The Achyranthes bidentata polypeptide k fraction enhances neuronal growth in vitro and promotes peripheral nerve regeneration after crush injury in vivo
Qiong Cheng, Chunyi Jiang, Caiping Wang, Shu Yu, Qi Zhang, Xiaosong Gu, Fei Ding
Jiangsu Key Laboratory of Neuroregeneration, Co-innovation Center of Neuroregenration, Nantong University, Nantong, Jiangsu Province, China
The Achyranthes bidentata polypeptide k fraction enhances neuronal growth in vitro and promotes peripheral nerve regeneration after crush injury in vivo
Qiong Cheng, Chunyi Jiang, Caiping Wang, Shu Yu, Qi Zhang, Xiaosong Gu, Fei Ding
Jiangsu Key Laboratory of Neuroregeneration, Co-innovation Center of Neuroregenration, Nantong University, Nantong, Jiangsu Province, China
Qiong Cheng and Chunyi Jiang contributed equally to this work.
We have previously shown that Achyranthes bidentata polypeptides (ABPP), isolated from Achyranthes bidentata Blume (a medicinal herb), exhibit neurotrophic and neuroprotective effects on the nervous system. To identify the major active component of ABPP, and thus optimize the use of ABPP, we used reverse-phase high performance liquid chromatography to separate ABPP. We obtained 12 fractions, among which the fraction of ABPPk demonstrated the strongest neuroactivity. Immunocytochemistry and western blot analysis showed that ABPPk promoted neurite growth in cultured dorsal root ganglion explant and dorsal root ganglion neurons, which might be associated with activation of Erk1/2. A combination of behavioral tests, electrophysiological assessment, and histomorphometric analysis indicated that ABPPk enhanced nerve regeneration and function restoration in a mouse model of crushed sciatic nerve. All the results suggest that ABPPk, as the key component of ABPP, can be used for peripheral nerve repair to yield better outcomes than ABPP.
nerve regeneration; Achyranthes bidentata polypeptides; neuroactive component; dorsal root ganglion; neurite outgrowth; crush injury; sciatic nerve; peripheral nerve regeneration; neural regeneration
Funding: This study was supported by a grant from National Key Basic Research Program of China (973 Program), No. 2014CB542202; a grant from Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) in China.
Cheng Q, Jiang CY, Wang CP, Yu S, Zhang Q, Gu XS, Ding F. The Achyranthes bidentata polypeptide k fraction enhances neuronal growth in vitro and promotes peripheral nerve regeneration after crush injury in vivo. Neural Regen Res. 2014;9(24):2142-2150.
Introduction
Peripheral nerve injury commonly occurs because of accidental trauma, acute compression, or iatrogenic injury, leading to neuronal dysfunction, and affects quality of life. In clinical practice, surgical repair is usually required for treating nerve transection injuries, while drug administration, such as neurotrophins and vitamins, is a useful therapy of choice for treating nerve crush injuries (Camara-Lemarroy et al., 2008; Roglio et al., 2008; Britt et al., 2010; Li et al., 2010; Shim and Ming, 2010). Accordingly, the development of neurotrophic and neuroprotective drugs/agents, including those derived from natural medicinal herbs, has attracted research attention.
Achyranthes bidentata Blume (Amaranthaceae family), listed in the Chinese Pharmacopoeia, is a natural herb used in traditional Chinese medicine with multiple therapeutic effects (Li et al., 2007). In previous studies, we have shown that an aqueous extract of Achyranthes bidentata Blume accelerated peripheral nerve regeneration of rabbit common peroneal nerve after a crush injury (Ding et al., 2008), and reduced glutamate-induced cell apoptosis in primary cultured hippocampal neurons (Zhou et al., 2009). Later, we isolated Achyranthes bidentata polypeptides (ABPP) from the aqueous extract of Achyranthes bidentata Blume, and found that ABPP protected primary culture of rat hippocampal neurons against N-methyl-D-aspartate (NMDA)-induced excitotoxicity (Shen et al., 2008), stimulated neurite outgrowth of rat dorsal root ganglia (DRGs), and promoted peripheral nerve regeneration in rats and rabbits (Yuan et al., 2010; Wang et al., 2013; Cheng et al., 2014).
Although considerable work has been done on revealing the neurotrophic and neuroprotective action of ABPP, the effective components contained in ABPP and their effects on the nervous system are still unknown. To identify the major active component of ABPP, we used a reverse-phase high performance liquid chromatography (RP-HPLC) method to isolate different components from ABPP and examined their neuroactivities by using a neuronal survival assay. Among a total of 12 RP-HPLC fractions, the 11thfraction, code-named ABPPk, exhibited the strongest neuroactivity. We then investigated the effects of ABPPk on neurite growth in an in vitro DRG neuronal model and on nerve regeneration and functional recovery in an in vivo animal model of peripheral nerve crush injury.
Materials and Methods
Culture of DRG explants and DRG neurons
DRG explants were harvested from spinal and peripheral roots of postnatal day 1 Sprague-Dawley rats, and plated on poly-L-lysine-coated cover slips for incubation in DMEM medium supplemented with 5% fetal bovine serum (FBS), 5% horse serum, 2 mmol/L L-glutamine, and 100 U/mL penicillin/streptomycin (Sigma, St. Louis, MO, USA).
Primary DRG neurons were obtained by a differential adhesion technique as described previously (Fudge and Mearow, 2013). In brief, the procured DRG tissues were digested with 0.1% collagenase type II (Gibco, Grand Island, NY, USA) and 0.25% trypsin/ethylenediamine tetraacetic acid (EDTA; Sigma) at 37°C. Tissue was transferred to DMEM supplemented with 10% FBS and antibiotics (Sigma) for trituration with a fi re-polished Pasteur pipette until the suspension was homogeneous. The cell suspension was fi ltered through a cell-strainer (40 µmol/L, BD Biosciences, Bedford, MA, USA) and centrifuged at 1,200 r/min for 5 minutes. The DRG pellets were resuspended in neurobasal medium plus 2 mmol/L L-glutamine (Gibco), and placed onto pre-coated plates for 30 minute incubation at 37°C and 5% CO2. Then, non-adherent DRG neurons were collected, and re-suspended in fresh medium.
