Comparative Study on the Allergenicity of Different Litopenaeus vannamei Extract Solutions
WU Lisha, LIN Haixin, WANG Guoying, LU Zongchao, CHEN Guanzhi, LIN Hong, and LI Zhenxing,
1) Food Safety Laboratory, College of Food Science and Engineering, Ocean University of China, Qingdao 266003, P. R. China
2) Departments of Immunology, Qingdao University, Qingdao 266383, P. R. China
3) The Affiliated Hospital of Medical College, Qingdao University, Qingdao 266003, P. R. China
Comparative Study on the Allergenicity of Different Litopenaeus vannamei Extract Solutions
WU Lisha1), LIN Haixin1), WANG Guoying2), LU Zongchao1), CHEN Guanzhi3), LIN Hong1), and LI Zhenxing1),*
1) Food Safety Laboratory, College of Food Science and Engineering, Ocean University of China, Qingdao 266003, P. R. China
2) Departments of Immunology, Qingdao University, Qingdao 266383, P. R. China
3) The Affiliated Hospital of Medical College, Qingdao University, Qingdao 266003, P. R. China
Allergen extracts are widely used for allergy diagnosis and treatment. The application of shrimp extract is hampered due to the low protein concentration and the inconsistent allergenicity. Extracting solutions are considered to be the primary limiting factor of protein extraction from crustaceans. This study aimed to select an optimal solution for shrimp protein extraction by comparing the allergenicity of different shrimp extracts. The effect of 7 existing or modified extracting solutions were evaluated, including the glycerol-NaCl solution, the glycerol Cocaine’s solution, the buffered saline solution, the Cocaine’s solution, the Glucose leaching solution, 1 mol L−1KCl solution, and 0.01 mol L−1phosphate buffered saline solution with and without dithiothreitolor (DTT). The quantitative (protein concentration) and qualitative parameters (SDS-PAGE protein patterns and immuno-reactivity) were determined using the sodium dodecyl sulfate polyacrylamide gel electrophoresis, enzyme linked immunosorbent assay and immunoblotting assay. Results showed that the 1 mol L−1KCl solution with DTT was optimal for shrimp protein extraction, which yielded high concentration and allergenicity in the protein extract, including major and minor allergens. The 1 mol L−1KCl solution with DDT is proposed for preparation of shrimp extract and associated allergy diagnosis, as well as potential applications for other crustaceans.
shrimp; allergen; protein extract; extracting solution; allergenicity
1 Introduction
In recent years, the incidence of food allergic disease has substantially increased and food allergy has become an important food safety issue. The Food and Agriculture Organization of the United Nations (FAO) has identified 8 categories of food allergens which can easily cause human allergic reactions, including crustaceans (shrimps and crabs) (Deanet al., 1997). In shrimp sensitized individuals, the allergic reactions are characterized by the development of urticaria, vomiting, diarrhea, angioedema, asthma, and even life-threatening anaphylactic reactions (Daulet al., 1994). Natural allergenic products are commonly used for the diagnosis and treatment of allergic diseases. Laboratory diagnostic testing of food allergy relies on the detection of food allergen-specific IgE antibody in the skin (skin prick test) or in the serum. Double-blind, placebo-controlled oral food challenges are currently the standard for food allergy diagnosis (Bocket al., 1988; Sichereret al., 2006). The allergen extracts are needed as the prick test fluid and fixed antigen for determination of the serum IgE specificity.
Ideally, a standardized extract must contain defined and consistent amounts of all the major and minor allergens with biologically activity (Jonaet al., 1997). In shrimps, the identified major allergens are proteins with molecular mass of 35–38 kDa, including the 34–39 kDa tropomyosin and a myofibrillar protein (Shantiet al., 1993; Daulet al., 1994; Leunget al., 1994). In addition, the shrimps contain minor allergens such as triosephosphate isomerase (28 kDa) (Bauermeisteret al., 2008), sarcoplasmic calcium-binding protein (20 kDa) (Shiomiet al., 2008; Ayusoet al., 2009), myosin light chain (20 kDa) (Ayusoet al., 2008), Troponin C (18 kDa) (Yuet al., 2008), and arginine kinase (40 kDa) (García-Orozcoet al., 2007). To ensure the thorough extraction of all the allergens capable of causing allergenic activities from shrimps, it is worthy selecting an optimal extracting solution.
