A comparative study on antioxidant potentials,inhibitory activities against key enzymes related to metabolic syndrome,and anti-inflammatory activity of leaf extract from different Momordica species
Gunasekaran Nagarani,Arumugam Abirami,Perumal Siddhuraju
Bioresource Technology Lab,School of Life Sciences,Department of Environmental Sciences,Bharathiar University,Coimbatore 641046,Tamil Nadu,India
Abstract Momordica species are vegetable crops widely distributed in warmer regions of the world.In this work,we describe the antioxidant,enzyme inhibitory and anti-inflammatory effects of the leaves from three different species of Momordica.The present investigation was initially carried out to explore the possible link between antioxidant,enzyme inhibitory property of Momordica leaf extract and their total phenolic and flavonoid contents.The anti-inflammatory activity was tested by using carrageenan-induced paw edema.Our results illustrated an enhanced antioxidant power of wild species comparable with a commercial variety.In addition,the leaf extract of M.dioica (200 mg/kg) presented a significant anti-inflammatory activity toward carrageenan-induced paw edema in Wistar rats in comparison to indomethacin (10 mg/kg).The contents of flavonoids and total phenolic compounds could be correlated with the antioxidant and enzymes inhibition activities.The major bioactive compounds of phenolic acids and flavonoids such as gallic acid,chlorogenic acid,caffeic acid,ferulic acid,ellagic acid,catechin,rutin,and quercetin were identified.Our findings suggest that wild Momordica species contains higher potential antioxidant and anti-inflammatory activities than a commercial variety does,which could be tested as drug candidates against oxidative and inflammation-related pathological processes.
Keywords: Momordica;Antioxidant;In vitro antidiabetic;Cholinesterase;Anti-inflammatory
1.Introduction
Indigenous plants have been used for many years by different cultures around the world for the management of several ailments.In recent years,investigation on these wild species has become progressively important in the search for new,effective and safe therapeutic agents for the treatment of oxidative diseases and metabolic disorders.MomordicaL.genus belonging to Cucurbitaceae family is native to Asia and includes approximately 825 species of annual and perennial plants distributed mainly in warmer tropics including The Amazon,East Africa,The Caribbean,and South America.Well identified species in India areM.charantiaL.,M.balsaminaL.,M.dioicaRoxb.,M.sahyadrica,M.cochinchinensis(Lour.),M.subangulataandBlumesubsprenigera[1].The large fruit bitter gourd variety,which is available in the markets,is inferior in edible/qualitative traits (such as non-bitterness),abiotic stress tolerance (e.g.,drought tolerance),and resistance to several insect pests when compared with these wild variant/species of this genus[2].The green fruits and leaves are consumed as a vegetable as traditions for a time in Asia.Their leaves have many uses in traditional folk medicine.Leaf decoction has been used as antifungal,anti-inflammatory,anti-malarial,anti-parasitic,anti-septic,carminatives,digestive stimulant,febrifuge,menstrual stimulator,vermifuge purgative,and wound healer.In addition,it has been effectively used to treat diabetes and cancer.Pharmacological investigations found thatMomordicaleaves possessed many bioactivities,such as anti-tumor,hypoglycemic,anti-diarrhoeal,antioxidant,hepato-protective activity and so on [3–5].Phytochemical studies revealed the presence of alkaloid,flavonoids,sterols,anthraquinones,and phenols,which represented the main active components inM.charantialeaves[6].In addition,Kuguacin J and karavilagenin D which belonged to triterpenoid have been reported for the treatment of carcinogenesis and prostate cancer[7].However,inMomordicafamilyM.charantiais the large fruit-cultivated variety and has been widely recognized by people,while other varieties and wild species which are less popular are unknowingly getting eradicated from the ecosystem.
Oxidation reaction induced the production of free radicals which act as a causative agent for several degenerative diseases by adducting with biomolecules such as lipid,protein,and DNA.Hence animals maintain complex systems of multiple types of antioxidants in their body.Even though living organisms have become more susceptible to more free radicals by exposure to pollutants,radiation,drugs,inappropriate food processing and cosmetics,it has been shown that plant polyphenols are renowned for their abilities to quench these free radicals and protect the body[8].Inflammation is a physiological defense mechanism and a symptom of many diseases including asthma,obesity,and cardiovascular diseases.Numerous synthetic drugs have been used to treat these infectious diseases but sometimes these are associated with adverse side effects such as hyper sensitivity,allergic reaction and immunosuppression in human body[9].Hence this situation forced researchers to discover novel anti-inflammatory agents from natural resources.
Type 2 diabetes mellitus (T2DM) and Alzheimer’s disease(AD)are both more prevalent with aging.Studies suggested that anti-diabetic drugs might be beneficial in treating AD patients.Decreased level of acetylcholine in the brain,which is responsible for learning,memory,behavior and emotional responses,causes several neuropathological conditions and AD.Acetylcholine transferase catalyses the production of Ach and the expression occurred in insulin and IGF-I receptor-positive cortical neurons.Therefore insulin impairment leads to reduction of Ach,which explains the possible relationship between diabetes mellitus and AD[10].At the same time acetylcholine esterase enzyme also reduces the acetylcholine production so it is necessary to inhibit acetylcholine esterase enzyme activity[11].In another way,lack of insulin secretion and/or insulin action disturbed the carbohydrate,fat and protein metabolism that results in inadequate glucose production and finally leads to several medical complications.One of the beneficial and conventional treatments for diabetes is to increase glucose absorption through the inhibition of carbohydrate hydrolyzing enzymes such asαamylase andα-glucosidase in the digestive organs.Tyrosinase enzyme is responsible for the production of melanin in human skin.Hyper activity of this enzyme leads to dermatological disorders such as melasma,freckles,and age spots[12].This study initiates researches toward comparing and understanding the reasons beneath the medicinal uses of leaf extracts from differentMomordicaspecies by using a range ofin vitroandin vivoassays.As of now far the antioxidant,enzymes inhibitory,and anti-inflammatory potential of this wild species has not been evaluated.
