Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones
Alexner Gosslu Kung Yu Chen Chi-Tng Ho Shiming Li
a Department of Science,BMCC-City University of New York,New York,NY 10007,United States
b Department of Chemistry and Chemical Biology,Rutgers University,Piscataway,NJ 08854-8087,United States
c Department of Food Science,Rutgers University,New Brunswick,NJ 08901-8520,United States
d College of Chemistry and Chemical Engineering,Huanggang Normal University,Huanggang 438000,China
Abstract In view of the potential of polymethoxyflavones(PMFs)and hydroxylated PMFs(OH-PMFs)as bioactives against inflammation,we prepared six different orange peel extracts(OPEs).The major compounds of these extracts were characterized and quantified by high performance liquid chromatography(HPLC).Effects on inflammation were analyzed by nutrigenomics using a human cell-based TPA-induced monocyte–macrophage differentiation model employing U-937 cells and inflammatory surrogate genes.Dose response and kinetics analysis of OPEs with different chemical profiles revealed less cytotoxic effects of PMFs as compared to OH-PMFs as demonstrated by the MTT-method.Noteworthy,a comparison of two PMF members such as 3,5,6,7,3',4'-hexamethoxyflavone(HexaMF)and 3,5,6,7,8,3',4'-heptamethoxyflavone(HeptaMF)exhibited less cytotoxic effects of HeptaMF as compared to HexaMF.A specific OPE enriched with HeptaMF,PMFs and OH-PMFs at low concentrations (10 μg/mL)significantly down-regulated the expression of a panel of genes involved in inflammatory response,including COX-2,TNF-α,ICAM-1,NFκB,IL-1β,IL-6,and IL-8 with an inflammatory index of −0.55.The strong anti-inflammatory effects were then validated in a mouse carrageenan-induced paw edema model.Oral intake of OPE reduced paw edema significantly in a dose-dependent manner.Importantly,a dosage of 250 mg/kg gave an anti-inflammatory effect comparable to ibuprofen.A preliminary clinical study showed that OPE was well tolerated showing no adverse side effects.In summary,enrichment of phyto extracts such as OPEs with specific polymethoxyflavones as anti-inflammatory bioactives is a promising strategy to find naturally derived extracts that are effective against diseases associated with inflammation.
Keywords: Citrus flavonoids;Edema;Gene expression;Inflammation;Nutrigenomics
1.Introduction
Chronic inflammation is widely recognized as a major underlying cause of various degenerative diseases including cardiovascular,Alzheimer’s,diabetes,and cancer [1–5].In contrast,acute inflammation is a beneficiary response by promoting vasodilatation which enables rolling,adhesion,and endothelial transmigration of leukocytes toward infected tissue.Acute inflammation is initiated by the activation of a variety of inflammatory genes encoding for adhesion molecules (e.g.ICAM-1,VCAM-1),chemokines (e.g.IL-8),and metabolites generated in the arachidonic acid(AA)pathway[1,2,6].Phospholipase A2provides AA as a substrate for cyclooxygenase-2(COX-2)and 5-lipoxygenase(5-LOX)which generate a variety of prostaglandins and leukotrienes,respectively,triggering chemotaxis and vasodilation [1,2,6].Activation of neutrophils enables phagocytosis and intracellular degradation of the ingested material mediated through lysosomal enzymes and oxidative burst.Oxidative burst is characterized by enzymatic generation of electrophilic species(ES)such as reactive oxygen species(ROS)and reactive nitrogen species(RNS)generated by NADPH oxidase,myeloperoxidase and inducible nitric oxide synthase (iNOS).Chronic inflammation is characterized by prolonged duration of persistent infections,immune-mediated inflammatory diseases,or prolonged exposure to toxic reagents.Monocytes differentiation to macrophages is an important event in chronic inflammation.Macrophages as the dominant cellular player activate several cytokines (e.g.IFN-γ,TNF-α,IL-1β,andIL-12) and chemokines (e.g.IL-8,monocyte chemotactic protein-1,and macrophage inflammatory protein-1) to perpetuate the inflammatory response [1–3,6,7].Activation of the transcription factor NFκB plays a central role in the induction of many key inflammatory genes such asPLA2,COX-2,iNOS,ICAM-1,IL-1β,IL-6,andIL-8[2,3,6,7].The function and interplay of key pro-inflammatory mediators in the inflammation cascade as well as the balance between their up-regulation and down-regulation may ultimately determine the degree of inflammation[1,2,8].It is generally believed that during severe chronic inflammation,accumulation of tissue destruction caused by ES coupled with damage induced by proteolytic metalloproteinases leads to pathological conditions of various diseases including cardiovascular,Alzheimer’s,diabetes,and cancer[1–5].
The limitation of current anti-inflammatory therapies is widely acknowledged and evident in the continuous efforts in the pharmaceutical industry to develop drugs targeting specific steps in the inflammatory cascade.Natural products have the potential to fill this therapeutic gap addressing the complexity in the inflammatory cascade thereby reducing side effects and compensatory reactions requiring secondary treatment [8].Orange peel is rich in flavonoids including methylated derivatives such as polymethoxyflavones (PMFs).PMFs have been shown to exhibit strong anti-inflammatory effects both at the level of gene expression and enzyme activity [9–16].In addition,induction of apoptosis by PMFsmediated calcium-signaling may attenuate inflammation [17].Flavonoids are typically found throughout the whole fruit whereas PMFs are found exclusively in the peels ofCitrusgenus,particularly in the peels of sweet oranges (Citrus sinensis) and mandarin oranges (Citrus reticulate).As the most abundant PMFs in orange peel extract (OPE),tangeretin and nobiletin have been demonstrated to have strong anti-inflammatory effects as indicated by inhibition ofPLA2,COX-2,iNOS,TNF-α,15-LOX,IL-1β,IL-6,and NADPH oxidase in different cell-based and animal models[10,12–16,18,19].Down-regulation of inflammatory genes by PMFs corresponded to suppression of NFκB,AP-1,and CREB[18].Noteworthy,strong anti-inflammatory activities were found for 3,5,6,7,8,3',4'-heptamethoxyflavone[20].Strong antiinflammatory activities were found also for OH-PMFs derived from OPEsuch as 5-hydroxy-3,6,7,8,3',4'-hexamethoxyflavone,3'-demethylnobiletin (3'-dNob),4'-demethylnobiletin (4'-dNob),and 3',4'-didemethylnobiletin (3',4'-dNob) which attenuatediNOS,TNF-α,andCOX-2expression[10,15,21–23].
