenced cells + PLGA group. This result indicatesthat the transplanted cells were able to repair the spinal nerve fi bers in the mild hypothermia + NgR-silenced cells + PLGA group. The BBB scores and inclined plate test angle results showed that the rats in the mild hypothermia + NgR-silenced cells + PLGA group performed better than the animals in the simple PLGA scaffold group, NSCs + SCs + PLGA group, and NgR-silenced cells + PLGA group at 4 weeks after injury, with significant differences among the groups. The somatosensory evoked potentials and motor evoked potentials in the mild hypothermia group showed the most significant recovery at 8 weeks after injury, and the increase in amplitude was more apparent than that in the other groups. The differences between groups were statistically signi fi cant. Together, these results suggest that mild hypothermia facilitates the development of electrical signal conduction time from the hindlimbs to the scalp, and that conduction pathway was smoother and better recovered.
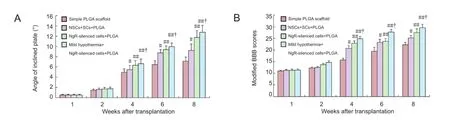
Figure 3 Effect of mild hypothermia combined with transplantation of the NgR-silenced cell scaffold on hindlimb motor function in rats after spinal cord injury.
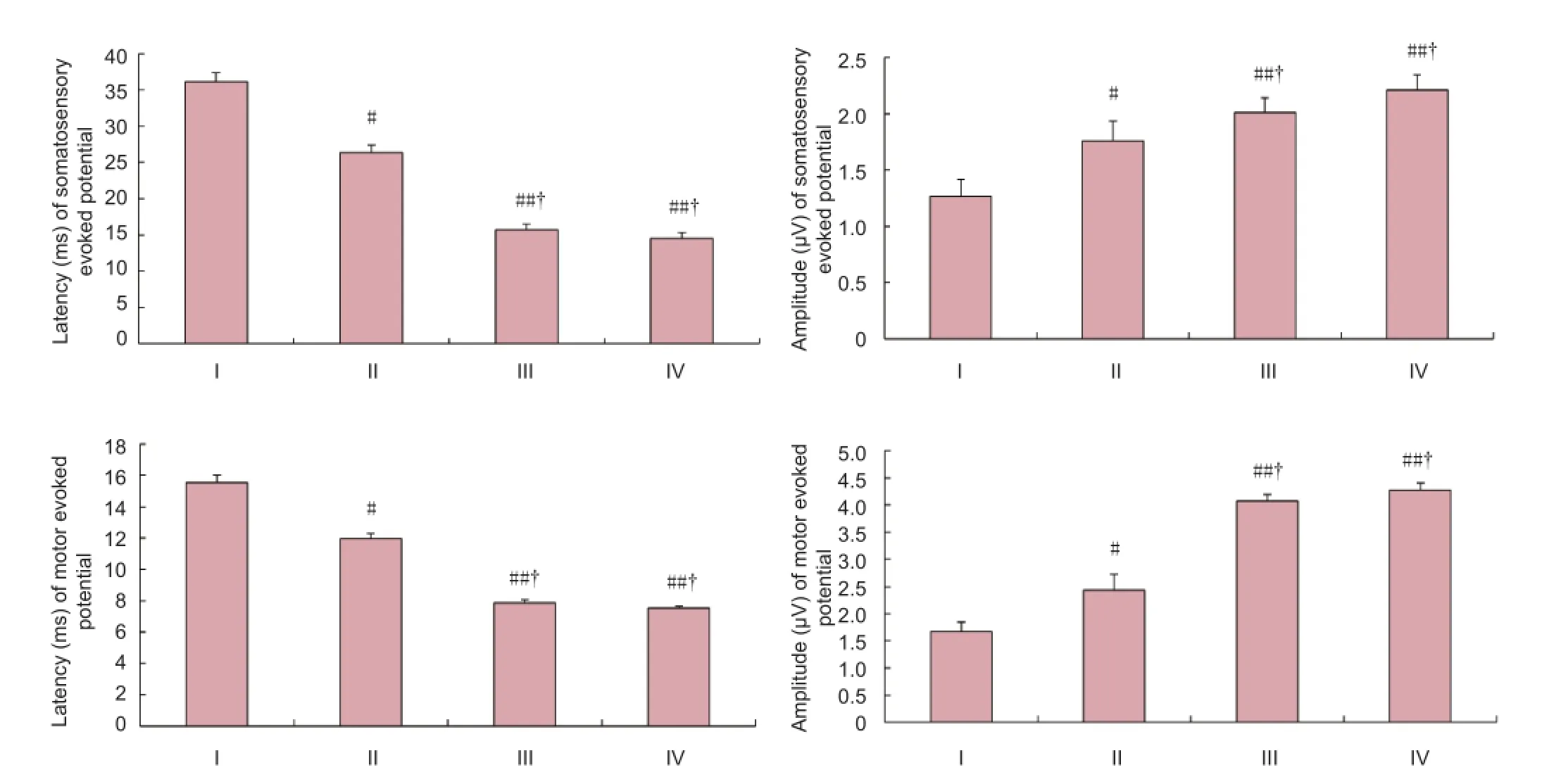
Figure 4 Effect of mild hypothermia combined with transplantation of the NgR-silenced cell scaffolds on the somatosensory evoked potential and motor evoked potential in rats after spinal cord injury.