2.Materials and methods
2.1.Plant materials
Fresh plant leaf materials of indigenousM.dioicaand cultivatedM.charantiawere collected from Kodiakarai and Thamaraipulam,Nagapattinam district,Tamilnadu,India during January 2011,whereas indigenousM.charantiavar.muricatawas collected from Buvanagiri,cuddalore district,Tamil Nadu,India in August 2011.The identity was confirmed by comparing voucher specimens available in the Botanical Survey of India,Coimbatore.After collection,the plant materials were washed under running tap water to remove the surface pollutants and dried at(45±2)°C depending on the dryness of samples.Time was maintained and the final moisture content of the leaves ofM.dioica,M.charantiaandM.charantiavar.muricatawere 10.45%,7.1%,and 8.4%,respectively,which were determined using MA35 moisture analyzer (Sartorius AG,Germany) at 105°C.The driedMomordicaleaves were milled to a particle size of 70–150 μm using laboratory blender and stored in separate screw cap bottles at −4°C.
2.2.Solvent extraction
The leaf samples(10 g)were subjected to successive solvent extraction with 95%of ethanol(1:7,m/V)for 48 h by maceration at room temperature and filtered through Whatman No.4 filter paper.The residues were re-extracted with 95% ethanol (1:5,m/V),as described above,for 24 h.The solvent combined extract was evaporated at 40°C and stored at 4°C for further analysis.
2.3.Chemicals
All the chemicals used in this study were of analytical grade.2,2-Azinobis (3-ethyl benzothiazoline-6-sulfonic acid)diammonium salt (ABTS),BHA (butylated hydroxy anisole),2,2'-diphenyl-1-picryl-hydrazyl (DPPH),β-carotene,linoleic acid,2,2'-azobis(2-amidinopropane)dihydrochloride(AAPH),α-amylase,α-glucosidase,acetylthiocholine iodide,tyrosinase,β-glucuronidase enzyme and indomethacin were purchased from Sigma Chemicals Co.(St.Louis,MO,USA).All the other chemicals were obtained from HiMedia Laboratories(Mumbai,Maharashtra,India).
2.4.Determination of total phenolics and tannins contents
The total phenolics and tannins were measured [13]from tannic acid calibration curve (3–15 μg range;y=0.08x–0.01;R2=0.99).One mL the sample extract was transferred to a test tube and 0.5 mL Folin–Ciocalteu reagent and 2.5 mL sodium carbonate solution(20%,m/V)were added.After an incubation period of 40 min in the dark,the absorbance was recorded at 725 nm with UV-visible spectrophotometer(Cyberlab-UV100,USA) against the reagent blank.Using the same extracts and method,the content of tannins were estimated after treatment with polyvinylpolypyrrolidone(PVPP).
2.5.Determination of total flavonoids content
Total flavonoid content was measured according to the method of Zhishen et al.,[14],outlined by Siddhuraju and Becker [15].Sample extract was added with 0.3 mL of 5%sodium nitrite and was mixed well.After 5 min of incubation,0.3 mL of 10% aluminum chloride solution was added.After 6 min,2 mL of 1 mol/L sodium hydroxide was added to the mixture and the volume was made up to 10 mL with water.The absorbance was measured at 510 nm with UV–vis spectrophotometer.Total flavonoids were measured from rutin(20–100 μg)standard curve and expressed as mg rutin equivalents/g extract.
2.6.Separation of phenolics in Momordica leaf by HPLC
2.6.1.Extraction and hydrolysis
The phenolic acid and flavonoids in investigated samples were extracted using the procedure described by Siddhuraju and Becker[15].The ethanolic extract of the leaf samples(10 mg)were weighed in a 10 mL Erlenmeyer flask and then dispersed in 2 mL of 62.5%aqueous methanol containing 200 mg/L BHA.The mixture was then ultrasonicated for 5 min,and 1 mL of 6 mol/L HCl was added to this extract with careful mixing.The extraction solution consisting of 1.2 mol/L HCl in 50%aqueous methanol(V/V)was thus obtained.The sample was bubbled with nitrogen for 40–60 s,after which the flask was sealed tightly.Hydrolysis was carried out in a water bath at 90°C for 2 h with shaking.After hydrolysis,the extract was cooled,filtered,subsequently made up to 5 mL with methanol,and sonicated for 5 min.Approximately 2 mL was filtered through a 0.45 μm membrane filter prior to injection in HPLC.
2.6.2.Identification and quantification of phenolics
Chromatographic separation of phenolics was done by HPLC equipped with Shimadzu LC-6AD pumps,SPD-20A prominence UV-vis detector and a LUNA C-118 column(4.6 mm×250 mm,5 μm).Gradient elution was employed for phenolic acid and flavonoids.Solution A contained 3%aqueous acetic acid,and Solution Bcontained a mixture of 3%acetic acid,25%acetonitrile and 72%water.Gradient elution was employed as follows:0–40 min,100%to 30%A,70%B at a flow rate of 1 mL/min;40–55 min,30%to 10%A,70%to 90%B at a flow rate of 1 mL/min [16].Operating conditions were as follows:column temperature 40°C;injection volume 20 μL;UV-diode array detection at 280 nm.
2.7.In vitro antioxidant activities
2.7.1.Free radical scavenging activity on DPPH•
The antioxidant activity of extracts and standards(BHA,rutin and tannic acid) was measured in terms of hydrogen donating ability using a stable,commercially available organic and nitrogen centered DPPH•by the method of Brand-Williams et al.[17]with slight modifications.Sample extracts prepared in methanol were mixed with 3.9 mL methanol containing DPPH•(0.025 g/L)and incubated in the dark for 30 min.The absorbance was measured at 515 nm with UV–visi spectrophotometer.The trolox standards were prepared in the range of 0–2.5 mmol/L.The concentration of DPPH•was calculated from a trolox standard curve and expressed as mmol trolox equivalents/g extract.