RP-HPLC of ABPPk
The root of Achyranthes bidentata Blume was purchased from a local Chinese medicine grocer and identi fi ed by Professor Zhao HR from the China Pharmaceutical University. ABPP was prepared from Achyranthes bidentata Blume as previously described (Yuan et al., 2010). The aqueous solution of ABPP was subjected to HPLC on a Waters System (Waters, Milford, MA, USA) consisting of Waters Alliance e2695 and Waters 2996 Photodiode Array Detector. A C18 reverse phase HPLC column (4.6 × 250 mm, 5 µm i.d. Waters, Milford, MA, USA) was applied, and a linear gradient elution was performed with 0.1% trifluoroacetic acid in water/acetonitrile (water ratio, 80–47% by volume) at a fl ow rate of 1.0 mL/min. The eluted 12 fractions were characterized by UV spectrophotometry at 220 nm. They were centrifuged and concentrated in a vacuum freeze drying machine to yield powders, which were easily dissolved in aqueous solution to achieve a desired concentration. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was then used to test the effects of 12 RP-HPLC fractions of ABPP on neuronal survival. After DRG neurons were plated onto 96-well plates at a density of 5 × 105cells/mL in serum-free neurobasal medium, cells were exposed to a combination of either fraction (250 ng/mL), ABPP at 250 ng/mL or 1 µg/mL (positive control), or no additive (negative control). Cells were allowed to incubate for 24 hours. MTT solution was then added at a fi nal concentration of 0.5 mg/mL, and cells were allowed to incubate for 4 hours at 37°C before sodium dodecyl sulfate (SDS) solution was added to dissolve the formazan precipitate. The optical density was measured by spectrophotometer at 570 nm with an ElX-800 Microelisa reader (Bio-Tek Inc., Burlington, VT, USA) to determine cell viability. MTT assay aided the selection of the 11thfraction of ABPP, named ABPPk, for further investigations.
Measurement of neurite length
DRG explants or DRG neurons were transferred to a serum-free neurobasal medium supplemented with 2 mmol/L L-glutamine (Gibco) containing ABPPk at 10, 50, or 250 ng/mL, with ABPP at 1 µg/mL (positive control), or with no added ABPP (negative control) for 72-hour incubation. To investigate for the involvement of Erk1/2 signaling in the effects of ABPPk, 10 µmol/L of PD98059 (Calbiochem, San Diego, CA, USA), a specific inhibitor of Erk1/2, was used to co-treat DRGs with 250 ng/mL of ABPPk. After these treatments, DRG explants or DRG neurons were fi xed in 4% paraformaldehyde, and blocked with 2% goat serum/0.3% Triton prior to incubation with a mouse anti-green associated protein GAP-43 or anti-β-tubulin III monoclonal antibody (both 1:200; Sigma) at 4°C overnight. After this incubation, FITC-conjugated goat anti-mouse IgG (1:200; Sigma) was incubated for 2 hours at room temperature to allow for signal visualization. Neurite outgrowth from DRG explants was quanti fi ed as described previously, with some minor modi fi cations (Bilsland et al., 1999). In brief, non-overlapping images were taken at 5× magni fi cation on a fl uorescence microscope (Leica, Wetzlar, Germany), and digitized using a CCD video camera to make the body and neurites of an explant visible. Two concentric circles were manually drawn first around the body and then around the halo of neurites to give the exclusion zones for measuring the neurite-occupied area by using the grain counting function of the Leica Qwin Image Analysis System (Leica). Neurite growth of DRG neurons was quanti fi ed by manual tracing with the Leica Qwin software. The length of the longest neurite per cell for the cell population in a fi eld having the identi fi able neurite and a visible neurite arbor was measured. At least 10 DRG explants and 30 DRG neurons were analyzed in each group, all experiments were repeated at least three times using cultures prepared on separate days.
Western blot analysis
DRG neurons were collected for homogenization in an icecold cell lysis buffer containing protease inhibitors. Total proteins were quanti fi ed by BCA analysis, and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Separation was followed by transferring to a polyvinylidene di fl uoride membrane, which was blocked with 5% non-fat powdered milk in 0.05% Tween-20-containing Tris-buffered saline (TBST), and allowed to incubate overnight at 4°C with primary antibodies (all from Abcam, Cambridge, MA, USA) diluted in TBST. Antibodies included: mouse anti-phosphorylated-Erk1/2 monoclonal antibody (1:10,000) and rabbit anti-total-Erk1/2 polyclonal antibody (1:1,000). After washing with TBST, horseradish peroxidase-labeled anti-mouse or rabbit IgG secondary antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was applied to react at room temperature for 2 hours. Signal detection was performed by exposure to enhanced chemiluminescent reagents (Roche Diagnostics, Basel, Switzerland) and then an X-ray film (Eastman Kodak Co., Rochester, New York, NY,USA) was used to capture the emitted signal. Analysis was performed using Adobe Photoshop 7.0 (Adobe, San Jose, USA) to measure the pixel density of the bands.
Animal surgery and treatment
The experimental procedures involving animals were performed in accordance with the Guide for the Care and Use of Laboratory Animals National Institute of Health (NIH) and approved by the Administration Committee of Experimental Animals, Jiangsu Province, China. Fifty male ICR mice weighing 22–25 g were provided by the Experimental Animal Center of Nantong University. All animals were deeply anesthetized with an intraperitoneal injection of xylazine (10 mg/kg), ketamine (95 mg/kg) and acepromazine (0.7 mg/kg). The sciatic nerve was exposed through a 1.0-cmlong incision on the left hind limb, and the 3-mm nerve was crushed by clamping for 30 seconds with a smooth-jawed forceps (Chung et al., 2012). The distal end of the crush site was marked with a 9-0 nylon suture. The sciatic nerve on the contralateral side was exposed but kept intact. After surgical incisions were closed, animals were randomly divided into fi ve groups (n = 10/group) to receive daily intravenous injection of saline/vehicle (negative control), 2.5, 5.0 and 10.0 mg/kg of ABPPk, and 16.0 mg/kg of ABPP (positive control), respectively. The treatment lasted for 21 days.
Walking track analysis
At 5, 10, 15, and 20 days after surgery, walking track analysis was performed as described previously (Inserra et al., 1998) with minor modi fi cations. Brie fl y, hind paws were dipped in red ink and animals were allowed to walk down a narrow and long corridor, making footprints on a white paper. The toe spread and paw length were measured, and the sciatic function index (SFI) was calculated according to the formula: SFI = 118.9(ETS – NTS)/NTS – 51.2(EPL – NPL)/NPL – 7.5, where ETS is experimental toe spread, NTS is normal toe spread, EPL is experimental paw length, and NPL is normal paw length. An SFI value of close to zero indicates normal nerve function, and a value of –100 indicates total impairment.