In view of the growing evidence of anti-inflammatory bioactives in orange peel extracts,we have prepared six different OPEs containing different concentrations of PMFs and OH-PMFs.Effects of different OPEs on cell viability by the MTTmethod were evaluated and correlated to different chemical profiles.The nutrigenomic method[24,25]was used as measure for anti-inflammatory bioactivity using a subset of inflammatory surrogate genes in a human monocyte–macrophage differentiation model [8].The OPE enriched with bioactive polymethoxyflavones showed strong anti-inflammatory effects as demonstrated in a cell-based humanin vitromonocyte–macrophage differentiation model and a paw edemain vivomouse model.
2.Materials and methods
2.1.Materials and chemicals
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco BRL(Gaithersburg,MD).Cell culture flasks,dishes,and 24-well plates were from Falcon (Becton-Dickinson,Franklin Lakes,NJ).For RNA isolation,RNeasyTMTotal RNA Kit (Qiagen,Chatsworth,CA)was used.Oligo-dT,dNTPs and SuperscriptTMII reverse transcriptase were purchased from Invitrogen,Life Technologies (Grand Island,NY).TaqMan qPCR probes,primers and master mix were from Applied Biosystems,Life Technologies (Grand Island,NY).Other chemicals were purchased from Sigma(St.Louis,MO).
2.2.Preparation and analysis of orange peel extracts
2.2.1.Preparation of OPEs
Different batches of sweet orange peel extract (OPE,from cold-pressed orange peel oil) were purchased previously from Florida Flavors Company(Lakeland,FL).The OPEs were further purified with a flash chromatography system on a silica gel column to remove majority of essential oils as previous described [26]to obtain OPE with high content of polymethoxyflavones,such as OPE-6 and the precursors of OPE-1 to OPE-5.The obtained OPE precursors were suspended in absolute ethanol and further treated with concentrated hydrochloric acid for certain period of time monitored with HPLC system to get the desired content of hydroxylated PMFs.The reaction mixture was then cooled and concentrated in vacuum.The residue was dissolved in ethyl acetate and washed with water,1 mol/L sodium bicarbonate,water and brine.The organic layer was dried over aqueous sodium sulfate,filtered to collect liquid and concentrated to remove solvent.The resulting OPEs were lyophilized overnight to get final products(OPE-1 to OPE-5).
As an example,the purchased commercial OPE mixture(10 g)was dissolved in a mixture of methylene chloride(2 mL)and hexanes(2 mL)and loaded onto a 120 g pre-conditioned silica gel flash column.The isocratic solvent was 10%ethyl acetate and 90% hexanes and kept eluting for 30 min.Then,another isocratic solvent system with 85%of ethyl acetate and 15%of hexanes was introduced and kept eluting while collecting eluent as one fraction for another 30 min.OPE (8 g) without essential oils was obtained.To the obtained OPE 100 mg,20 mL of anhydrous ethanol was added and followed by the addition of 1 mL concentrated hydrochloric acid.The mixture was heated to boiling and remained reflux for 16 h.The reaction mixture was cooled and concentrated in vacuum.The resulted residue was redissolved in ethyl acetate and washed with water,1 mol/L sodium bicarbonate solution,water and brine.The organic layer was separated and dried over anhydrous sodium sulfate.After filtration,the filtrate was concentrated in vacuumand lyophilized for 24 h.Then,84 mg OPE-5 was obtained.
2.2.2.Separation and quantification of polymethoxyflavones
The HPLC was equipped with a reversed phase C16amide column (Ascentis RP-Amide,150 mm×4.6 mm,3 μm),from Supelco (Bellefonte,PA).Gradient elution was used with a mobile phase composed of water (solvent A) and acetonitrile(ACN,solvent B).The optimized condition is as follows: a 20 min gradient was started with 40% B,linearly increased to 55%B in 10 min,then linearly increased to 70%in 15 min,and finally ramped to 80% in 20 min.Flow rate was 1.0 mL/min and the column temperature was maintained at 35°C.Detection wavelength was 326 nm and the injection volume was 10 μL.
2.3.Cell culture and treatment
U-937 cells (CRL-1593.2,human histiocytic lymphoma)were obtained from the American Type Culture Collection(Rockville,MD).Cells were cultured in RPMI-1640 medium with 10%FBS at 37°C in a humidified,10%CO2atmosphere.Cells were subcultured in culture flasks (Falcon,Becton-Dickinson,Franklin Lakes,NJ)and passaged every 3 d.Before experiments,cells were seeded in 60 mm culture dishes or 24-well plates (Falcon,Becton-Dickinson,Franklin Lakes,NJ) as indicated for the different assays.OPEs or PMFs were applied directly to the medium to achieve final concentrations as indicated.
2.4.MTT cell proliferation assay
Cell proliferation was measured by the MTT (3(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium-bromide)method after treatment in 24-well plates for 3 h,24 h,or 5 d.The MTT-assay measures mitochondrial activity based on the conversion of the tetrazolium salt MTT to blue formazan by mitochondrial dehydrogenase activity[27].Color development was documented by a scanner (UMAX,Astra 2200) and the absorbance was determined spectrophotometrically at 570 nm using a multi-well spectrophotometer (Infinite M200,Tecan).Viability is given in percent of the control value (e.g.DMSO controls) and corresponding IC50values (e.g.50% of growth inhibition) are indicated in Section 3.In view of the concern that MTT may yield false-positive results for certain cell types when treated with flavonoids or polyphenols[28],proliferation data were verified by crystal violet dye staining[29].
2.5.Nutrigenomic analysis
2.5.1.Human cell-based model for inflammation
For analysis of anti-inflammatory potential,we used human U-937 cells as a monocyte–macrophage differentiation model for nutrigenomic analysis.The expression of a subset of inflammatory surrogate genes in human monocytes induced by the inflammatory stimulant 12-O-tetradecanoylphorbol-13-acetate (TPA) during differentiation to macrophages was used as measure for anti-inflammatory bioactivity [8,30–32].For nutrigenomic analysis,we employed a subset of seven inflammatory surrogate genes(COX-2,TNF-α,ICAM-1,NFκB,IL-1β,IL-6,andIL-8)which had been previously selected and validated throughout various cell-based,animal and clinical studies by whole genome Affymetrix microarray,focused Oligo microarray andTaqMan qPCR analysis [8,33,34].Gene expression analysis was performed by RT-PCR andTaqMan qPCR analysis.