2.7.2.Antioxidant activity by the ABTS•+assay
The ABTS•+decolorization assay was performed to evaluate the radical scavenging ability of crude extracts by the method of Re et al.[18]with slight modification made by Siddhuraju and Becker [15].ABTS•+was generated by adding 2.45 mmol/L potassium persulfate to 7 mmol/L ABTS and incubated in the dark at room temperature for 12–16 h.This stock solution of ABTS•+was diluted with ethanol to give an absorbance of(0.70±0.02)at 734 nm,which acted as a positive control.Ten microliters crude extract(prepared in ethanol)was mixed with 1.0 mL diluted ABTS•+solution and incubated at 30°C for 30 min.The absorbance value was measured at 734 nm with UV–vis spectrophotometer.Trolox standard was also prepared(in ethanol: 0–1.5 mmol/L) to get the concentration response curve.The unit of trolox equivalent antioxidant activity(TEA)was defined as the concentration of trolox with theequivalent antioxidant activity expressed as mmol/g of extracts.The TEA of BHA,rutin and tannic acid was also measured by ABTS•+method for comparison.
2.7.3.Ferric reducing antioxidant power(FRAP)assay
FRAP assay can be used to evaluate the electron donating ability of antioxidants according to the method of Pulido et al.[19].An aliquot of 30 μL sample was mixed with 90 μL water and 900 μL FRAP reagent (2.5 mL of 20 mmol/L of 2,4,6-tri-2-pyridinyl-1,3,5-triazine(TPTZ)in 40 mmol/L of HCl,2.5 mL of 20 mmol/L of ferric chloride,25 mL of 0.3 mol/L of acetate buffer(pH 3.6))and incubated at 37°C for 30 min.After incubation,the absorbance values were recorded at 593 nm with UV–vis spectrophotometer.Known ferrous sulphate contents ranging from 400 to 2000 μmol were used to generate the calibration curve.From the curve,the ferrous ions reduced by the sample were calculated using regression equation.The antioxidant activity was expressed as amount of extract required to reduce 1 mmol of ferrous ions.The antioxidant activity of samples was compared with the following standards: BHA,rutin and tannic acid.
2.7.4.Nitric oxide radical scavenging activity
Nitric oxide radical scavenging activity was measured by the method of Marcocci et al.[20].All sample extracts or standards (ascorbic acid and quercetin) (500 μg) were mixed with sodium nitroprusside (final concentration 5 mmol/L) in phosphate buffered saline,pH 7.4 to the final volume of 1 mL and incubated at 25°C for 150 min.After incubation,the reaction mixture was mixed with Griess reagent(1%sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride in 5% ortho phosphoric acid).The absorbance was measured at 540 nm with UV–vis spectrophotometer.The degree of nitric oxide radical scavenging activity(NRSA)was calculated as follows:
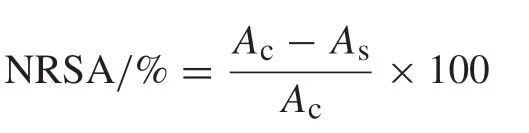
whereAcis the absorbance of the control;Asis the absorbance of the sample.
2.7.5.Superoxide anion radical scavenging assay
The superoxide anion radical(O2•−)scavenging capacity of standards(BHA,catechin,trolox and rutin)and sample extracts was determined by the method of Martinez et al.[21]for the determination of superoxide dismutase with some modifications made by Dasgupta and De[22]in the riboflavin-light-nitroblue tetrazolium system.Each 3 mL of reaction mixture consisting of 50 mmol/L phosphate buffer (pH 7.8),13 mmol/L methionine,2 μmol/L riboflavin,100 μmol/L EDTA,75 μmol/L NBT and 1 mL extract/standard was kept for 10 min under illumination with 20 W fluorescent lamps.The production of blue formazan was monitored and recorded at 560 nm with UV-vis spectrophotometer.The degree of superoxide anion radical scavenging activity(SRSA)was calculated as follows:

whereAcis the absorbance of the control;Asis the absorbance of the sample.The scavenging activity was compared with the positive standards(150 μg)of BHA,rutin and trolox.
2.7.6.Hydroxyl radical scavenging activity
Hydroxyl radical scavenging ability of extract and standard(catechin)was measured according to the method of Klein et al.[23].Samples and standards were mixed with 1 mL iron–EDTA solution (0.13% ferrous ammonium sulfate in 0.26% EDTA),0.5 mL of 0.018%EDTA and 1 mL DMSO solution(0.85%in phosphate buffered saline 0.1 mol/L,pH 7.4).The reaction was terminated by the addition of 1 mL ice cold trichloroacetic acid(TCA)(17.5,m/V).Then,3 mL Nash reagent(7.5 g ammonium acetate,0.3 mL glacial acetic acid,0.2 mL acetyl acetone and 100 mL distilled water) was added to the above mixture and incubated at room temperature for 15 min and the absorbance values were recorded at 412 nmwith UV–vis spectrophotometer.A control was also run with phosphate buffer instead of ascorbic acid.The percentage of hydroxyl radical scavenging activity(HRSA)was calculated using the following formula:
The activity was compared with the positive standard catechin(250 μg).
2.7.7.β-Carotene/linoleic acid bleaching activity
The antioxidant activity of sample extracts and standards(BHA,rutin and trolox)was analyzed according to the method of Taga et al.[24]with slight modifications.Two mgβ-carotene was dissolved in 1 mL chloroform containing 40 mg linoleic acid and 400 mg Tween 40.The chloroform was removed by rotary vacuum evaporator at 45°C for 4 min and 100 mL distilled water was added slowly to the semisolid residue with vigorous agitation to form an emulsion.A 5 mL aliquot of the emulsion was added to a tube containing standards (50 μg) and sample extracts(250 μg)and the absorbance was measured at 470 nm with UV–vis spectrophotometer,immediately against a blank consisting of the emulsion withoutβ-carotene.The tubes were placed in a water bath at 50°C and the absorbance measurements were conducted at 120 min.All determinations were carried out in triplicates.The antioxidant activity(AA)of the extracts was evaluated in terms of bleaching ofβ-carotene using the following formula:

whereAE0andAC0are the absorbance values measured at 0 min of the incubation for test sample and the control,respectively;AE120andAC120are the absorbances measured in the test sample and the control,respectively,after incubation for 120 min.