Foot re fl ex withdrawal test
Foot re fl ex withdrawal test was performed daily as described previously (Vogelaar et al., 2004). A small electrical current was applied to a well-characterized site on the central portion of the foot sole using two stimulation poles (spaced 3 mm apart). The current was increased stepwise from 0.1 mA to a maximum 1.0 mA in increments of 0.1 mA. A normal rat immediately withdraws its foot and spreads its toes when stimulated. Animals with crushed sciatic nerves do not retract their paws upon skin contact with the poles, with the reflex being restored upon reinnervation of target organs. The threshold value, i.e. the lowest current causing this re fl ex at the operated side, was noted.
Electrophysiological assessment
At 21 days post surgery, animals were anesthetized and sciatic nerves at both sides were re-exposed. Electrical stimuli were applied to the proximal and distal ends of the sciatic nerve, and the compound muscle action potential (CMAP) amplitude was respectively recorded on the gastrocnemius muscle by MYTO Portable Digital Electromyography (Esaoto, Hong Kong) using a Galileo NT System Software (Callizot et al., 2008).
Electron microscopy of regenerated nerves
At 21 days post surgery, animals in five groups were transcardially perfused with 4% paraformaldehyde in PBS. The distal portion of crushed and contralateral, uncrushed sciatic nerves were harvested. The nerve sample was fi xed with 2.5% glutaraldehyde, post- fi xed with 1% osmium tetraoxide solution at 4°C, dehydrated stepwise in increasing concentrations of ethanol, embedded in Epon 812 epoxy resin, and cut into transverse sections. The semi-thin section was stained with toluidine blue for measuring the cross-sectional area of the total nerve trunk area under light microscopy, and ultra-thin sections were stained with lead citrate and uranyl acetate for examination under transmission electron microscopy (JEOL Ltd., MA, USA). The electron micrographs from six random fi elds (5,000 × magni fi cation) of each nerve section were digitalized into a Q550 IW image analysis system (Leica Imaging Systems Ltd.) and analyzed with a Leica Qwin image analysis software package to obtain morphometric parameters, including myelin thickness and diameter of myelinated axons.
Hematoxylin and eosin staining of target muscles
Bilateral gastrocnemius muscles were harvested from animals post-sacri fi ce. The muscle sample from the mid-belly was post-fixed in buffered 4% paraformaldehyde for 24 hours, dehydrated in a graded ethanol series, cleared in xylene, embedded in paraf fi n and cut into 5-µm-thick transverse sections, which were processed with hematoxylin and eosin (HE) staining. Stained sections were photographed with a DC 300F color digital camera, and digitalized into a Q550 IW image analysis system (Leica Imaging System Ltd.). Six visual fields were selected randomly for each specimen and the cross-sectional areas (CSA) of the gastrocnemius muscle fi bers were measured by the aid of Leica Qwin Image Analysis software package.
Statistical analysis
All data are expressed as the mean ± SEM (for in vitro tests) or the mean ± SD (for in vivo tests). Statistical comparisons were performed with one-way analysis of variance (ANOVA) plus Scheffé post hoc test using SPSS 19.0 software package (IBM, San Francisco, USA). Values of P < 0.05 were considered signi fi cant.
Results
ABPPk was identi fi ed as the major component of ABPP
A representative RP-HPLC chromatogram of ABPP was recorded at 220 nm, which indicated that a total of 12 fractions were isolated from ABPP. These components were eluted at different retention times and code-named ABPPa, b, c, d, e, f, g, h, i, j, k, and l (Figure 1A).
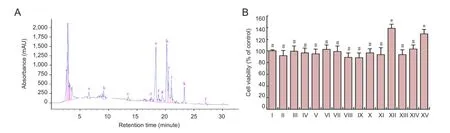
Figure 1 Effects of ABPP fractions on cell viability of DRG cells.
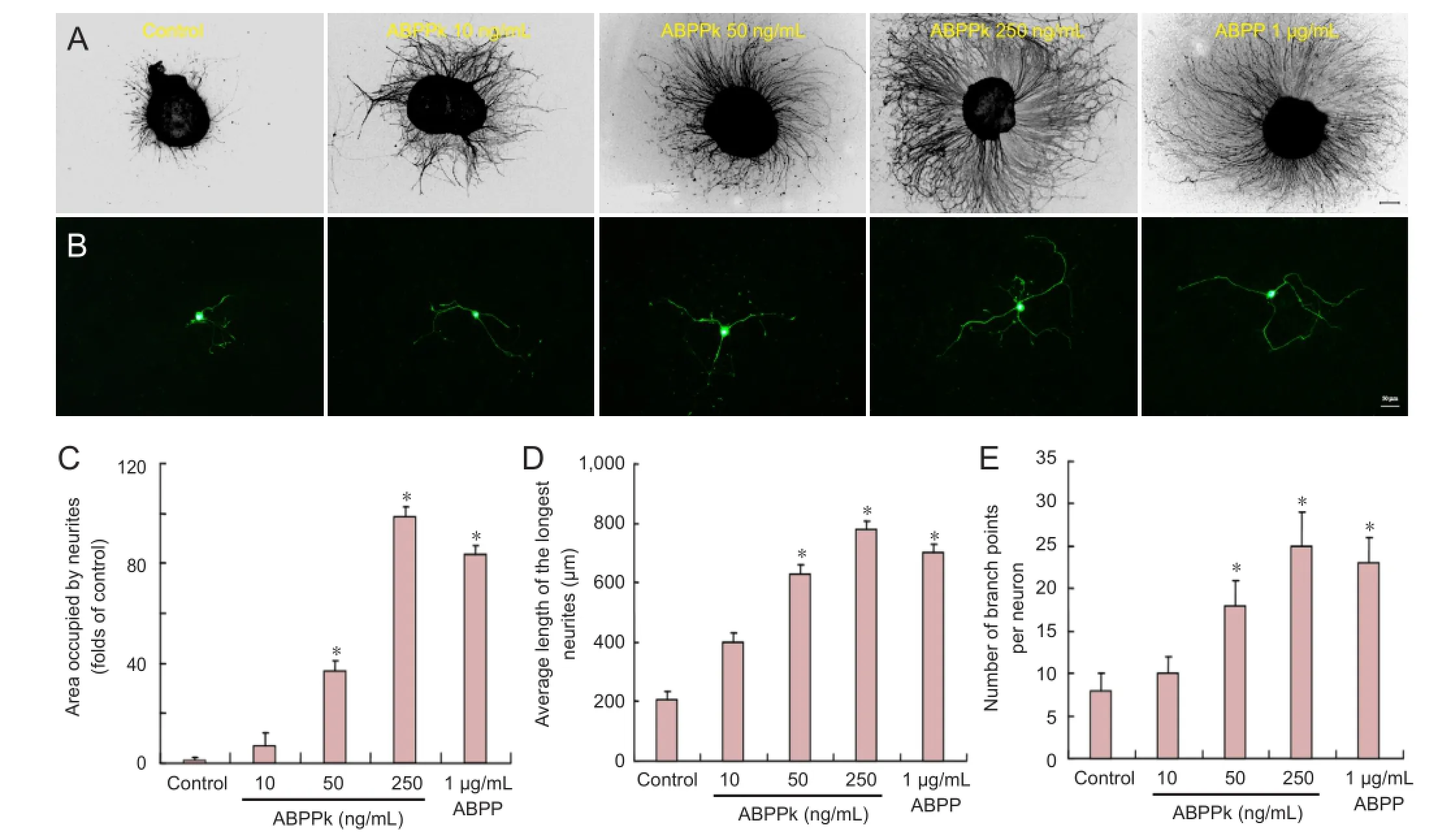
Figure 2 Effects of ABPPk on neuronal growth in cultured DRG explants and neurons.