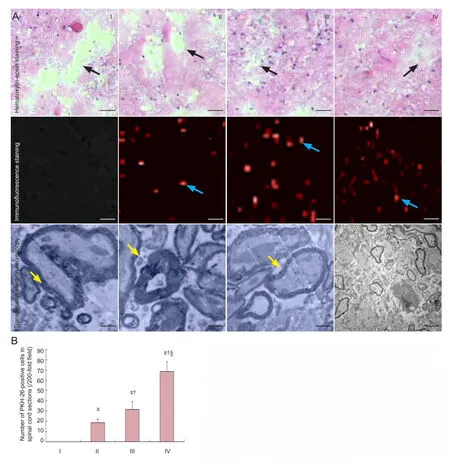
Figure 5 Effect of mild hypothermia combined with transplantation of the NgR-silenced cell scaffold on the pathological changes in spinal cord sections from rats after spinal cord injury.
In conclusion, mild hypothermia treatment prior to transplantation of scaffolds seeded with NgR gene-silenced cells can prolong the survival, proliferation, differentiation, and migration of transplanted neural stem cells at the site of injury, reduce glial scar formation, and promote the recovery of neurological function in rats after spinal cord injury, thereby contributing to the processes of restoration and regeneration following spinal cord injury.
Author contributions:Wang D, Sun WW and Liang JH were responsible for the study design, implementing data analysis and writing the manuscript. Zhang JJ revised the manuscript. Zhang JJ and Liu SH were responsible for statistical analysis. All authors read and approved the final version of the manuscript.
Con fl icts of interest:None declared.
Akbary K, Arora SS (2014) Biological modalities for treatment of acute spinal cord injury: a pilot study and review of the literature. Chin J Traumatol 17:157-164.
Antonic A, Sena ES, Lees JS, Wills TE, Skeers P, Batchelor PE, Macleod MR, Howells DW (2013) Stem cell transplantation in traumatic spinal cord injury: a systematic review and meta-analysis of animal studies. PLoS Biol 11:e1001738.
Ban DX, Kong XH, Feng SQ, Ning GZ, Chen JT, Guo SF (2009) Intraspinal cord graft of autologous activated Schwann cells efficiently promotes axonal regeneration and functional recovery after rat’s spinal cord injury. Brain Res 1256:149-161.
Batchelor PE, Skeers P, Antonic A, Wills TE, Howells DW, Macleod MR, Sena ES (2013) Systematic review and meta-analysis of therapeutic hypothermia in animal models of spinal cord injury. PLoS One 8: e71317.
Bazley FA, Pashai N, Kerr CL, All AH (2014) The effects of local and general hypothermia on temperature pro fi les of the central nervous system following spinal cord injury in rats. Ther Hypothermia Temp Manag 4:115-124.
Chi GF, Kim MR, Kim DW, Jiang MH, Son Y (2010) Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp Neurol 222:304-317.
Choi JS, Leem JW, Lee KH, Kim SS, Suh-Kim H, Jung SJ, Kim UJ, Lee BH (2012) Effects of human mesenchymal stem cell transplantation combined with polymer on functional recovery following spinal cord hemisection in rats. Korean J Physiol Pharmacol 16:405-411.
Dididze M, Green BA, Dalton Dietrich W, Vanni S, Wang MY, Levi AD (2013) Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord 51:395-400.
Du BL, Zeng X, Ma YH, Lai BQ, Wang JM, Ling EA, Wu JL, Zeng YS (2014) Graft of the gelatin sponge scaffold containing genetically-modified neural stem cells promotes cell differentiation, axon regeneration, and functional recovery in rat with spinal cord transection. J Biomed Mater Res A doi: 10.1002/jbm.a.35290.
Hiraizumi Y, Transfeldt EE, Kawahara N, Yamada H (1996) Differences in sensitivity between magnetic motor-evoked potentials and somatosensory-evoked potentials in experimental spinal cord lesions. Spine (Phila Pa 1976) 21:2190-2196.
Hou X, Liang Q, Wu Y (2013) Transplantation of Schwann cells co-cultured with brain-derived neurotrophic factor for the treatment of experimental autoimmune neuritis. J Neuroimmunol 263:83-90.
Karimi-Abdolrezaee S, Eftekharpour E (2012) Stem cells and spinal cord injury repair. Adv Exp Med Biol 760:53-73.
Kim JW, Ha KY, Molon JN, Kim YH (2013) Bone marrow-derived mesenchymal stem cell transplantation for chronic spinal cord injury in rats: comparative study between intralesional and intravenous transplantation. Spine 38:E1065-1074.
Kobbe P, Lichte P, Wellmann M, Hildebrand F, Nast-Kolb D, Waydhas C, Oberbeck R (2009) Impact of hypothermia on the severely injured patient. Unfallchirurg 112:1055-1061.