2.8.In vitro antidiabetic activity
2.8.1.α-Amylase inhibition activity
The sample extracts (250 μg) were mixed with 100 μL of 0.02 mol/L sodium phosphate buffer (pH 6.9) and 100 μLαamylase solution(4.5 units/mL/min)and pre-incubated at 25°C for 10 min.Then,100 μL of 1%starch solution was added and incubated at 25°C for 30 min and the reaction was stopped by the addition of 1.0 mL dinitrosalicylic acid reagent (1 g 3,5-dinitrosalicylic acid in 20 mLof 2 mol/LNaOH+50 mLdistilled water+30 g Rochelle salt.The contents were dissolved and made up to 100 mL with distilled water).The test tubes were then incubated in a boiling water bath for 5 min and then cooled at room temperature.The reaction mixture was then diluted 10 times with distilled water and the absorbance was measured at 540 nm.The readings were compared with the control(extract was replaced by the buffer)andα-amylase inhibition activity/%was calculated[25].
2.8.2.α-Glucosidase inhibition activity
The sample extracts (250 μg) were mixed with 100 μL 0.1 mol/L phosphate buffer(pH 6.9)and 100 μLα-glucosidase solution(1 unit/mL/min)and incubated at 25°C for 5 min.After the pre-incubation,100 μLp-nitrophenyl-α-D-glucopyranoside(5 mmol/L) solution was added and the reaction mixture was incubated at 25°C for 10 min.After the incubation,the absorbance was recorded at 405 nm andα-glucosidase inhibition/%was calculated[25].
2.9.Tyrosinase inhibitory activity
Tyrosinase inhibitory activity was determined according to the modified method of Chang et al.[26].An aliquot of 20 μL of tyrosinase (1000 U/mL in 50 mmol/L phosphate buffer,pH 6.8)was mixed with 100 μL extract(dissolved in DMSO)and 1.9 mL of 50 mmol/L phosphate buffer (pH 6.8).The reaction mixture was incubated at 25°C for 5 min.Then,880 μLL-3,4-dihydroxyphenylalanine(L-DOPA)as the substrate in the same buffer was added to start the reaction.The increase in absorbance at 475 nm was monitored with the spectrophotometer.Kojic acid was used as a positive control.The tyrosinase inhibitory activity was calculated using the formula

whereA0is the absorbance at 475 nm with DMSO instead of the tested sample andA1is the absorbance at 475 nm with the tested sample.
2.10.Determination of anti-acetylcholinesterase activity
Acetylcholinesterase (AchE) inhibitory activity was measured by slight modification of spectrophotometric method of Ellman et al.[27].An aliquot of 300 μL of 100 mmol/L sodium phosphate buffer (pH 8.0),10 μL sample solution and 40 μL AChE (5.32×10−3U) solution were mixed and incubated for 15 min at 25°C,and then 20 μL 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) (0.5 mmol/L) was added.The reaction was then initiated by the addition of acetylthiocholine iodide(0.71 mmol/L).The hydrolysis of this substrate was monitored spectrophotometrically at 412 nm.Percentage of inhibition of AChE or butyrylcholinesterase(BChE)enzymes was determined by comparison of reaction rates of samples relative to blank samples using the formula

whereEis the activity of enzyme without the test sample,andSis the activity of enzyme with the test sample.Eserine was used as a reference compound.
2.11.Experimental animals
Male Wistar rats (120–150 g) were procured from animal house of Nandha College of Pharmacy and Research Institute,Erode,TN,India.The animals were provided with adequate environmental conditions (temperature–(24±2)°C;relative humidity–(50±10)%;and (12:12) light:dark cycle) with the standard commercial pellets(M/s.Hindustan Lever Ltd.,Mumbai,Maharashtra,India) and purified water ad libitum.All the experiments were performed with the permission from Institutional Animal Ethics Committee(688/2/C-CPCSEA)and were in accordance with the guidelines of Committee for the purpose of control and supervision of experiments on animals (CPCSEA).
2.12.Anti-inflammatory activity
For the experiment,the male Wistar albino rats(120–150 g)were divided into eight groups (n=6).Animals were fasted overnight and were divided into control,standard and different test groups.The first group received distilled water(10 mL/kg),while the second group was treated with indomethacin(10 mg/kg).Remaining groups were administered with the two different concentrations of ethanol extract ofMomordicaleaves(100 and 200 mg/kg body weight).Acute inflammation was induced by the subplantar administration of 0.1 mL of 1%carrageenan (in 1% CMC,m/V) in the right hind paw of the rats.The animals were pretreated with the drug 1 h before the administration of carrageenan.The thickness(mm)of the paw was measured immediately and at 0,1,2,3 h interval after the carrageenan injection using vernier caliper (Model 2061,Mututoyo Digimatic Caliper,Japan) [28].The percent inhibition of paw thickness for treated groups was calculated by comparing with mean paw thickness of control group.Inhibition/%=100(1 −Vt/Vc),(Vc–control mean paw thickness,Vt–test mean paw thickness).
2.13.Statistical analysis
The results were expressed as mean±SD and statistical analysis was carried out by analysis of variance(ANOVA)followed by Dunnet’s test and Duncan’s multiple tests.P<0.01 andP<0.05 were considered as indicative of significance,as compared to the control group.All calculations were performed using SPSS(Statistical Package for the Social Sciences)version 13.0(SPSS Inc.,Chicago,Illinois,USA)and Graph Pad Instant software version 3.01.
3.Results and discussion
3.1.Total phenolic content of leaf extracts
The extraction yields,total phenolics,tannins and total flavonoid contents ofMomordicaleaf extracts were shown in Table 1.The total phenolic content ofMomordicaleaf extracts was in the range of 33.9-49.31 TAE/g extracts (Table 1).The tannin levels inM.charantiaLinn,M.charantiavar.muricataandM.dioicawere 8.51,19.25 and 14.9 TAE/g extract respectively(Table 1).Kubola and Siriamornpun[16]reported higher levels of total phenolics inM.charantialeaf than the present results,which could be due to the differences in concentration of the samples.The results revealed that marked differences were observed among wild and cultivated species ofMomordica.This was in agreement with the reports of Petkovse et al.[29]who found that wild species of strawberry,raspberry,and blackberry had 2- to 5-fold more total phenolics compared to cultivated plants.The differences in total phenolic content of plants were attributed to the factors such as genotype,site location,climatic conditions,technological measures,year,and so on[29].Total flavonoid contents of different leaf extracts ofMomordicaspecies ranged from 72.83–94.16 mg of RUE/g extract and variations across species were significant (Table 1).As compared with the cultivated form,both wild species possessed higher total phenolics,tannins and flavonoid contents and would justify their comparative advantage over the common cultivable species.