2.5.2.Reverse transcription-polymerase chain reaction(RT-PCR)
U-937 were treated with TPA (20 nmol/L) either alone or in combination with OPE (10 μg/mL) for 3 h.After treatment in 60 mm culture dishes,cells were harvested at indicated times and the total RNA was prepared using RNeasyTMTotal RNA Kit.Then,total RNA(1 μg)was reverse transcribed into cDNA by incubating with SuperScriptTMRNase H reverse transcriptase using oligo(dT)12–18as primer.For PCR amplification of the humanCOX-2gene,gene specific primers,both sense (5'-TTCAAATGAGATTGTGGGAAAAT-3') and antisense(5'-AGATCATCTCTGCCTGAGTATCTT-3')were used.The expression of the house-keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control (sense: 5'-TGAAGCTCGGAGTCAACGGATTTG-3';antisense:5'-CATGTGGGCCATGAGGTCCACCAC-3').PCR conditions were chosen to ensure that the yield of the amplified products was linear with respect to the amount of input cDNA.PCR products were analyzed by electrophoresis on a 1%agarose gel and visualized with ethidium bromide.COX-2andGAPDHexpression was quantified by densitometry using the Image J software(NIH,Bethesda,MD).COX-2expression was then normalized toGAPDHand expressed as the ratio of the mean experimental channel(TPA+OPE)to the mean control channel(TPA alone).
2.5.3.TaqMan qPCR analysis
After treatment of U-937 cells with TPA(20 nmol/L)either alone or in combination with OPE(10 μg/mL)for 3 h,total RNA was isolated using the QIAcube from Qiagen(Chatsworth,CA)according to manufacturer’s protocols.Total RNA was then reverse transcribed using standard protocols and reagents from Invitrogen,Life Technologies (Grand Island,NY).TaqMan qPCR was run on the Roche 480 Lightcycler for 50 cycles with concentrations ranging from 100 ng to 0.01 ng for the standard curve.Gene expression ofCOX-2(Hs01573471_m1),TNF-α(Hs00174128_m1),ICAM-1 (Hs99999152_m1),NFκB(Hs00153294_m1),IL-1β (Hs00174097_m1),IL-6(Hs00174131_m1),IL-8 (Hs00174103_m1),andGAPDH(Hs99999905_m1) was analyzed using the probes,primers and master mix from Applied Biosystems,Life Technologies(Grand Island,NY;for details see the Assay IDs as indicated above in brackets next to the respective genes).After normalization toGAPDH,gene expression was calculated according to the delta–delta CT method as the ratio of the mean experimental channel(TPA+OPE)to the mean control channel(TPA alone).Besides ratios,degree of gene expression was also expressed as“inflammatory index”which was calculated as Log 2 values of the ratios.

Fig.1.Chemical profile of OPE-4.(A) HPLC trace shows a representative chemical profile of OPE-4.HPLC conditions: Supelco Ascentis Amide column,4.6 mm×150 mm,3 μm,100 A;UV 326 nm;mobile phase A,water,mobile phase B,acetonitrile;flow rate,1 mL/min.Peaks: (1) sinesetin;(2) 3,5,6,7,3',4'-hexamethoxyflavone;(3) nobletin;(4) 3,5,7,4'-tetramethoxyflavone;(5) 3,5,6,7,8,3',4'-heptamethoxyflavone;(6) tangeretin;(7–12) 5-demethylated PMFs.(B)Structure of 3,5,6,7,3',4'-hexamethoxyflavone(HexaMF).(C)Structure of 3,5,6,7,8,3',4'-heptamethoxyflavone(HeptaMF).
2.6.Mouse paw edema model
The carrageenan-induced mouse hind paw edema model was used for determination of anti-inflammatory activity by measuring the degree of edema formation [35].Male albino mice (6 weeks old) were group housed (6 per cage) and fed standard mouse chow and tap water ad libitum.All animal experiments were approved by the Ethics Committee for Animal Experiments of Rutgers University and were performed in accordance with the Guideline for Animal Experiments of the laboratories(protocol no.87-115).For experiments,mice were grouped in five different cohorts and treated by a single oral dose of different concentrations of OPE-4(e.g.125,250,and 500 mg/kg)and compared to the vehicle control(tocopherol-stripped cornoil).As positive control we included ibuprofen (100 mg/kg)demonstrated to have strong anti-inflammatory effects in the paw edema model in other studies[36].1 h after treatment,all groups received an injection of 0.1 mL of carrageenan (1% in water) into the plantar side of the right hind paw of the mice.The mice were then euthanized 1,2,4,and 8 h after carrageenan injection and hind paws were taken.The degree of swelling was determined by paw volume displacement measured by a digital hydroplethysmometer.Results were expressed as area under the curve(AUC)units or volume of hind paw(mL)at indicated times,respectively.
2.7.Statistics
Results were presented as means±standard deviation(SD)of at least three independent experiments unless otherwise indicated.Statistical comparisons of data were performed using Student’st-test and analysis of variance(ANOVA).
3.Results
3.1.Preparation and characterization of different orange peel extracts
The commercial sweet orange peel extracts made from cold pressed oil contain certain amount of essential oil.Intention has been given to remove the majority of essential oils to obtain the highest content of PMFs and OH-PMFs in the OPEs.This process was achieved with silica gel flash chromatography using 10%of ethyl acetate and 90%of hexanes as eluting solvents.Other methods or other solvent system may reach the same goal of this de-oil process,but the process we used is at our convenience and non-complexity and it also has high efficiency in terms of time and cost.The various contents of OH-PMFs in a mixture of OPE was closely monitored in the process of 5-desmethylation of PMFs in OPEs with a validated HPLC method [37].The control of reaction time is crucial to obtain the desired OH-PMF content in targeted OPE samples.By following the established procedures of 5-desmethylation of OPE[15]and the monitoring HPLC method[37],we prepared six OPE samples ranging from non-desmethylation process(OPE-6) to the maximum 5-desmethylated sample of OPE-5.Concentrations of individual as well as total PMFs and OHPMFs were analyzed by HPLC as described in Section 2 and were expressed in percent of the individual OPEs (Table 1).Fig.1 shows the HPLC profile of one of the OPE samples(e.g.OPE-4;A) and the structures of two individual PMFs such as 3,5,6,7,3',4'-hexamethoxyflavone (HexaMF;B) and 3,5,6,7,8,3',4'-heptamethoxyflavone(HeptaMF;C).