According to MTT assay, treatment with ABPPk at 250 ng/mL significantly increased cell viability of DRG neurons when compared with treatment without additives, but also compared with treatment with the other 11 components of ABPP or with ABPP (all at the same concentration of 250 ng/mL). ABPPk displayed the strongest neuroactivity (i.e., the neuron survival-promoting effect) among the 12 components of ABPP. Moreover, ABPPk had stronger neuroactivity compared with ABPP (its parent product) at the same concentration (250 ng/mL). ABPP (1 µg/mL), a 4-fold concentration,showed similar neuroactivity to that of 250 ng/mL of ABPPk (Figure 1B). All these results suggested that ABPPk was the major neuroactive component of ABPP and more potent than ABPP.
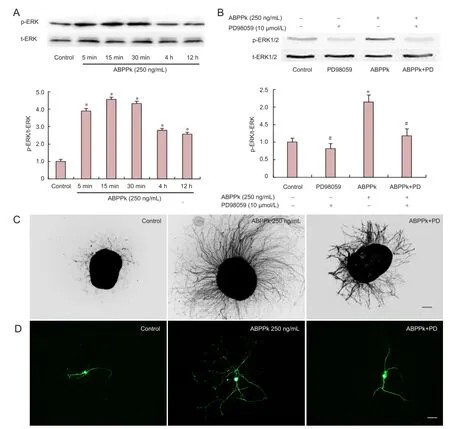
Figure 3 ABPPk-induced activation of phosphorylation of ERK1/2 in cultured DRG neurons.
ABPPk promoted neurite outgrowth of DRG explants and neurons and activated Erk1/2
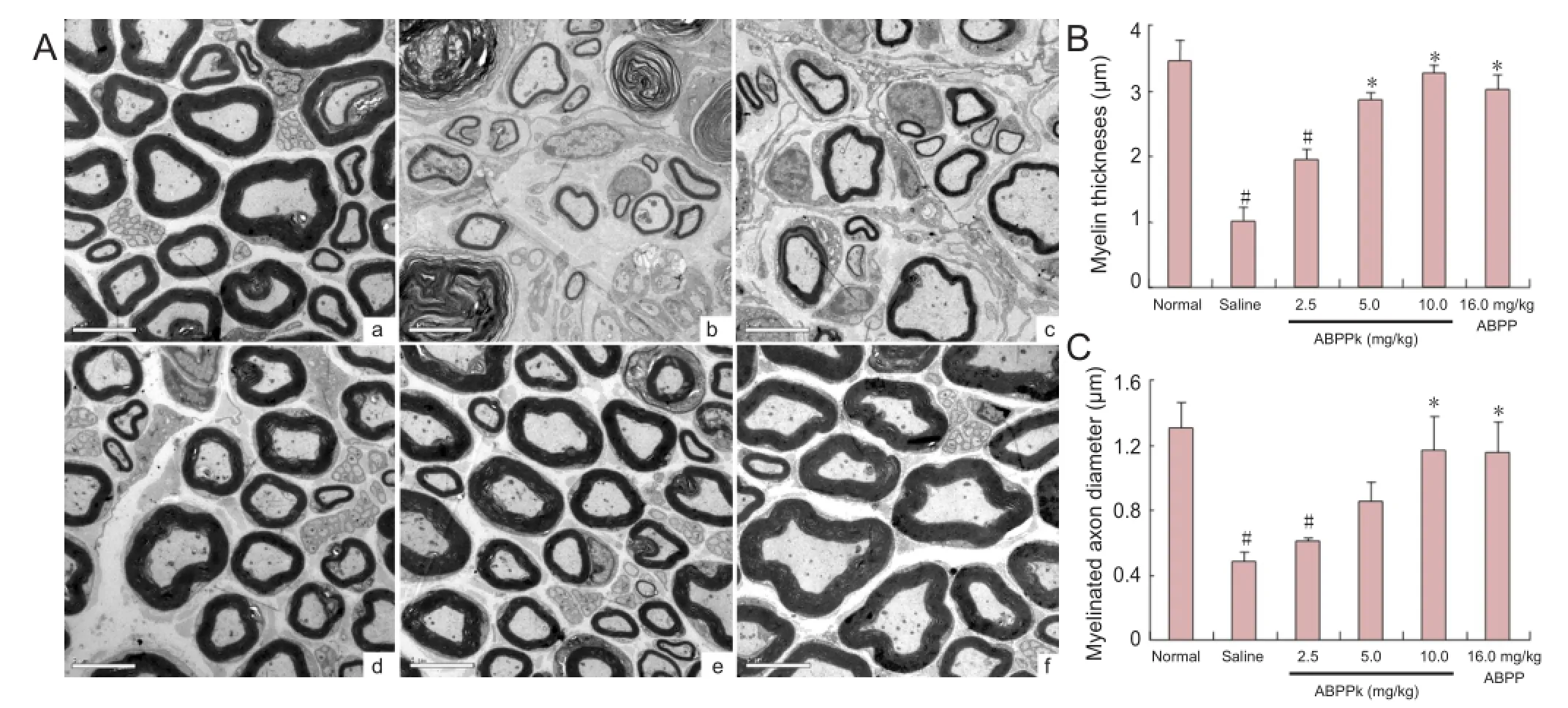
Figure 4 Nerve regeneration in mice after sciatic nerve crush injury.