3.2.Identification of phenolic compounds in Momordica leaves
A chromatogram of phenolic compounds identified inMomordicaleaf samples are illustrated in Fig.1.On the basis of the HPLC data,the following phenolic acid and flavonoid compounds including gallic acid,chlorogenic acid,catechin,caffeic acid,ferulic acid,quercetin and ellgic acid have been identified and quantified (Fig.1 and Table 2).Some major phenolic acid and flavonoids such as chlorogenic acid,caffeic acid,catechin and quercetin were found in both cultivated and indigenous variety ofM.charantia.However,higher amounts of phenolic acid and flavonoids were found inM.charantiavar.muricatathan other species.The main components ofM.dioicawere gallic acid,rutin,ferulic acid and ellagic acid.When compared to both varieties ofM.charantia,M.dioicahad lower concentration of phenolic acid;at the same time some unidentified peaks were found which might be responsible for the bioactivities ofM.dioica.Gallic acid,catechin,caffeic acid and ferulic acid contents ofM.charantiawere found to be higher in present study than previously reported by Kubola and siriamornpun [16].This difference may be attributed to locality of the samples,genetic variability,analytical method used and solvent used for the extraction procedure.Interestingly,the phenolic acids and flavonoids identified in these wild species have been proved as potent antioxidant agents in many plants[30].
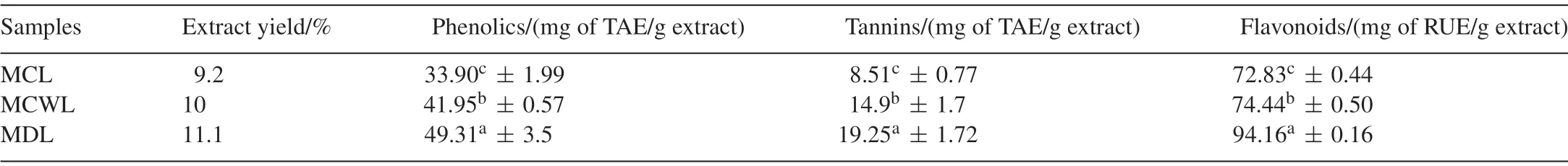
Table 1 Extract yield,total phenolics,tannins and flavanoid content of leaf from wild and cultivated variety of Momordica sp.
3.3.DPPH• scavenging activity
DPPH•is a stable organic-free radical with absorbance at 517 nm.The scavenging effect of samples ranged from 6358.48 to 6790.35 mmol TE/g extract (Table 3).Higher scavenging capacity was shown inM.dioica(6790.35 mmol TE/g extract)than other leaf extracts.The present results forM.dioicashowed similar DPPH•scavenging activity with that of Jain et al.[31].When compared with standards,the scavenging activity of the extract was found to be low (P<0.05) because of the amount of extractable phenolics,molecular weight of phenolics,the number of aromatic rings,nature of hydroxyl group substitution and the formation of complexes with proteins[32].Antioxidant activity toward DPPH•was found to be positively correlated with their phenolics,flavonoids and tannin contents(R2=0.975,0.968,0.843,respectively)of leaf samples,which can donate more hydroxyl groups for reducing a number of DPPH•(Table 3).The results presented above are in agreement with the fact that the total phenolics,tannin and flavonoid contents are major contributors to the antioxidant activity of many vegetables,which is in accordance with the report of Sasipriya et al.[33].
3.4.ABTS•+assay
Several studies reported the fact that phenolics act as potent scavenger of ABTS•+through hydrogen atom donation,electron transfer or even a combination of the two mechanisms[34].The results of the ABTS•+scavenging activity are as shown in Table 3.Similar to the findings in DPPH•assay,M.dioicashowed the highest antioxidant activity (22,463.81 mmol TE/g extract),andM.charantiacultivated form showed the lowest radical scavenging activity (12,790.64 mmol TE/g extract).These results seem to correlate with the total phenolics,tannin and flavonoids contents of leaf extracts and the correspondingR2values are 0.874,0.830,and 0.994,respectively.Interestingly,the results of antioxidant activity ofM.charantiaextracts by ABTS•+assay in our report are two-fold higher than that in the previous report[35].
3.5.FRAP assay
The reducing abilities of different leaf extracts determined by FRAP method were measured spectrophotometrically by their absorbance at 700 nm and summarized in Table 3.FRAP is an excellent method based on the electron transfer mechanismand very useful to distinguish dominant mechanisms of antioxidant action,in combination with the other methods [36].The antioxidants present in theMomordicaleaf extracts caused their reduction of Fe3+/ferricyanide complex to the ferrous form,and thus proved the reducing power.The highest reducing ability was observed inM.dioica(14,832.98 mmol (FeII)/g extract),and significant differences existed among the reducing ability of all the extracts (Table 3).This variation may be attributed to the varied phytochemical contents.The reducing power of leaf extracts in this investigation that was positively correlated with phenolics,tannins and flavonoids(R2=0.967,0.951,0.937,respectively),which might act as electron donors.

Table 2 Contents of phenolic acid and flavonoid compounds(n=3).