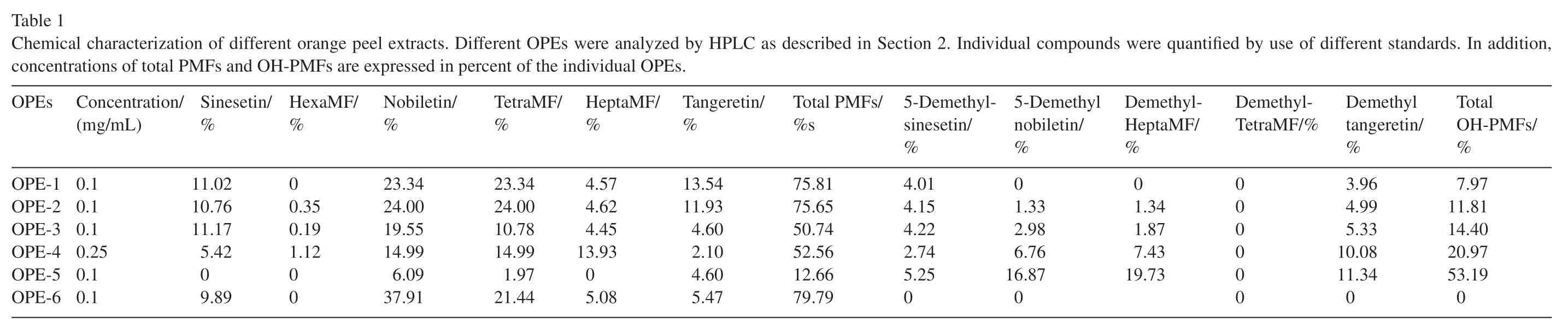
Table 1 Chemical characterization of different orange peel extracts. Different OPEs were analyzed by HPLC as described in Section 2.Individual compounds were quantified by use of different standards.In addition,concentrations of total PMFs and OH-PMFs are expressed in percent of the individual OPEs.OPEs Concentration/(mg/mL)Sinesetin/%HexaMF/%Nobiletin/%TetraMF/%HeptaMF/%Tangeretin/%Total PMFs/%s 5-Demethylsinesetin/%5-Demethyl nobiletin/%Demethyl-HeptaMF/%Demethyl-TetraMF/%Demethyl tangeretin/%Total OH-PMFs/%OPE-1 0.1 11.02 00.35 23.34 23.34 4.57 13.54 75.81 4.01 01.33 01.34 000000 3.96 7.97 OPE-2 0.1 10.76 24.00 24.00 4.62 11.93 75.65 4.15 4.99 11.81 OPE-3 0.1 11.17 0.19 19.55 10.78 4.45 4.60 50.74 4.22 2.98 1.87 5.33 14.40 OPE-4 0.25 5.42 1.12 14.99 14.99 13.93 2.10 52.56 2.74 6.76 7.43 10.08 20.97 OPE-5 0.1 09.89 00 6.09 1.97 05.08 4.60 12.66 5.25 16.87 19.73 11.34 53.19 OPE-6 0.1 37.91 21.44 5.47 79.79 0 0 0 0
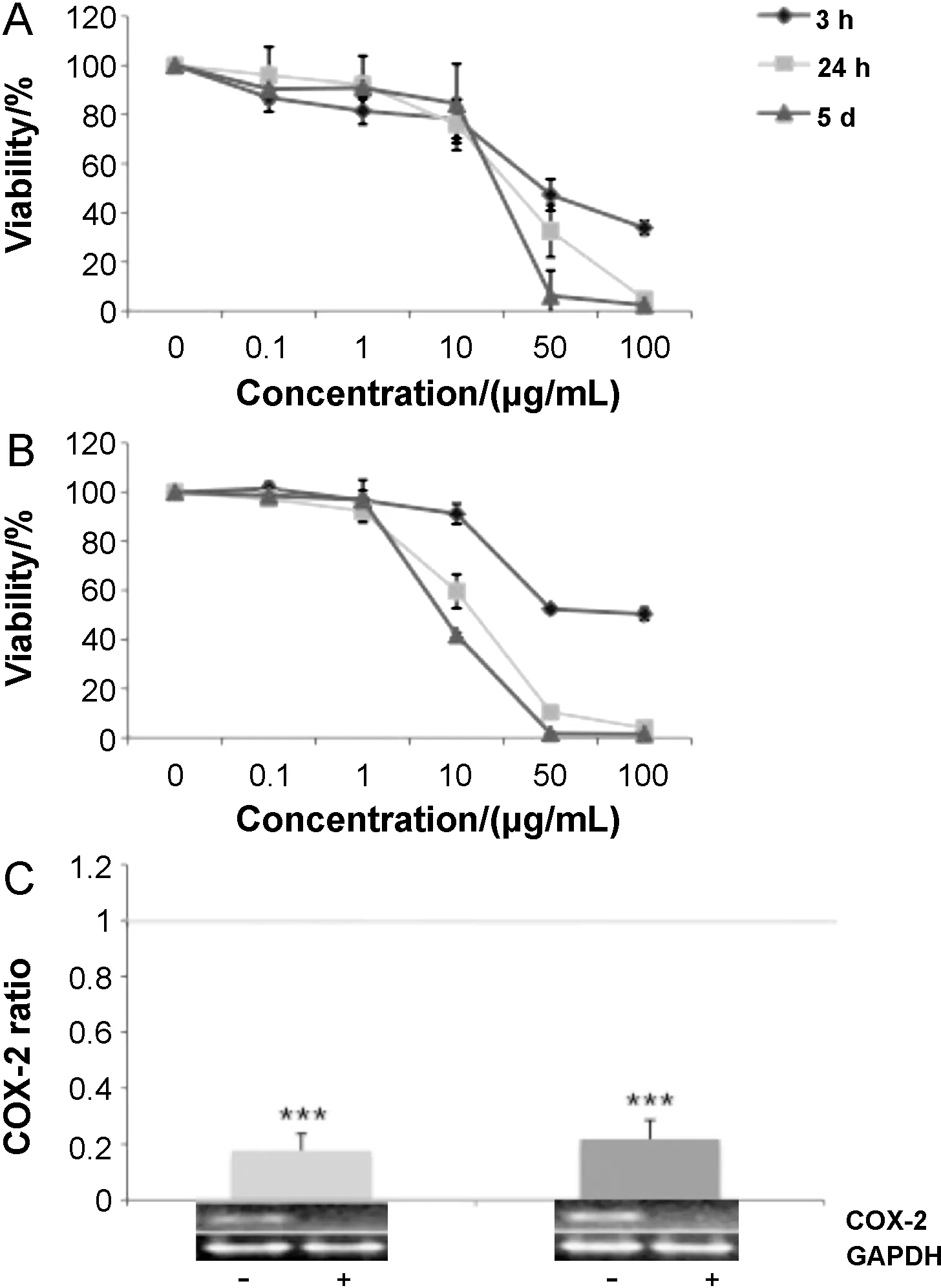
Fig.2.Effects of two characterized OPEs on cell viability and COX-2 expression in U-937 cells.Human monocytes(U-937)were subcultured at 1:20 dilution and then treated with OPE-1(A)and OPE-2(B)at indicated concentrations for 3 h,24 h,and 5 d as indicated by different symbols(rhombus,square,and triangle),respectively.The cell growth was monitored by the MTT assay as described in Section 2.Mean values±standard deviation of three independent experiments are shown.(C)Effects of OPE-1 and OPE-2 on TPA-induced COX-2 expression.U-937 cells were treated by TPA (20 nmol/L) either alone or in combination with OPE-1 or OPE-2 (50 μg/mL) for 3 h.Thereafter,expression of COX-2 was analyzed by RT-PCR.The expression of glyceraldehyde-3-phosphate dehydrogenase(GAPDH)was used as internal control.COX-2 expression normalized to GAPDH was expressed as the ratio of the mean experimental channel(TPA+OPE) to the mean control channel (TPA alone)±standard deviation.Representative blots of six independent experiments are shown.
3.2.Effects of two characterized OPEs on cell viability and COX-2 expression in U-937 cells
For initial screening of effects on cell viability and inflammation,we chose OPE-1 and OPE-2 which contain similar amount of total PMFs(around 76%)but differ in OH-PMFs(around 8%and 12% for OPE-1 and OPE-2,respectively;Table 1).Fig.2 shows the effect of these two OPEs on cell viability of human monocytic U-937 cells.Dose response analysis was performed in the range from 0.1 to 100 μg/mL after 3 h,24 h,and 5 d of exposure.For OPE-1,the IC50values(half-maximal inhibition of viability)were around 25,30,and 50 μg/mL for 5 d,24 h,and 3 h after exposure,respectively(A).OPE-1 showed slightly less cytotoxic effects as compared to OPE-2 as indicated by lower IC50values after long-term incubation for 5 d(around 8 μg/mL)and 24 h(around 18 μg/mL)for OPE-2(B).For short-term exposure of 3 h,both OPEs showed IC50values around 50 μg/mL.Interestingly,higher concentrations of OPE-2 appear to be less toxic for short-term incubation (3 h) as compared to OPE-1 as indicated by a horizontal slope between 50 and 100 μg/mL for OPE-1.In view of the concern that MTT may yield false-positive results for certain cell types when treated with flavonoids or polyphenols[28],we verified our proliferation data by the use of the crystal violet dye assay[29]and achieved similar results(data not shown).Since both OPEs are complex extracts with similar concentrations of total PMFs(Table 1),we can only speculate whether higher concentrations of OH-PMF in OPE-2 are responsible for the differential effects on cell viability.