Lepore AC, Neuhuber B, Connors TM, Han SS, Liu Y, Daniels MP, Rao MS, Fischer I (2006) Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience 142:287-304.
Meletis K, Barnabé-Heider F, Carlén M, Evergren E, Tomilin N, Shupliakov O, Frisén J (2008) Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 6:e182.
Nout YS, Beattie MS, Bresnahan JC (2012) Severity of locomotor and cardiovascular derangements after experimental high-thoracic spinal cord injury is anesthesia dependent in rats. J Neurotrauma 29:990-999.
Ok JH, Kim YH, Ha KY (2012) Neuroprotective effects of hypothermia after spinal cord injury in rats: comparative study between epidural hypothermia and systemic hypothermia. Spine 37:E1551-1559.
Pallini R, Vitiani LR, Bez A, Casalbore P, Facchiano F, Di Giorgi Gerevini V, Falchetti ML, Fernandez E, Maira G, Peschle C, Parati E (2005) Homologous transplantation of neural stem cells to the injured spinal cord of mice. Neurosurgery 57:1014-1025.
Papastefanaki F, Chen J, Lavdas AA, Thomaidou D, Schachner M, Matsas R (2007) Grafts of Schwann cells engineered to express PSANCAM promote functional recovery after spinal cord injury. Brain 130:2159-2174.
Pearse DD, Sanchez AR, Pereira FC, Andrade CM, Puzis R, Pressman Y, Golden K, Kitay BM, Blits B, Wood PM, Bunge MB (2007) Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia 55:976-1000.
Peng Y, Zhang QL, Xu D, Wang YP, Qin XY (2010) Small hairpin RNA interference of the Nogo receptor inhibits oxygen-glucose deprivation-induced damage in rat hippocampal slice cultures. Neuropathology 30:565-573.
Peterson SL, Anderson AJ (2014) Complement and spinal cord injury: traditional and non-traditional aspects of complement cascade function in the injured spinal cord microenvironment. Exp Neurol 258: 35-47.
Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH, Rahimi-Movaghar V, Raza M, Firouzi M (2008) Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes. Neurosci Lett 443:46-50.
Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY (2002) Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A 99:3024-3029.
Wang D, Zhang JJ, Yang ZX (2010) Treatment of spinal cord injury by transplanting neural stem cells with NgR gene silencing. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 22:28-31.
Xiao ZM, Hu MZ, Lv HB, Zhuo XL, Xu DQ, Wang SX, Li JH (2012) The effect of tetramethylpyrazine on the expression of NF-κB and I-KBa after acute spinal cord injury in the rat model. Jichu Yixue yu Linchuang 32:407-412.
Xu CJ, Xu L, Huang LD, Li Y, Yu PP, Hang Q, Xu XM, Lu PH (2011) Combined NgR vaccination and neural stem cell transplantation promote functional recovery after spinal cord injury in adult rats. Neuropathol Appl Neurobiol 37:135-155.
Yan Q, Ruan JW, Ding Y, Li WJ, Li Y, Zeng YS (2011) Electro-acupuncture promotes differentiation of mesenchymal stem cells, regeneration of nerve fi bers and partial functional recovery after spinal cord injury. Exp Toxicol Pathol 63:151-156.
Young W (2002) Spinal cord contusion models. Prog Brain Res 137: 231-255.
Zaminy A, Shokrgozar MA, Sadeghi Y, Norouzian M, Heidari MH, Piryaei A (2013) Transplantation of schwann cells differentiated from adipose stem cells improves functional recovery in rat spinal cord injury. Arch Iran Med 16:533-541.
Zhang D, He X (2014) A meta-analysis of the motion function through the therapy of spinal cord injury with intravenous transplantation of bone marrow mesenchymal stem cells in rats. PLoS One 9:e93487.
Zhou QZ, Zhang G, Long HB, Lei F, Ye F, Jia XF, Zhou YL, Kang JP, Feng DX (2014) Effect of spinal cord extracts after spinal cord injury on proliferation of rat embryonic neural stem cells and Notch signal pathway in vitro. Asian Pac J Trop Med 7:562-567.
Copyedited by McCarty W, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.147952
Wenwen Sun, Department of Neurosurgery, the Fourth Center Clinical College of Tianjin Medical University, Tianjin Fourth Central Hospital, Tianjin 300140, China, wd5609@hotmail.com.
http://www.nrronline.org/
Accepted: 2014-11-25
- 中国神经再生研究(英文版)的其它文章
- Hydrogen sul fi de controls peripheral nerve degeneration and regeneration: a novel therapeutic strategy for peripheral demyelinating disorders or nerve degenerative diseases
- Activities of nicotinic acetylcholine receptors modulate neurotransmission and synaptic architecture
- A novel arti fi cial nerve graft for repairing longdistance sciatic nerve defects: a self-assembling peptide nano fi ber scaffold-containing poly(lactic-co-glycolic acid) conduit
- The effects of claudin 14 during early Wallerian degeneration after sciatic nerve injury
- Transplantation of human amniotic epithelial cells repairs brachial plexus injury: pathological and biomechanical analyses
- Long-term treatment with PP2 after spinal cord injury resulted in functional locomotor recovery and increased spared tissue