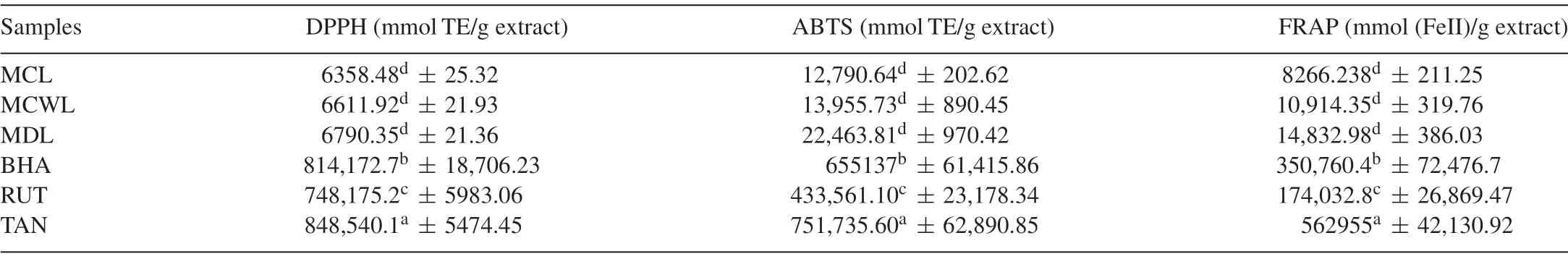
Table 3 DPPH•,ABTS•+ and FRAP assay of leaf extract of M.charantia Linn.,M.charantia var.muricata and M.dioica(n=3).
3.6.Nitric oxide scavenging activity
The nitric oxide (NO) scavengers from the extracts compete with oxygen,leading to reduced production of nitrite ions.Fig.2 presents the scavenging ability of different leaf extracts ofMomordicaagainst NO.Out of the three leaf extracts,M.charantiavar.muricataextracts showed the highest nitric oxide inhibition ability (78%) compared to other extracts and standards,ascorbic acid and quercetin.No significant correlation was found between NO radicals scavenging activity and phenolics.Hence,bioactive substances other than phenolics,tannins and flavonoids may be the reason for scavenging activity inMomordicaleaves.
3.7.Hydroxyl radical scavenging activity
The results obtained in this study demonstrate that leaf extract ofMomordicaspecies had appreciable hydroxyl radical scavenging activity.As shown in Table 3,results obtained by hydroxyl radical scavenging assay once again revealed that wild species had the highest antioxidant activity,whereas commercial variety had the lowest activity.M.dioicashowed the highest hydroxyl radical scavenging ability(80%)(Fig.2).Our results varied from those reported by Kubola and Siriamornpun[11]who found lower hydroxyl radical scavenging activity of aqueous extracts ofM.charantialeaf.These differences could be mainly due to the different procedures used to prepare sample extracts.Phenolics (R2=0.905,P<0.01),tannins (R2=0.916,P<0.05) and flavonoids (R2=0.619,P<0.05) inMomordicaleaves highly correlated with hydroxyl radical scavenging activity.
3.8.Superoxide anion-scavenging activity
Although superoxide anion is a weak oxidant,it plays an important role in the formation of other reactive oxygen species,such as hydrogen peroxide,hydroxyl radical,or singlet oxygen in living systems which contribute to oxidative stress.The scavenging ability of various leaf extracts on superoxide radicals are shown in Fig.2.Compared to BHA and rutin,the superoxide scavenging activity of the extract was found to be low(P<0.05).In spite of this,M.dioicaextract behaves as a powerful superoxide anion scavenger(51%)and showed comparatively similar activity to trolox.The present study suggested that there is a strong correlation between superoxide radical scavenging activity and phenolics,tannins and flavonoid contents of the plant(R2=0.970,0.946,0.938,respectively).
3.9.β-Carotene/linoleate bleaching assay
The antioxidant capacity by usingβ-carotene bleaching assay inMomordicaleaf extract is shown in Fig.2.The antioxidant capacity of leaf samples was determined by stabilizing the yellow colorβ-carotene and hindering the extent of bleaching by hydrogen peroxide radical through the heat-induced oxidation of linolenic acid.The biological function ofβ-carotene was destroyed via free radical-mediated oxidation,which breaks the 11 pairs of sensitive double bonds[37].On oxidation it loses its color,but in the presence of leaf extracts bleaching ofβ-carotene is inhibited by neutralizing the linoleate free radical in the system.Results showed thatM.dioicaleaf extract has the highest potential to inhibit free radical-mediated oxidation ofβ-carotene(64%),followed byM.charantiavar.muricata(56%) andM.charantia(47%).
3.10.Carbohydrate-hydrolyzing enzymes inhibition
α-Amylase andα-glucosidase are the key enzymes in the digestive system,which hydrolyze the oligosaccharide into monosaccharide prior to absorption.They are correlated with increase in post-prandial glucose levels in small intestine.Hence retardation of carbohydrate digestion by inhibition of enzymes such asα-amylase andα-glucosidase would play a key role in reducing the formation of glucose monomers,which controls the risk of diabetes and obesity[38].Theα-amylase inhibitor prevents starch digestion by completely blocking the access to the active site and promotes weight loss concurrently,thereby it is used to manage type 2 diabetes.In an array to explore thein vitroanti-diabetic activity,various leaf extracts fromMomordicaspecies were screened for theα-amylase andα-glucosidase inhibitory property.Initial screening of various extracts showed that the ethanol extract ofM.dioicaleaf had significantly higherα-amylase(73.5%)andα-glucosidase(50.4%)inhibition activity (Fig.3).The ability ofMomordicaleaves to inhibit both enzymes may be related to phenolic contents since total extracts showed the highest inhibiting activity.The inhibitory activity ofα-amylase andα-glucosidase is strongly correlated with the contents of flavonoids and phenolics (R2=0.996,0.842) and(r2=0.960,0.956,0.921).Several studies revealed that the plant phytochemicals such as phenolics,flavonoids,tannin,saponin,terpenes and glycoproteins are responsible for the inhibitory action of these enzymes[39].The obtained results are better than those obtained forM.charantiaseeds with maximum inhibition of 38%forα-amylase and 53%forα-glucosidase respectively.At the same time polypeptide-k isolated from seeds ofM.charantiawas demonstrated to inhibitα-amylase andα-glucosidase with a percentage of 35%and 79%respectively[40].