Dithiothreitolor (DTT) is a strong reducing agent commonly used for allergen extraction from a variety of animals and plants (Kathleenet al., 1984; Fredet al., 1980), particularly shrimps (Patricket al., 1994; Shantiet al., 1993). However, it remains unclear whether DTT affects the concentration and allergenicity of the protein extracts. Thus, this study compared the protein extraction effect of 7 extracting solutions with DTT and withoutDTT using biochemical and immunochemical methods. Results were used to select an optimal solution for allergen extraction from shrimps as well as other crustaceans.
2 Materials and Methods
2.1 Materials
The shrimps (Litopenaeus vannamei) were purchased from a local market in Qingdao, China. After removal of the shells and heads, the shrimps were stored at −20℃prior to use. Seven different extracting solutions were prepared (Table 1), including the glycerol-NaCl solution, the glycerol Cocaine’s solution, the buffered saline solution, the Cocaine’s solution, the Glucose leaching solution, the 1 mol L−1KCl solution, and the 0.01 mol L−1phosphate buffered saline (PBS) solution. Distilled water was used as control. The extracting solutions were used in two groups with and without dithiothreitol (DTT, Sigma, Missouri, USA), respectively.

Table 1 The composition of shrimp protein extracting solutions
Protein standards for SDS-polyacrylamide gel electrophoresis (SDS-PAGE) were from Fermentas (Lithuania). Rabbit antiserum against tropomyosin was prepared by the Beijing Genomics Institute. Goat anti-human IgE and goat anti-rabbit IgG conjugated with peroxidase were purchased from Sigma (Missouri, USA) for Western blotting and indirect ELISA assays. Solid-phase enzyme immunoassays were using 96-well microtiter plates (Nunc, Denmark) and a Multiskan MK3 ELISA reader (Thermo Labsystems). Unless otherwise stated, all reagents were analytical grade.
Sera were obtained from 4 patients in the Affiliated Hospital of Medical College of Qingdao University, Qingdao. The patients were selected based on their past clinical history of shrimp allergy,e.g., urticaria and diarrhea after ingestion of shrimps. All sera were stored at−80℃ until used.
2.2 Methods
2.2.1 Preparation of acetone powder
Acetone powder was prepared from the shrimps used the method described by Greaser and Gergely (1971). Peeled shrimps were weighed and homogenized in a 0.85% sodium chloride (NaCl) solution (1 g mL−1). The homogenate was transferred to cold acetone (−20℃ precooling) at the ratio of 1:4. After stirring at 4℃ in 30 min, the mixture was centrifuged at 4000 r min−1for 15 min. The pellet was suspended in cold acetone again, and then centrifuged till the supernatant was clear. The supernatant was transferred to a clean filter paper and dried at room temperature, then ground and sieved through a 200 mesh. The obtained acetone powder was stored in a sealed glass container prior to use.
2.2.2 Protein extraction
The acetone powder (3 g) was extracted overnight with 30 mL of extracting solutions at 4℃. After centrifugation at 4000 r min−1for 30 min, the supernatant was dialyzed against double-distilled water for 24 h at 4℃. Then, the protein concentration was determined and the solution was lyophilized and stored at −20℃ prior to use.
2.2.3 Protein concentration determination
The protein concentration was determined using bicinchoninic acid (BCA, Sigma, Missouri, USA) according to the Smith’s method (Lianget al., 2008) with slight modifications. Bovine serum albumin (BSA, Sigma, Missouri, USA) was used as the protein standard. The absorbance was measured at 590 nm, and each sample was measured in triplicate. Results are presented as the athrimetic mean values. One-dimensional variance analysis was carried out using SPSS 18.0.