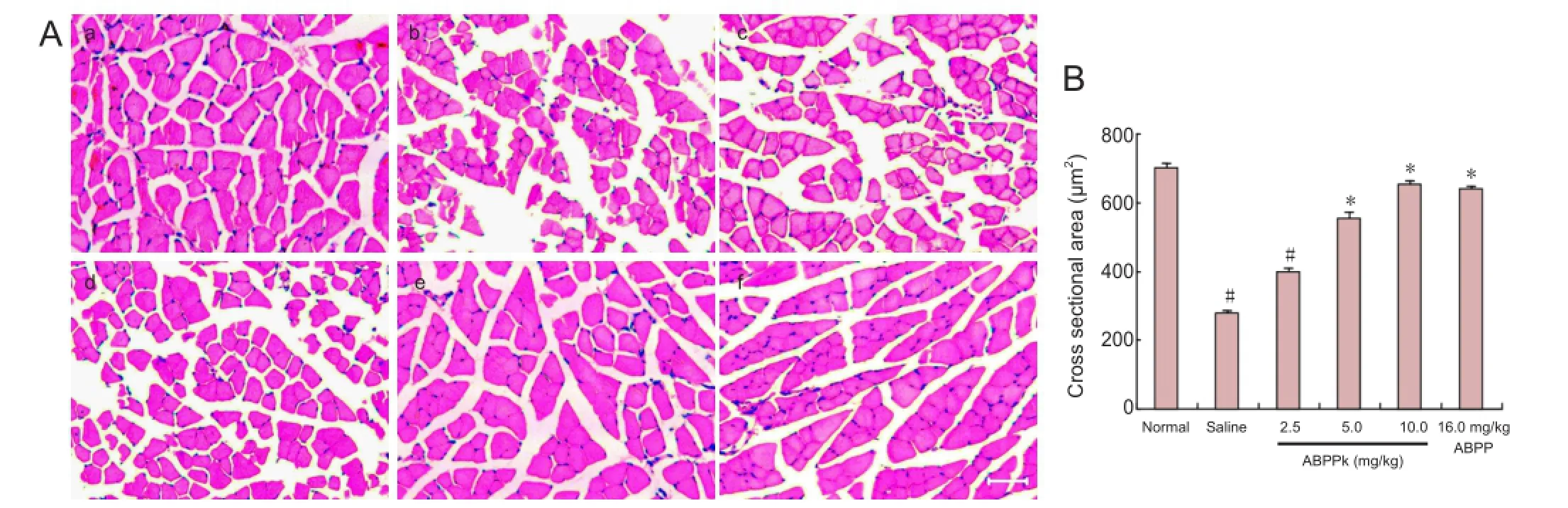
Figure 5 Target muscle reinnervation in mice after sciatic nerve crush injury.
To determine the effects of ABPPk on neurite growth, primary cultures of DRG neurons were treated with control (vehicle), 10, 50, 250 ng/mL of ABPPk, or 1 µg/mL ABPP for 72 hours, and their effects on neurite outgrowth were assessed. GAP-43 and β-tubulin III were used as two markers to determine neurite outgrowth. Immunostaining with anti-GAP-43 or anti-β-tubulin III showed that neurites emanated radially from the edge of DRG explants (Figure 2A), or from DRG neurons (Figure 2B), both of which were treated with different concentrations of ABPPk or with a single concentration of ABPP for 72 hours. The area occupied by neurites was signi fi cantly larger in DRG explants treated with ABPPk (50 or 250 ng/mL) or with ABPP (1 µg/mL) than in non-treated DRG explants (Figure 2C). The length or the number of branch points, which was determined for the longest neurite extended by randomly selected DRG neurons, was significantly greater (P < 0.05) in DRG neurons treated with 50 or 250 ng/mL of ABPPk or with 1 µg/mL of ABPP than in DRG non-treated neurons (Figure 2A, E), suggesting that ABPPk promoted neurite outgrowth of DRG explants and enhanced neurite elongation and branching of DRG neurons. The effect of ABPPk showed a concentration-dependent pattern, and was greater by more than 4 fold when compared with ABPP.
To investigate the possible involvement of signaling molecules, we examined the level of Erk1/2 phosphorylation in primary cultured DRG neurons after cell treatment. Western blot analysis showed that the expression ratio of phosphorylated Erk1/2 (p-Erk1/2) to total Erk1/2 (t-Erk1/2) in DRG neurons was signi fi cantly increased by ABPPk (250 ng/mL) treatment for 5 minutes–24 hours and reached a peak value after 15 minutes of treatment as compared with control (Figure 3A). ABPPk (250 ng/mL)-induced Erk1/2 phosphorylation was attenuated by PD98059 (10 µmol/L;Figure 3B).Immunostaining with GAP-43 and β-tubulin III showed that the enhancing effect of ABPPk on neurite outgrowth was blocked by 10 µmol/L PD98059 treatment (Figure 3C, D), suggesting that ABPPk promotion of neurite outgrowth was associated with activation of Erk1/2.