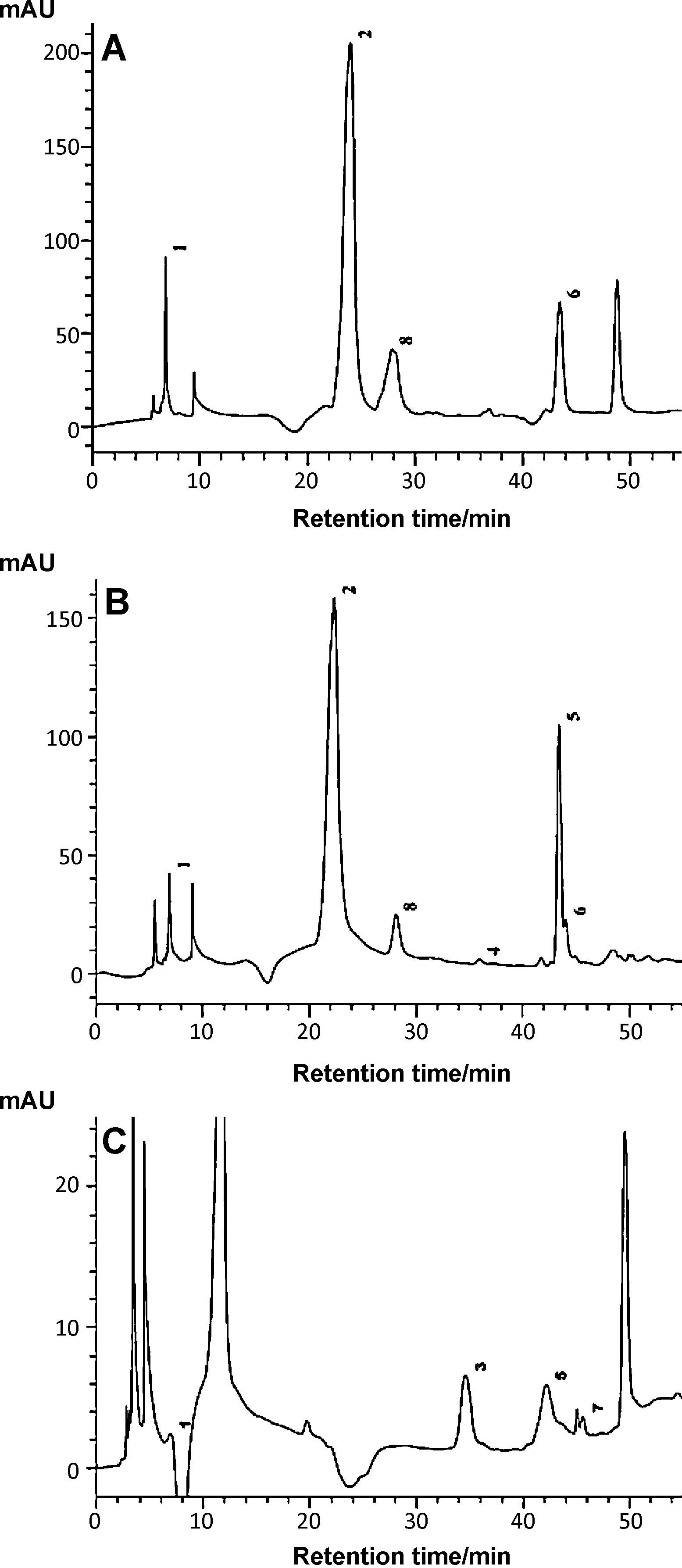
Fig.1.HPLC chromatograms of Momordica leaves recorded at 280 nm:A.M.charantia;B. M.charantia var. muricata;C. M.dioica.Peak: 1.Gallic acid;2.Chlorogenic acid;3.Rutin;4.Caffeic acid;5.Ferulic acid;6.Quercetin;7.Ellagic acid;8.Catechin.

Fig.2.Nitric oxide,superoxide,hydroxyl radical scavenging activity and inhibition of β-carotene bleaching of M.charantia Linn.M.charantia var.muricata and M.dioica leaf extract (n=3).ASC,ascorbic acid;QUE,quercetin;CAT,catechin;TRO,trolox;BHA,butylated hydroxyanisole;RUT,Rutin.

Fig.3. α-Amylase, α-glucosidase,acetyl cholinesterase,tyrosinase inhibitory activity of M.charantia Linn. M.charantia var. muricata and M.dioica leaf extracts(n=3).
3.11.Anti-AChE activity
Acetylcholinesterase catalyses the breakdown of acetylcholine,one of the neurotransmitter in the synaptic cleft of brain,which leads to several neurological disorders such as Alzheimer’s disease,senile dementia,ataxia,and myasthenia gravis [41].The leaf extract of threeMomordicaspecies was tested against AChE enzyme and the data obtained underlined an appreciable inhibition rate.All the extract showed comparable activity sinceM.dioica(78.8%)showed the highest AChE inhibitory activity,followed byM.charantia(77.6%)(Fig.3).It was previously reported that flavonoid derivatives were potent inhibitors of AChE enzyme [42].In agreement with that fact,M.dioicahad the highest flavonoid content than other extracts.Besides,Lopez and Pascual-Villalobos [43]reported that terpenoids is also a potent AChE inhibitor.Several terpenoids such as Kuguacin J isolated fromM.charantialeaf could be suggested for the higher inhibitory activity of AChE[7].
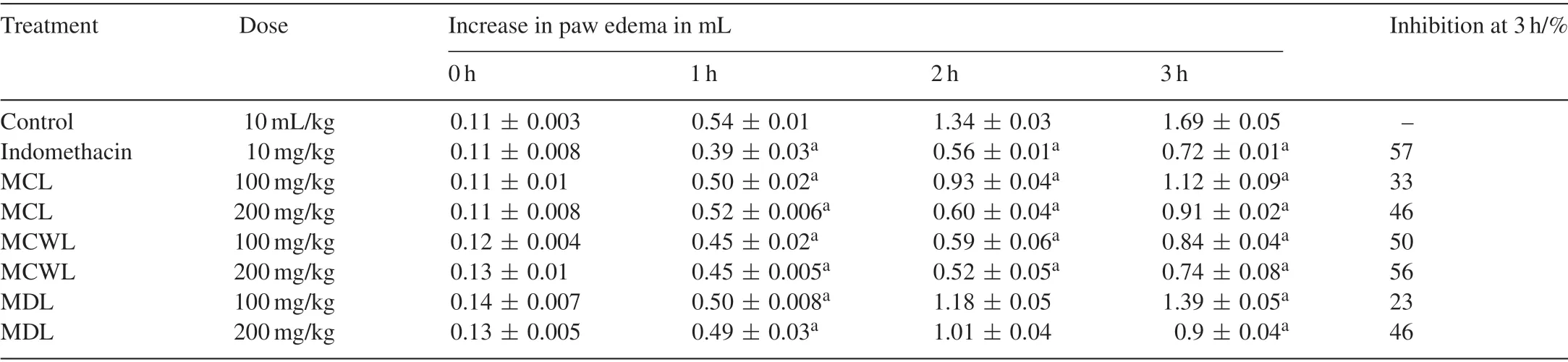
Table 4 Anti-inflammatory activity of different Momordica leaves extracts on carrageenan-induced paw edema in rats.