2.2.4 SDS-PAGE and immunoblotting assay
SDS-PAGE was performed according to the method of Laemmliet al. (1970). Protein samples were mixed with ¼ volume of 1× Laemmli buffer (2% SDS, 25% glycerol, 14.4 mmol L−1β-mercaptoethanol (Sigma, Missouri, USA), and 0.1% bromphenol blue in 1 mol L−1Tris-HCl, pH 6.8), heated at 100℃ for 7 min, and then loaded to a 12% analytical SDS-polyacrylamide gel (15 μL/lane) on a vertical electrophoresis system (BIO-RAD). The gel was stained with Coomassie Brilliant Blue R-250 (Smithet al., 1988) or transferred to a 450 nm polyvinylidene fluoride membrane (PVDF, Pall-Gelman, USA). For western blotting,the gel was transferred by electroblotting at a constant current of 60 mA for 3.5 h according to Towbin and Gordon (1994) with some modifications. The membrane was stained with Ponceau S (Sigma, Missouri, USA) to verify the transfer of proteins. The blotting was ceased with 5% skimmed milk in PBST (pH 7.4, 0.01 mol L phosphate buffer, pH 7.4, containing 0.15 mol L NaCl, 0.05% Tween 20) for 2 h at 37℃. The membrane with blots was incubated with patients sera (1:20 in 2.5% skimmed milk) overnight at 4℃. After washed with PBST, the polyclonal goat anti-human IgE antibody conjugated with peroxidase (1:1000 in 2.5% skimmed milk) for 2 h and then washed again with PBST. Immunoreactive bands were developed using enhanced chemiluminescent (ECL) after final washing with PBST. Nonspecific binding of the anti-IgG antibody conjugate was measured in a similar blotting procedure.
Coomassie blue stained gels and the developed PVDF membranes were scanned using Tanon-4200 automatic translation of a digital gel image analysis system. The low-range pre-stained SDS-PAGE protein mixture (Fermantas, Lithuania) was used as standard.
2.2.5 Indirect ELISA
The protein extracts (1 µg well−1) were immobilized on microtiter plates using 100 µL of carbonate coating buffer (pH 9.6) and then incubated overnight at 4℃. The coating buffer without protein extracts was incubated as blank control. The plates were washed and free binding sites were blocked with blocking buffer (5% BSA in PBST) for 2 h at 37℃. After being washed three times with PBST, rabbit anti-tropomyosin antibodies (1:20000 in PBS, containing 0.01 mmol L−1phosphate buffer, pH 7.4, containing 0.15 mmol L−1NaCl) was added for 1.5 h incubation at 4℃. The PBS without rabbit anti-tropomy- osin antibodies was incubated as negative control. The plates were washed again and 100 µL of goat anti-rabbit IgG conjugated with peroxidase (1:10000 in PBS) was added for 1 h incubation at 37℃. The plates were washed with PBST followed by the addition of 3, 3’, 5, 5’-tetrame-thylbenzidine (TMB, Sigma, Missouri, USA) as substrate, then incubated in dark for 15 min. The optical density (OD value) was measured at the 450 nm using an ELISA reader. All ELISA experiments were performed in triplicate.
2.2.6 Statistical analysis
All analyses were carried out in triplicate and data were expressed as means standard deviation. A one-way analysis of variance (ANOVA) was performed to calculate significant differences in treatment means. A probability value ofP<0.05 was considered significant, and only significant differences were considered unless stated otherwise.
3 Results
3.1 Protein Concentration of Different Shrimp Extracts
Comparison of shrimp extracts in the 7 extracting solutions showed that in the presence of DTT, the protein concentration was significantly higher in S6 than in other extracting solutions (P<0.01). Addition of DTT resulted in significantly higher protein concentrations in most of the shrimp extracts except for S3 (Fig.1).