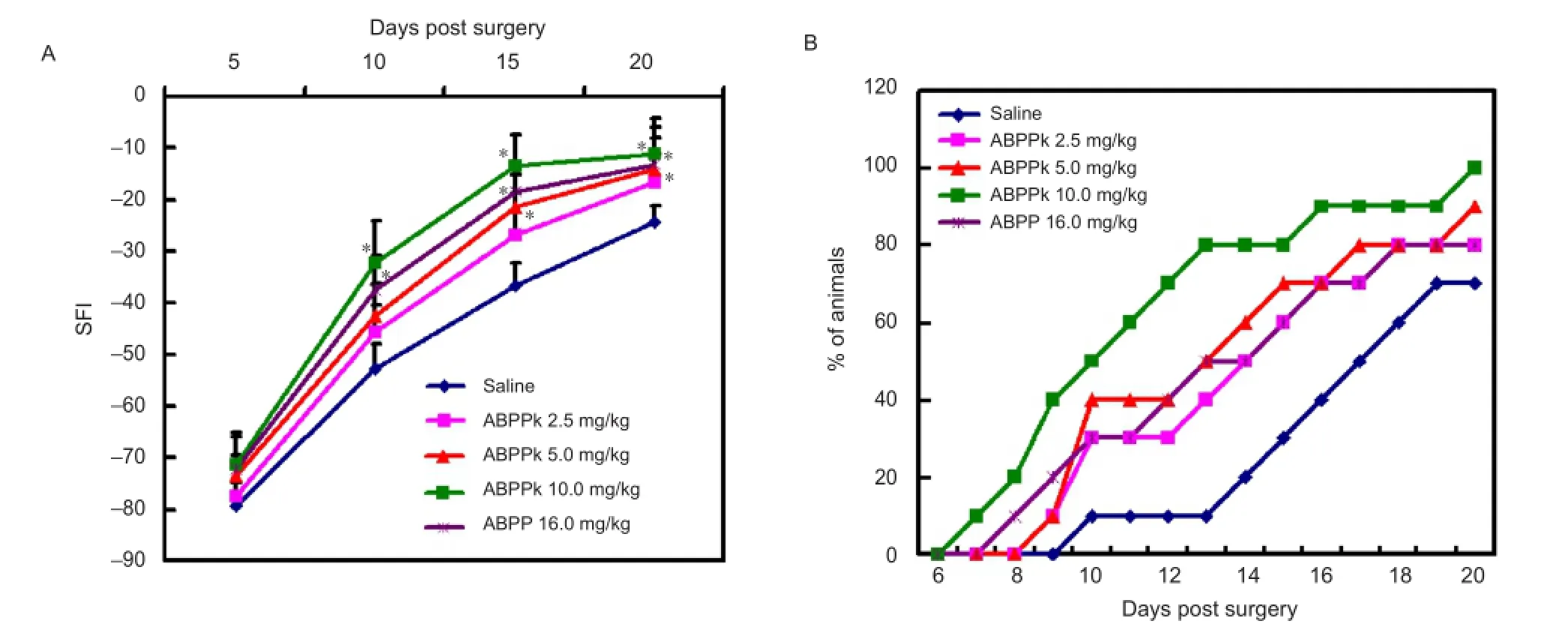
Figure 6 Walking track analysis and foot re fl ex withdrawal test for mice after sciatic nerve crush injury.
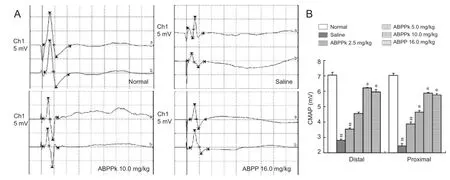
Figure 7 Electrophysiological assessments of mice after sciatic nerve crush injury.
ABPPk promoted regeneration of crushed nerve and alleviated atrophy of target muscle
Transmission electron microscopy was used to show the morphological features of regenerated sciatic nerves in the different groups at 21 days after nerve crush. The regenerated nerve fibers in mice treated with 10.0-mg/kg ABPPk were dispersed densely in clusters, and the myelinated axons were surrounded by a thick, clear, and electron-dense myelin sheath and a perfect basal membrane of Schwann cells. The myelin thickness was thinner than that of the contralateral, uncrushed sciatic nerve (Figure 4A). Morphometric analysis also revealed that myelin thickness and diameter of myelinated axons was signi fi cantly higher in mice treated with ABPPk (10.0 mg/kg) or with ABPP (16.0 mg/kg) than in mice treated with saline (Figure 4B, C). These observations suggested that ABPPk accelerated the morphological recovery of crushed peripheral nerve.
HE staining and morphometric analysis were performedto show the cross-sectional area (CSA) of gastrocnemius muscle fibers after treatment for 21 days. Microscopic observation indicated that despite the atrophied gastrocnemius muscle seen in saline-treated mice, the muscle fi bers in ABPPk-treated mice were distributed evenly with basal laminae being close to each other (Figure 5A). CSA was signi fi cantly higher in mice treated with ABPPk (10.0 mg/kg) or with ABPP (16.0 mg/kg) than in mice treated with saline (Figure 5B), indicating that ABPPk attenuated atrophy of the target muscle after nerve injury.
ABPPk improved functional recovery after sciatic nerve injury
To evaluate the possible effects of ABPPK on motor functional recovery, we performed walking track analysis to assess the recovery of motor function after crush injury in mice. At 5 days post surgery, no significant difference in the SFI value was observed among mice receiving different treatments. From 5 to 20 days post surgery, the SFI value in all animals increased steadily, but the rate of increase was different among mice receiving different treatments. At 10, 15 and 20 days post surgery, the SFI value was significantly higher in mice treated with 10.0 mg/kg ABPPk or with 16.0 mg/kg ABPP than in mice treated with saline. At 15 and 20 days post surgery, the SFI value was signi fi cantly higher in mice treated with 5.0 mg/kg ABPPk compared with mice treated with saline. The SFI value was higher in mice treated with 2.5 mg/kg ABPPk compared with mice treated with saline only at 20 days post surgery (Figure 6A). These results suggest that ABPPk accelerated the recovery of motor function in a dose-dependent manner after sciatic nerve crush injury. We also performed foot re fl ex withdrawal testing to evaluate the recovery of sensory function. At 10 days post surgery, 3, 3, 4, 5, and 1 of the 10 mice treated with 2.5, 5.0, 10.0 mg/ kg of ABPPk, 16.0 mg/kg of ABPP, and with saline, respectively, showed a response to 0.1-mA electric current. At 15 days post surgery, 6, 6, 7, 8, and 3 of the 10 mice treated as above showed a response to 0.1-mA electric current (Figure 6B), suggesting that ABPPk accelerated recovery of sensory function.
Electrophysiological assessments were performed at 21 days after nerve crush. Although the compound muscle action potential (CMAP) value recorded at either a proximal or distal portion of regenerating sciatic nerves was signi fi cantly lower on the crushed side than the contralateral uncrushed side for all animals, the CMAP value was signi fi cantly higher in ABPPk- or ABPP-treated animals than that in saline-treated animals (Figure 7). These results were similar to SFI data, suggesting that ABPPk improved functional recovery after peripheral nerve crush injury.