It is well established that PMF have anti-inflammatory effects[9–16].Since OPE-1 and OPE-2 both contain high concentrations of PMF(around 75%),we were interested in the effects of both OPEs on inflammation.For initial nutrigenomic screening,we chose cyclooxygenase-2 (COX-2) as inflammatory gene.COX-2has become an important pharmacological target for inflammatory diseases [38,39]and previous studies indicated a suppression of TPA-inducedCOX-2expression in cell-based and animal studies by PMFs [12,15,16,18,19,23].To examine the effects of OPE-1 and OP-2 onCOX-2expression,we performed RT-PCR analysis in U-937 cells.Cells were treated with TPA (20 nmol/L) either alone or in combination with OPE-1 and OPE-2 (50 μg/mL) for 3 h.Glyceraldehyde-3-phosphate dehydrogenase(GAPDH)was used as internal control.Fig.2C shows the expression ofCOX-2at the mRNA level as the ratio of the mean experimental channel (TPA+OPE) to the mean control channel(TPA alone)normalized toGAPDH.Although both OPEs were applied at high concentrations(e.g.50 μg/mL)which are in the range of IC50values(Fig.2A and B),inhibitory effects on gene transcription are less likely as indicated by unchangedGAPDHexpression in response to both OPEs.We observed a dramatic decrease of TPA-inducedCOX-2expression in response to both OPE-1 and OPE-2(P<0.001).For OPE-1,there appears to be slight stronger anti-inflammatory effects as compared to OPE-2 as indicated by ratios of 0.18 and 0.22,respectively,although the differences between OPE-1 and -2 were not statistically significant.
3.3.Comparison of the effects of different OPEs and Hexaand HeptaMF on viability of U-937 cells
Our analysis of OPE-1 and OPE-2 showed a similar chemical profile for PMFs and slight differences for OH-PMFs(Table 1).Cell viability analysis showed slight differences between OPE-1 and -2 by comparing long-term and short-term kinetics which might be correlated to the slight differences in OH-PMF content(see Section 3.2).We were now interested in the effects of other OPEs (OPE-3–OPE-6) containing different concentrations of PMFs and OH-PMFs(Table 1).As described in Section 3.2,we used the MTT cell proliferation assay for kinetic analysis of the different OPEs after 3 h,24 h,and 5 d of exposure with a dose response analysis in the range from 0.1 to 100 μg/mL.OPE-3(Fig.3A) and OPE-4 (Fig.3B) showed similar effects on cell viability as indicated by corresponding IC50values around 7,12,and 50 μg/mL for OPE-3 or around 8,15,and 50 μg/mL for OPE-4 after long-term exposures for 5 d,24 h,and 3 h,respectively.OPE-5 (Fig.3C) showed more cytotoxic effects as indicated by lower IC50values around 5 and 7 μg/mL for long-term kinetics after 5 d and 24 h,respectively.An incubation of 10 μg/mL for 5 d was lethal to all of the cells.On the other hand,an IC50value around 50 μg/mL after 3 h of exposure was similar to that of OPE-3 and OPE-4.As compared to the other OPEs,OPE-6 (Fig.3D) was prominently less cytotoxic after long termincubation for 5 d and 24 h showing IC50values around 25 and 30 μg/mL,whereas the IC50value around 70 μg/mL after 3 h was similar to that of OPE-3(A)and OPE-4(B).Our chemical profiling(Table 1)revealed that all of the orange peel extracts(with the exception of OPE-5)contain the two individual PMFs,3,5,6,7,3',4'-hexamethoxyflavone(HexaMF,Fig.1B)and 3,5,6,7,8,3',4'-heptamethoxyflavone (HeptaMF,Fig.1C),latter reported to play a role as anti-inflammatory bioactive[20].