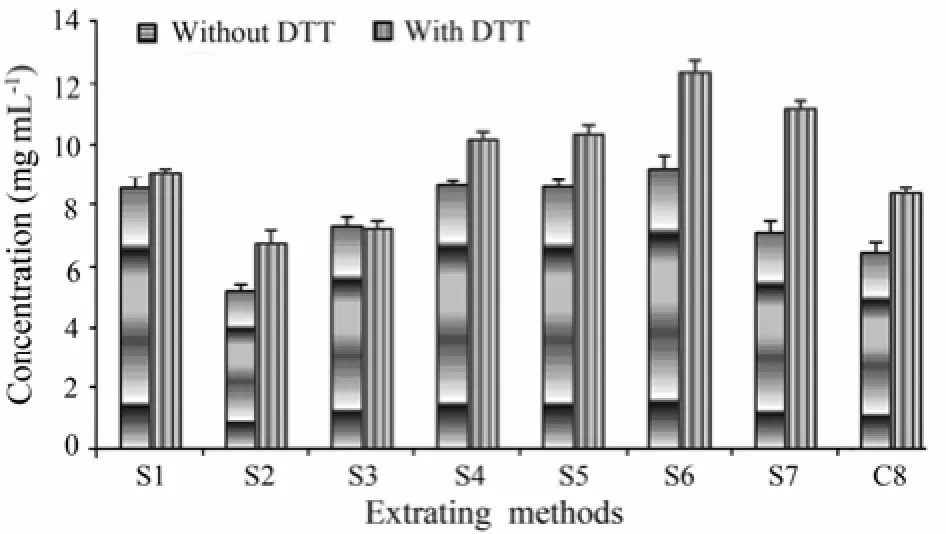
Fig.1 The concentration of proteins in different shrimp extracts determined by BCA assay. S1: glycerol-NaCl solution; S2: glycerol Cocaine’s solution; S3: buffered saline solution; S4: Cocaine’s solution; S5: Glucose leaching solution; S6: 1 mol L−1KCl; S7: 0.01 mol L−1PBS; and C8: distilled water (control).
3.2 Protein Composition of Different Shrimp Extracts
SDS-PAGE assay showed that shrimp extracts of the same protein concentration without DTT yielded contained more components in the solutions S1, S6 and S7 than in the other solutions (Fig.2). The most dominant protein components (36 kDa and 20 kDa) appeared in the solutions S1, S3–S7 and C8, but not S2.

Fig.2 SDS-PAGE/Coomassie blue-staining of different shrimp extracts of the same protein concentration without DTT. M, Molecular weight marker; Lanes 1: glycerol-NaCl solution (S1); 2: glycerol Cocaine’s solution (S2); 3: buffered saline solution (S3); 4: Cocaine’s solution (S4); 5: Glucose leaching solution (S5); 6: 1 mol L−1KCl (S6); 7: 0.01 mol L−1PBS (S7); and 8: distilled water (C8, control).
In the presence of DTT, more protein components were extracted in S1, S6 and S7. The 36 kDa and 20 kDa proteins remained as the dominant components (Fig.3). Comparison of the Figs.2 and 3 showed a lack of difference in the protein composition of each lane.
3.3 Tropomyosin Allergenicity of Different Shrimp Extracts
The tropomyosin allergenicity of different shrimp extracts was evaluated by indirect ELISA using the rabbit antiserum against shrimp tropomyosin. The shrimp proteins at the same concentration showed significantly higher binding ability in the extracting solutions with DTT (Fig.4). The OD values of shrimp protein extracts with DTT showed the same trend compared to the extracts without DTT. Overall, the OD value of S1 extract had the highest allergenicity than the other extracts.

Fig.4 Indirect ELISA of tropomyosin in different shrimp protein extracts. S1, glycerol-NaCl solution; S2, glycerol Cocaine’s solution; S3, buffered saline solution; S4, Cocaine’s solution; S5, Glucose leaching solution; S6, 1 mol L−1KCl; S7: 0.01 mol L−1PBS; and C8: distilled water (control).
3.4 Allergenicity of Different Shrimp Extracts Without DTT
The allergenicity of different shrimp protein extracts without DTT was confirmed by immunoblotting assay using 4 human sera of shrimp allergy patients. The results of immunoblotting assay (Fig.5) supported the conclusion that the shrimp protein extracts obtaiend wtih different extracting solutions had different allergenicity to the tested patient sera. With the sera of patients A and B, the extracts of S3 to C8 showed the same immunogenicity. The IgE-bands of 6, 7 and 8 immunized by the sera of patients A, B and D showed substantially higher allergenicity than the other bands.