Discussion
To date, much effort has been devoted to the synthesis and/ or isolation of neurotrophic and neuroprotective drugs/ agents for treating peripheral nerve crush injury. On the basis of our previous work concerning ABPP, this study aimed to fi nd the major active component of ABPP to advance the therapeutic application of Achyranthes bidentata Blume-derived plant peptides in peripheral nerve repair.
RP-HPLC was used to separate ABPP, and the 12 eluted fractions were screened for their neuroactivity on neuron viability using MTT method. In this way, ABPPk was identi fi ed as the major neuroactive component of ABPP. To investigate the in vitro neural actions of ABPPk, cultured DRG explants and DRG neurons were subjected to immunostaining and western blot analysis. GAP-43 is an axonal membrane protein involved in neuronal outgrowth and synaptic plasticity of developing and regenerating neurons, and it is extensively used as a marker of neuritogenesis and synaptogenesis (Pfenninger et al., 1991; Mahalik et al., 1992). β-Tubulin III, as a member of the tubulin family, is found in the brain and root ganglia and localized to the nervous system, where its expression increases during neurite outgrowth (Carre et al., 2002). β-Tubulin III is therefore a highly speci fi c marker for neurons. In this study, immunostaining with anti-GAP-43 and anti-β-tubulin III con fi rmed that ABPPk could encourage neurite growth in cultured DRG explants and neurons in a concentration-dependent manner. This effect was greater than that induced by an identical concentration of ABPP (positive control). It suggested that ABPPk might present the major neurotrophic and neuroprotective effect of ABPP.
Phosphorylated Erk activates CREB, Cdk5, GAP-43, and other neuritogenesis-related genes (Yang et al., 2012). The Mek/Erk pathway can be activated through phosphorylation by neurotrophins, and Erk activation induces neurite extension and branching of DRG neurons (Markus et al., 2002; Minano et al., 2008). The aqueous extract of Achyranthes bidentata Blume has been found to induce neuronal differentiation of PC12 cells through activation of Erk1/2 (Ding et al., 2004). Similarly, western blot analysis in this study showed that ABPPk increased the phosphorylation of Erk1/2 in cultured DRG neurons, while application of PD98059 (an Erk 1/2 inhibitor) attenuated activation of Erk1/2. Additionally, immunostaining with two neuronal markers provided further evidence that the effect of ABPPk on neurite growth might be associated with activation of Erk signaling molecules.
To investigate the in vivo neural actions of ABPPk, mice with sciatic nerve crush were used, which represented a commonly used animal model for developing new drugs/agents to cure peripheral nerve crush injury. A combination of behavioral studies, electrophysiological assessment, and histological analysis of regenerated nerves and target muscles was used to evaluate the repair outcomes of ABPPk treatments.
The SFI value is a reliable index for examining motor function recovery of injured peripheral nerves (Varejao et al., 2003). Treatment of animals with a high concentration (10.0 mg/kg) of ABPPk caused a signi fi cant acceleration in recovery of motor function starting from 10 days post surgery. The efficacy of 10.0-mg/kg ABPPk was similar to that of 16.0 mg/kg ABPP. The foot re fl ex withdrawal test is commonly used to assess sensory function of injured peripheral nerves (Vogelaar et al., 2004). At 20 days post surgery, the mice treated with high concentration (10.0 mg/kg) of ABPPkor ABPP (16.0 mg/kg) showed a full foot withdrawal response to current stimuli. By contrast, only 70% of saline-treated animals exhibited functional recovery. A signi fi cant difference occurred between ABPPk and saline treatment, but no signi fi cant difference occurred between treatment with 10.0 mg/kg ABPPk and 16.0 mg/kg ABPP. Electrophysiological assessments were further used to compare the restoration of electrophysiological properties induced by ABPPk or ABPP treatments with that by saline treatment. Also, histological observation and morphometric analysis clearly demonstrated that ABPPk treatment, similar to the higher dose of ABPP treatment, supported axon regrowth and helped reinnervation of target gastrocnemius muscle in an effective way. The in vivo nerve regeneration-promoting effect of ABPPk was also dose-dependent, and comparable with that produced by the higher dose of ABPP (positive control).
To summarize, this study identi fi ed ABPPk as the key neuroactive component of ABPP. ABPPk was found to promote neuronal growth in vitro and to enhance peripheral nerve regeneration in vivo. The comparison on an equal concentration basis con fi rmed that ABPPk was a more potent agent used for peripheral nerve repair than ABPP. The underlying mechanism of ABPPk on neuroactivity is still need to be further investigated.
Acknowledgments:We thank Professor Jie Liu, Jiangsu Key Laboratory of Neuroregeneration, Nantong University, China, for assistance in manuscript preparation.
Author contributions:Cheng Q and Ding F conceived and designed the study. Cheng Q, Jiang CY, Wang CP, Yu S, and Zhang Q collected the data. Gu XS and Ding F revised the manuscript. Cheng Q wrote the manuscript. All authors approved the final version of this article.
Con fl icts of interest:None declared.
Bilsland J, Rigby M, Young L, Harper S (1999) A rapid method for semi-quantitative analysis of neurite outgrowth from chick DRG explants using image analysis. J Neurosci Methods 92:75-85.
Britt JM, Kane JR, Spaeth CS, Zuzek A, Robinson GL, Gbanaglo MY, Estler CJ, Boydston EA, Schallert T, Bittner GD (2010) Polyethylene glycol rapidly restores axonal integrity and improves the rate of motor behavior recovery after sciatic nerve crush injury. J Neurophysiol 104:695-703.
Callizot N, Andriambeloson E, Glass J, Revel M, Ferro P, Cirillo R, Vitte PA, Dreano M (2008) Interleukin-6 protects against paclitaxel, cisplatin and vincristine-induced neuropathies without impairing chemotherapeutic activity. Cancer Chemother Pharmacol 62:995-1007.
Camara-Lemarroy CR, Guzman-de la Garza FJ, Barrera-Oranday EA, Cabello-Garcia AJ, Garcia-Tamez A, Fernandez-Garza NE (2008) Celecoxib accelerates functional recovery after sciatic nerve crush in the rat. J Brachial Plex Peripher Nerve Inj 3:25.
Carre M, Andre N, Carles G, Borghi H, Brichese L, Briand C, Braguer D (2002) Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J Biol Chem 277:33664-33669.