We were now interested in the effects of Hexa-and HeptaMF on cell viability.HexaMF showed IC50values around 25,30,and 500 μmol/L after 5 d,24 h,and 3 h of incubation,respectively(Fig.3E).Interestingly,we observed less cytotoxic effects of HeptaMF(Fig.3F)as compared to HexaMF for long-time incubation showing IC50values around 140 and 190 μmol/L for 5 d and 24 h incubation,respectively.On the other hand,short-term exposure (3 h) showed similar effects on cell viability of HeptaMFas well as HexaMF(e.g.IC50value around 500 μmol/L).In summary,we observed less cytotoxic effects of OPEs containing higher amounts of total PMFs after long-term exposure for 5 d and 24 h.On the other hand,OH-PMFs appeared to have slightly stronger cytotoxic effects as indicated by OPE with increasing concentration of OH-PMFs(e.g.OPE-5 containing around 53%of total OH-PMFs)showing lower IC50values after long-term exposure.This is in line with our observation that OPE-2(around 12%OH-PMFs)exhibited lower IC50values after 5 d(8 μg/mL)and 24 h (18 μg/mL) as compared to OPE-1 (around 8% OHPMFs)showing IC50values around 25 and 30 μg/mL after 5 d and 24 h,respectively(see Section 3.2).
3.4.Effects of OPE-4 on expression of inflammatory genes in U-937 cells
For further analysis of effects against inflammation we chose OPE-4 for several reasons: OPE-4 containing high amounts of HeptaMF (around 14%) was reported to have strong antiinflammatory activity [20].In addition,our viability analysis showed less cytotoxic effects of HeptaMF as compared to HexaMF(see Section 3.3).Most importantly,OPE-4 contains high amounts of PMFs (52%) and OH-PMFs (21%) both reported to have strong anti-inflammatory effects[10,12–16,21–23].For experiments,we used the U-937 cell model for inflammation employing a subset of seven inflammatory surrogate genes(COX-2,TNF-α,ICAM-1,NFκB,IL-1β,IL-6,andIL-8).U-937 cells were treated for 3 h with TPA(20 nmol/L)either alone or in combination with OPE-4(10 μg/mL),a concentration showing more than 90%cell viability using the MTT-method(Fig.3B).Gene expression ofCOX-2,TNF-α,ICAM-1,NFκB,IL-1β,IL-6,andIL-8was analyzed byTaqMan qPCR analysis and normalized toGAPDH.Gene expression is shown as the ratio of the mean experimental channel (TPA+OPE-4) to the mean control channel(TPA alone)or expressed as“inflammatory index”calculated as Log 2 values of the ratio as described in Section 2.OPE-4 showed strong anti-inflammatory effects (Fig.4C)as indicated by an inflammatory index of −0.55 by integrating all seven genes (COX-2,TNF-α,ICAM-1,NFκB,IL-1β,IL-6,andIL-8).Noteworthy,all seven genes showed a downregulation in response to OPE-4 as compared to the TPA control as indicated by negative inflammatory indices.In another set of experiments we validated our results of a down-regulation ofCOX-2andTNF-α,two important genes in the inflammatory cascade [1,2,4,5,8].Expression ofCOX-2(Fig.4A) andTNF-α(Fig.4B)was expressed as ratios(left columns)as well as inflammatory indices(right columns).Treatment with OPE-4(10 μg/mL)induced a significant down-regulation ofCOX-2andTNF-α(bothP<0.05) as indicated by low ratios of 0.61 and 0.45 which corresponded to inflammatory indices of −0.72 and −1.16 forCOX-2andTNF-α,respectively.In summary,we observed a strong anti-inflammatory potential of OPE-4 as indicated by significant down-regulation of TPA-induced expression of inflammatory surrogate genes.

Fig.3.Comparison of the effects of different OPEs and Hexa-and HeptaMF on viability of U-937 cells.Human monocytes(U-937)were subcultured at 1:20 dilution and then treated with different OPEs,HexaMF,and HeptaMF at indicated concentrations for 3 h,24 h,and 5 d as indicated by different symbols(rhombus,square,and triangle),respectively.The cell growth was monitored by the MTT assay as described in Section 2.Mean values±standard deviation of three independent experiments are shown.(A)OPE-3;(B)OPE-4;(C)OPE-5;(D)OPE-6;(E)3,5,6,7,3',4'-hexamethoxyflavone(HexaMF);(F)3,5,6,7,8,3',4'-heptamethoxyflavone(HeptaMF).