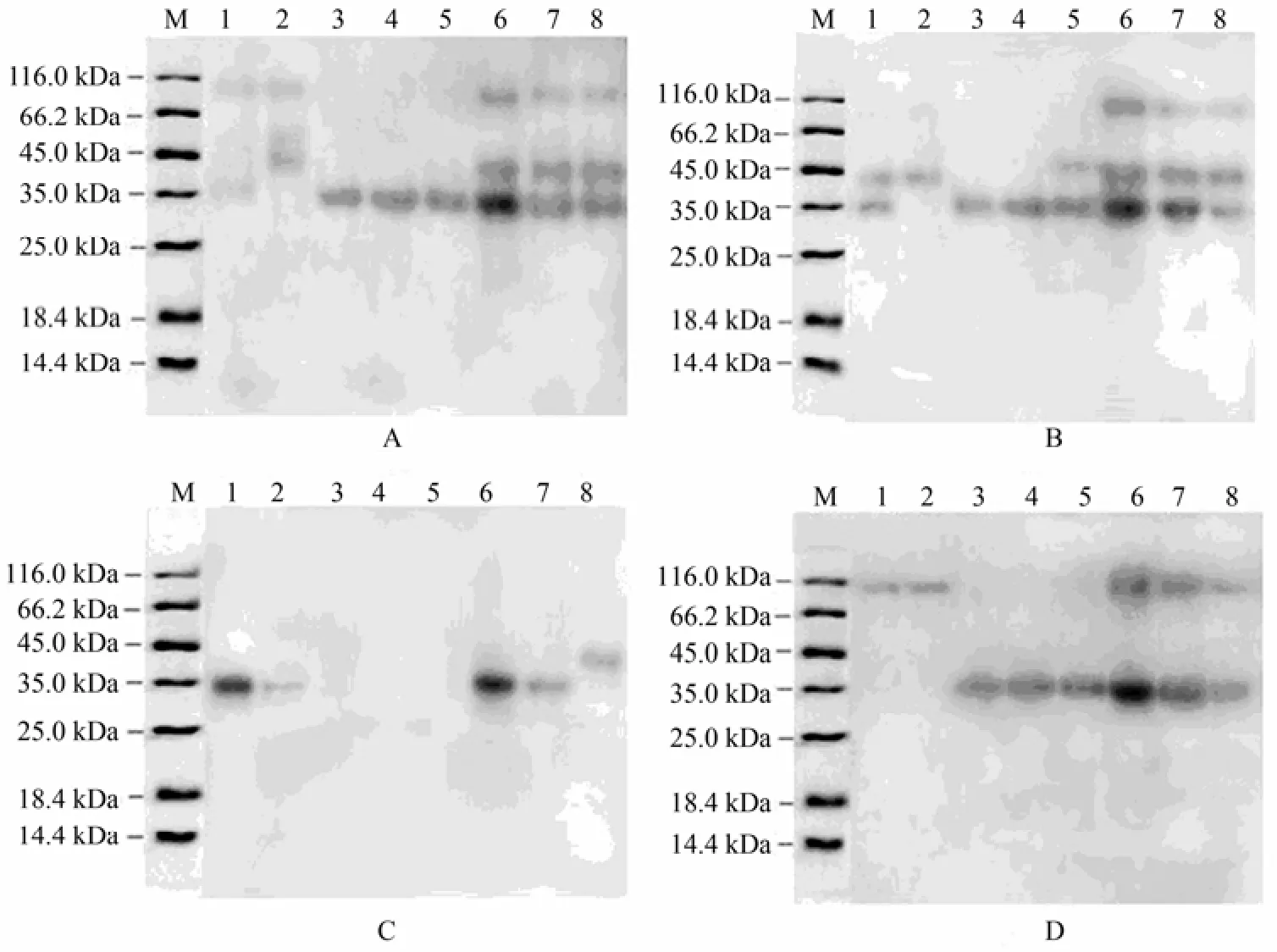
Fig.5 The allergenicity of different shrimp protein extracts detected by western blotting assay. Samples were separated by SDS-PAGE followed immunological detection using the sera of 4 shrimp allergic patients (A, B, C, and D). M, molecular weight marker; Lanes 1: glycerol-NaCl solution (S1); 2: glycerol Cocaine’s solution (S2); 3: buffered saline solution (S3); 4: Cocaine’s solution (S4); 5: Glucose leaching solution (S5); 6: 1 mol L−1KCl (S6); 7: 0.01 mol L−1PBS (S7); and 8: distilled water (C8, control).