3.12.Tyrosinase inhibition
Malanogenesis plays an important role in protecting the skin from sun-related injuries and is principally responsible for skin color,but at the same time abnormal hyperpigmentation such as freckles,chloasma,and lentigines can be serious esthetic problems [44].To sort out this problem several tyrosinase inhibitors have been widely used to regulate hyperpigmentation in cosmetic industry.Tyrosinase is one of the major targets in screening inhibitors of melanin synthesis.In addition,some studies reported that melanogenisis have been associated with oxidative stress and the effects of antioxidant in down-regulating UV-induced melanogenesis [45].The effects ofMomordicaleaves extract on tyrosinase activities are shown in Fig.3.It was found that all extracts had potent inhibitory effects onL-DOPA oxidase activity of tyrosinase enzyme,and the highest activity was registered inM.dioicawith 74% of inhibition.The highest inhibitory activity was strongly correlated with phenolics,tannins and flavonoids (R2=0.973,0.864,and 0.821,respectively).Flavonoid-derived compounds are competitive inhibitors of tyrosinase substrates [46].Peng et al.[47]reported that compounds having higher antioxidant activity and radical scavenging ability may be linked with tyrosinase inhibitory effects.In the present study the potent inhibition activity ofMomordicaleaves is probably extracts due to their higher antioxidant activity.
3.13.Anti-inflammatory activity
TheMomordicaleaves extract exhibited a dose-dependent anti-inflammatory activity and significantly (P<0.01) reduced the rat paw edema volume induced by carrageenan.It was found that theM.charantiavar.muricata(200 mg/kg) had significant inhibitory effect on the edema formation with a value of 56%at 3 h after carrageenan administration(Table 4).The other two species ofMomordicaleaves also significantly inhibited the carrageenan-induced paw edema obviously (P<0.05) in a dose-dependant manner.The development of edema induced by carrageenan can be divided into three different phases.The early (0–90 min) phase takes place with the release of histamine,serotonin and kinins.The second phase is associated with bradykinin(90–180 min).The third phase is mediated by prostaglandins,and the continuity between the two phases is provided by kinins,in which the edema reaches its highest volume[48].The release of histamine leads to outward movements of proteins and fluid into the extracellular spaces,leading to the initial stage of inflammation,while the later phase induces the production of prostaglandin which is responsible for the formation of edema.The three leaves extracts fromMomordicaspecies exhibited a moderate inhibitory activity at the early phase but effectively inhibited the paw volume during the last phase(3 h after carrageenan injection).Based on this fact,it may be suggested that the potent inhibitory activity of leaves is due to the inhibition of prostaglandin,one of the powerful vasodilator in acute inflammation.In comparison to the earlier studies on aqueous and alcoholic extracts of leaves and aerial parts ofM.charantia,the extract in our study was found to be an efficacious and potent anti-inflammatory agent [49].Recent studies revealed the possible relationship between the free radicals and inflammatory processes.The phytochemicals,flavonoids and triterpenoids isolated from plants have been effectively neutralized the free radicals which attenuate the inflammation and possess significant anti-inflammatory effects[28].The presence of different triterpenoids such as momordicoside,kuguaglycoside,karavilagenin,goyaglycosides and Kuguacin J were reported inM.charantialeaves [50].It is,therefore,possible that the anti-inflammatory effects observed with this extract may be attributable to its flavonoid and triterpenoid components.
4.Conclusions
It may be concluded that the dietary phenolic extracts of leaf samples from threeMomordicahave potential antioxidant,enzyme inhibition and anti-inflammatory activities.Some of the major phenolic acid and flavonoids have been identified as gallic acid,quercetin,catechin,etc.Additional work is needed to determine whether these phenolic constituents are responsible for the antioxidant activity of theMomordicaleaf extract.In addition,a screening test of ethanolic extract ofMomordicaleaves appears to reveal greater activity in carageenan-induced paw edema.These effects have been correlated to the flavonoid and total phenolic contents of the leaf,indicating that phenolic compounds could be the major contributors to these activities.Furthermore,the quantitative and qualitative evaluation of other phenolic acid and flavonoids is needed and the toxical and functional assessment of these leaf extracts in food system are important.For example,Momordicaleaves paste is applied externally to prevent itching in anus and to remove intestinal worms in children,a practice followed by indigenous people.The reason for curing effect ofMomordicaleaves against worms should be investigated,and extended to ointment preparation.Considering the traditional knowledge with present scientific investigation of antioxidant,enzyme inhibition,andin vivoassessments,we have concluded that this polyphenolic richMomordicaleaves can be suitable for incorporation into functional food.Further,this study explains its extensive use in daily life and possible health-promoting properties related to food,cosmetic,and pharmaceutical applications.
Acknowledgments
The authors wish to thank University Grants Commission(UGC),New Delhi,India (F.No.34-2592008) for financial assistance.
- 食品科学与人类健康(英文)的其它文章
- About the Beijing Academy of Food Sciences
- Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones
- In vitro antioxidant,anti-diabetic,cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C.maxima fruits
- Effects of Co-60 gamma-irradiation and refrigerated storage on the quality of Shatang mandarin
- In vitro modulation of TH1 and TH2 cytokine expression by edible tuber of Dioscorea alata and study of correlation patterns of the cytokine expression
- GUIDE FOR AUTHORS