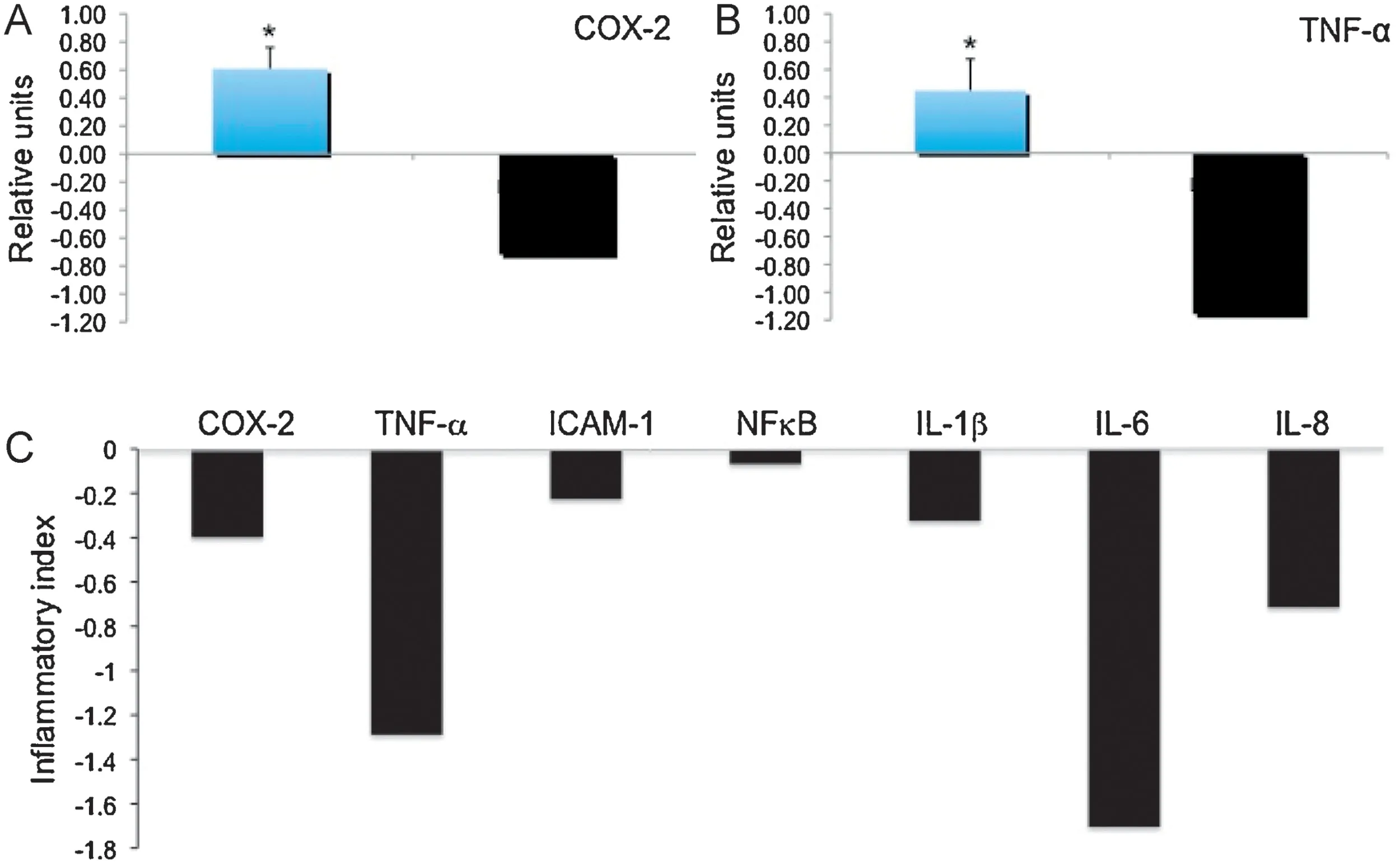
Fig.4.Effects of OPE-4 on expression of inflammatory genes in U-937 cells.Human monocytes (U-937) were treated by TPA (20 nmol/L) either alone or in combination with OPE-4(10 μg/mL)for 3 h.After RNA isolation and reverse transcription,expression of either(A)COX-2,(B)TNF-α,or(C)a subset of seven inflammatory surrogate genes(COX-2,TNF-α,ICAM-1,NFκB,IL-1β,IL-6,and IL-8)was analyzed by Taq Man qPCR and normalized to GAPDH.Gene expression is shown as the ratio of the mean experimental channel(TPA+OPE-4)to the mean control channel(TPA alone)or expressed as“inflammatory index”calculated as Log 2 values of the ratio.Ratios below 1 or inflammatory indices below 0 suggest anti-inflammatory activity as indicated by an inhibition of the expression of inflammatory genes.COX-2(A)and TNF-α(B)ratios(left columns)and inflammatory indices(right columns)are shown.(C)Inflammatory indices of seven genes(COX-2, TNF-α, ICAM-1, NFκB, IL-1β, IL-6,and IL-8) are shown.For OPE-4 an inflammatory index of −0.55 by integrating all seven genes was calculated.*indicates significant differences from the control group with P<0.05 as analyzed by ANOVA.
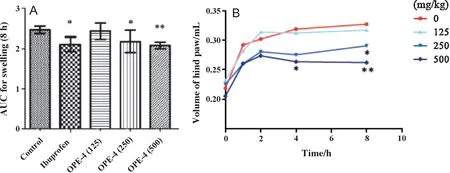
Fig.5.Effects of OPE-4 on carrageenan-induced paw edema in mice.Mice received a single oral dose of control vehicle(tocopherol-stripped corn-oil),different concentrations of OPE-4(e.g.125,250,and 500 mg/kg),or ibuprofen(100 mg/kg),respectively.1 h after treatment,all groups received an injection of 0.1 mL of carrageenan (1% in water) into the plantar side of the right hind paw.The mice were then euthanized 1,2,4,and 8 h after carrageenan injection.The degree of swelling was determined by paw volume displacement measured at indicated times by a digital hydroplethysmometer.Results are expressed as the mean±SD area under the curve(AUC)units after 8 h(A)or volume of hind paw/mL for different time points(B),respectively.*and**indicate significant differences from the control group with P<0.05 and 0.01,respectively as analyzed by ANOVA.
3.5.Effects of OPE-4 on carrageenan-induced paw edema in mice
Using a carrageenan-induced paw edema model,we investigated the effects of OPE-4 which showed strong antiinflammatory potential as indicated by strong down-regulation of inflammatory surrogate genes as demonstrated in our cell-based model for inflammation (see Section 3.4).For experiments,mice were treated by different concentrations of OPE-4 and compared to the vehicle control.As positive control,we included ibuprofen demonstrated to have strong antiinflammatory effects in the paw edema model in other studies[36].As shown in Fig.5A we observed a reduction of paw edema in a dose-dependent manner by OPE-4 as compared to the carrageen-treated control group 8 h after carrageenan injection.Significant effects of paw edema inhibition by OPE-4 started at 250 mg/kg (P<0.05) and increased in response to a higher dosage of 500 mg/kg(P<0.01).Importantly,OPE-4 at a dosage of 250 mg/kg showed anti-inflammatory effects comparable to ibuprofen(100 mg/kg).Kinetic analysis(Fig.5B)showed significant effects at high dosages of OPE-4 (500 mg/kg) as soon as 4 h after carrageenan injection (P<0.05).In summary,we observed a significant reduction of carrageenan-induced paw edema formation in a dose-dependent manner.Noteworthy,dosages around 250 mg/kg showed anti-inflammatory effects comparable to ibuprofen.Strong anti-inflammatory effects of OPE-4 correlated to nutrigenomic screening analysis(see Section 3.4) showing a strong down-regulation of TPA-induced inflammatory surrogate genes as obtained in our cell-based model for inflammation.