Cheng Q, Yuan Y, Sun C, Gu X, Cao Z, Ding F (2014) Neurotrophic and neuroprotective actions of Achyranthes bidentata polypeptides on cultured dorsal root ganglia of rats and on crushed common peroneal nerve of rabbits. Neurosci Lett 562:7-12.
Chung CL, Tsai HP, Lee KS, Chen KI, Wu SC, Kuo YH, Winardi W, Chen IC, Kwan AL (2012) Assisted peripheral nerve recovery by KMUP-1, an activator of large-conductance Ca(2+)-activated potassium channel, in a rat model of sciatic nerve crush injury. Acta Neurochir (Wien) 154:1773-1779.
Ding F, Cheng Q, Gu X (2008) The repair effects of Achyranthes bidentata extract on the crushed common peroneal nerve of rabbits. Fitoterapia 79:161-167.
Ding F, Qiang L, Liu M, Gu X, Gu X (2004) Effect of nerve regeneration factor on differentiation of PC12 cells and its signaling pathway. Prog Nat Sci 14:971-974.
Fudge NJ, Mearow KM (2013) Extracellular matrix-associated gene expression in adult sensory neuron populations cultured on a laminin substrate. BMC Neurosci 14:15.
Inserra MM, Bloch DA, Terris DJ (1998) Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery 18:119-124.
Li FQ, Fowler KA, Neil JE, Colton CA, Vitek MP (2010) An apolipoprotein E-mimetic stimulates axonal regeneration and remyelination after peripheral nerve injury. J Pharmacol Exp Ther 334:106-115.
Li J, Qi H, Qi LW, Yi L, Li P (2007) Simultaneous determination of main phytoecdysones and triterpenoids in radix achyranthis bidentatae by high-performance liquid chromatography with diode array-evaporative light scattering detectors and mass spectrometry. Anal Chim Acta 596:264-272.
Mahalik TJ, Carrier A, Owens GP, Clayton G (1992) The expression of GAP43 mRNA during the late embryonic and early postnatal development of the CNS of the rat: an in situ hybridization study. Brain Res Dev Brain Res 67:75-83.
Markus A, Zhong J, Snider WD (2002) Raf and akt mediate distinct aspects of sensory axon growth. Neuron 35:65-76.
Minano A, Xifro X, Perez V, Barneda-Zahonero B, Saura CA, Rodriguez-Alvarez J (2008) Estradiol facilitates neurite maintenance by a Src/Ras/ERK signalling pathway. Mol Cell Neurosci 39:143-151.
Pfenninger KH, de la Houssaye BA, Helmke SM, Quiroga S (1991) Growth-regulated proteins and neuronal plasticity. A commentary. Mol Neurobiol 5:143-151.
Roglio I, Bianchi R, Gotti S, Scurati S, Giatti S, Pesaresi M, Caruso D, Panzica GC, Melcangi RC (2008) Neuroprotective effects of dihydroprogesterone and progesterone in an experimental model of nerve crush injury. Neuroscience 155:673-685.
Shen H, Yuan Y, Ding F, Liu J, Gu X (2008) The protective effects of Achyranthes bidentata polypeptides against NMDA-induced cell apoptosis in cultured hippocampal neurons through differential modulation of NR2A- and NR2B-containing NMDA receptors. Brain Res Bull 77:274-281.
Shim S, Ming GL (2010) Roles of channels and receptors in the growth cone during PNS axonal regeneration. Exp Neurol 223:38-44.
Varejao AS, Cabrita AM, Geuna S, Melo-Pinto P, Filipe VM, Gramsbergen A, Meek MF (2003) Toe out angle: a functional index for the evaluation of sciatic nerve recovery in the rat model. Exp Neurol 183:695-699.
Vogelaar CF, Vrinten DH, Hoekman MF, Brakkee JH, Burbach JP, Hamers FP (2004) Sciatic nerve regeneration in mice and rats: recovery of sensory innervation is followed by a slowly retreating neuropathic pain-like syndrome. Brain Res 1027:67-72.
Wang Y, Shen W, Yang L, Zhao H, Gu W, Yuan Y (2013) The protective effects of Achyranthes bidentata polypeptides on rat sciatic nerve crush injury causes modulation of neurotrophic factors. Neurochem Res 38:538-546.
Yang SH, Liao CC, Chen Y, Syu JP, Jeng CJ, Wang SM (2012) Daidzein induces neuritogenesis in DRG neuronal cultures. J Biomed Sci 19:80.
Yuan Y, Shen H, Yao J, Hu N, Ding F, Gu X (2010) The protective effects of Achyranthes bidentata polypeptides in an experimental model of mouse sciatic nerve crush injury. Brain Res Bull 81:25-32.
Zhou S, Chen X, Gu X, Ding F (2009) Achyranthes bidentata Blume extract protects cultured hippocampal neurons against glutamate-induced neurotoxicity. J Ethnopharmacol 122:547-554.
Copyedited by Aprico K, Raye W, Li CH, Song LP, Zhao M
10.4103/1673-5374.147948
Xiaosong Gu, Jiangsu Key Laboratory of Neuroregeneration, Co-innovation Center of Neuroregenration, Nantong University, 19 Qixiu Road, Nantong 226001, Jiangsu Province, China, nervegu@ntu.edu.cn.
Fei Ding, Jiangsu Key Laboratory of Neuroregeneration, Co-innovation Center of Neuroregenration, Nantong University, 19 Qixiu Road, Nantong 226001, Jiangsu Province, China, dingfei@ntu.edu.cn.
http://www.nrronline.org/
Accepted: 2014-10-10
- 中国神经再生研究(英文版)的其它文章
- Hydrogen sul fi de controls peripheral nerve degeneration and regeneration: a novel therapeutic strategy for peripheral demyelinating disorders or nerve degenerative diseases
- Activities of nicotinic acetylcholine receptors modulate neurotransmission and synaptic architecture
- A novel arti fi cial nerve graft for repairing longdistance sciatic nerve defects: a self-assembling peptide nano fi ber scaffold-containing poly(lactic-co-glycolic acid) conduit
- The effects of claudin 14 during early Wallerian degeneration after sciatic nerve injury
- Transplantation of human amniotic epithelial cells repairs brachial plexus injury: pathological and biomechanical analyses
- Long-term treatment with PP2 after spinal cord injury resulted in functional locomotor recovery and increased spared tissue