4.Discussion
In this study,we prepared six different orange peel extracts and quantified major compounds by HPLC.By comparing the different OPEs,we looked for relationships between chemical profiles and cell viability profiles.Our analysis revealed cytoprotective effects of PMFs as indicated by an increase of IC50values for OPEs with increasing amounts of total PMFs after long-term exposure for 5 d and 24 h.On the other hand,OH-PMFs appear to have cytotoxic effects as indicated by a decrease of IC50values for OPE with increasing concentration of OH-PMFs.OPE-5 containing the highest amount of total OH-PMFs(around 53%)showed the lowest IC50value after long-term exposures of 24 h and 5 d.Since OPEs are complex extracts,we can only speculate whether higher concentrations of OH-PMF are responsible for the differential effects on cell viability.But our results correlate to pro-apoptotic effects of OH-PMFs as observed earlier[17,40].Despite of potential cytotoxic effects,OH-PMFs are reported to be strong agents against inflammation[10,15,21–23].To gain insights in possible structure-activity relationships,we compared effects on cell viability by 3,5,6,7,3',4'-hexamethoxyflavone and 3,5,6,7,8,3',4'-heptamethoxyflavone.Intriguingly,HeptaMF showed less cytotoxic effects as compared to HexaMF.Previously,it has been reported that HeptaMF have strong anti-inflammatory activity[20].In the light of HeptaMF,PMFs and OH-PMFs as anti-inflammatory bioactives,we selected OPE-4 for further evaluation of its anti-inflammatory effects since it contains high amounts of cytoprotective HeptaMF (14%),PMFs (52%) and OH-PMFs (21%) reported to have strong anti-inflammatory effects in cell-basedin vivoand animalin vivomodels[10,12–16,18–23].
Anti-inflammatory potential of OPE-4 was evaluated by the nutrigenomic method [24,25].In the past two decades,different cell-based and animal models have been used to analyze the effects of test extracts on expression of genes related to a variety of diseases,a discipline called nutrigenomics[24,25].In our human cell-based model for inflammation,we used the phorbol ester TPA to induce differentiation of the human monocytic cell line (U-937) to macrophages [31,32,41].During differentiation,a variety of inflammatory genes that are up-regulated play key roles in the inflammatory cascade and are relevant in chronic inflammatory diseases [1,2,4,5,8].Previously,we have selected a subset of seven inflammatory surrogate genes for nutrigenomic screening (COX-2,TNF-α,ICAM-1,NFκB,IL-1β,IL-6,andIL-8) which have been validated throughout various cell-based,animal and clinical studies by whole genome Affymetrix microarray,focused Oligo microarray andTaqMan qPCR analysis[8,33,34].Our present study revealed strong antiinflammatory effects of OPE-4 as indicated by a TPA-induced down-regulation ofCOX-2,TNF-α,ICAM-1,NFκB,IL-1β,IL-6,andIL-8in our cell-based human model for inflammation.Interestingly,we observed an attenuation of the p65 subunit ofNFκBin our gene expression analysis.Our non-significant effects correlate to high constitutive expression levels ofp65[7].Interestingly,a posttranslational inhibition ofNFκBthrough blocking ofIκBwas observed by PMFs[18,21,22].Activation ofNFκBplays a central role to initiate and promote the inflammatory response through induction of many key inflammatory genes.Therefore,NFκBhas been in the focus of anti-inflammatory therapy[1–3,7,42,43].In addition to our nutrigenomic analysis,we used the paw edema mouse model to evaluate anti-inflammatory bioactivity of OPE-4.The carrageenan-induced mouse paw edema model has been used for decades as a reliable model for studying the mechanisms acute and chronic inflammation[35].Strong anti-inflammatory effects of OPE-4 were confirmed in thein vivopaw edema mouse model showing a significant dose-dependent attenuation of edema.Importantly,effects at 250 mg/kg were comparable to the effects of ibuprofen,a well established drug against inflammation shown to inhibit paw edema[36].
Previously,we proposed a chemical characterization of natural extracts to be essential to insure adequate consistency in performance[8].Our data showed that an enrichment of orange peel extracts with specific polymethoxyflavones is a promising strategy to find natural-derived extracts effective against inflammation.Although the techniques in characterization of natural extracts are advancing,still a complete characterization of natural extracts is difficult.Complex natural extracts such as OPEs are rich in polymethoxyflavones and hydroxylated polymethoxyflavones.These bioactives may have potential synergistic effects but other unknown components may also have additive,even synergistic positive or negative effects which may account for its efficacy and effectiveness against inflammation.In another study,strong anti-inflammatory effects were observed for a citrus peel extract enriched with PMFs which correlated to inhibition of tumorigenesis [9].Previously,we found that PMF-enriched OPE was well tolerated in humans and showed no adverse reactions in a small colon cancer pilot study(data not shown).Thus,the overall content and formulation of this specific orange peel product presents a unique chemical profile leading to strong anti-inflammation activity in our tested cell-basedin vitroand mouse paw edemain vivomodel which qualifies as promising candidate extract against diseases associated with inflammation.
Acknowledgement
The authors wish to thank WellGen Inc.for support of materials.
- 食品科学与人类健康(英文)的其它文章
- About the Beijing Academy of Food Sciences
- A comparative study on antioxidant potentials,inhibitory activities against key enzymes related to metabolic syndrome,and anti-inflammatory activity of leaf extract from different Momordica species
- In vitro antioxidant,anti-diabetic,cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C.maxima fruits
- Effects of Co-60 gamma-irradiation and refrigerated storage on the quality of Shatang mandarin
- In vitro modulation of TH1 and TH2 cytokine expression by edible tuber of Dioscorea alata and study of correlation patterns of the cytokine expression
- GUIDE FOR AUTHORS