4 Discussion
At present, DTT is frequently used to reduce the disulfide bonds of proteins. It prevents the formation of intramolecular and intermolecular disulfide bonds between cysteine residues of proteins, further preventing the protein crystallization from water-soluble to water-insoluble forms (Ruegget al., 1977). This explains why the extract rate in solutions with DTT was higher than that in solutions without DTT (Fig.1). The characteristics of the major allergen tropomyosin of shrimps have been widely studied (Ruegget al., 1993; Shantiet al., 2007; Motoyamaet al., 1984). In the present study, the allergenicity of tropomyosin in different protein extracts with DTT was higher than that in the solutions without DTT except for S3 and S4 (Fig.4).
SDS-PAGE showed that the same protein components were obtained from the corresponding extracting solutions with or without DTT. The extracted proteins were treated with β-mercaptoethanol, a protein denaturant, which played a similar role as DTT. That is, β-mercaptoethanol could change the spherical proteins to linear forms (Wesselet al., 1984) while not changing the composition and molecular mass of the proteins.
Comparison of the concentration, composition and allergenicity of different protein extracts showed that 1 mol L−1solution KCl (S6) with DTT was the optimal extracting solution for the shrimpPenaeus vannamei. The extracting temperature and pH were not considered in the present study as previous work has indicated that variations in the extraction temperature or pH have no effect on the quality of extracted protein or the degradation of allergens in milk and hen’s egg, except for the strong alkaline conditions (Steinhoffet al., 2011).
In addition, the 1 mol L−1KCl solution (S6) with DTT was tested protein extraction from other crustacean. Extracts of crab, lobster and crayfish obtained using the optimized solution were found superior in terms of protein concentration and composition compared with those obtained using other solutions (data not shown).
In conclusion, this study presents an optimal extracting solution,i.e., 1 mol L−1KCl solution with DTT, for extraction of allergens from shrimp as well as other crustaceans. The 1 mol L−1KCl solution with DTT proved efficient in comparison to other existing or modified extracting solutions. It has great potential for preparation of various commercial crustacean extracts that can be used in allergy diagnosis and treatment.
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 31371730), and National Science & Technology Pillar Program (No. 2012BAD28B05).
Ayuso, R., Grishina, G., Bardina, L., Carrillo, T., Blanco, C., Ibaňez, M. D., Sampson, H. A., and Beyer, K., 2008. Myosin light chain is a novel shrimp allergen, lit v 3. Journal of Allergy and Clinical Immunology, 122 (4): 795-802.
Ayuso, R., Grishina, G., Ibáňez, M. D., Blanco, C., Carrillo, T., Bencharitiwong, R., Sánchez, S., Nowak-Wegrzyn, A., and Sampson, H. A., 2009. Sarcoplasmic calcium-binding protein is an EF-hand-type protein identified as a new shrimp allergen. Journal of Allergy and Clinical Immunology, 124 (1): 114-120.
Bauermeister, K., Wangorsch, A., Perono Garoffo, L., Reuter, A., Conti, A., Falk, S., Taylor, S. L., Vieths, S., Holzhauser, T., Ballmer-Weber, B.K., and Reese, G., 2008. Novel crustacean allergens identified in North Sea shrimp crangon and other crustacean species–sarcoplasmic calcium-binding protein, troponin C, troponin I, triosephosphate isomerase, and myosin light chain. http://www.ncbi.nlm.nih.gov/protein/ 238477329.
Björkstén, F., Halmepuro, L., Hannuksela, M., and Lahti, A., 1980. Extraction and properties of apple allergens. Allergy, 35 (8): 671-677.
Bock, S. A., Sampson, H. A., Atkins, F. M., Zeiger, R. S., Lehrer, S., Sachs, M., Bush, R. K., and Metcalfe, D. D., 1988. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: A manual. Journal of Allergy and Clinical Immunology, 82 (6): 986-997.
Daul, C. B., Slattery, M., Reese, G., and Lehrer, S. B., 1994. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Internatioanl Archives Allergy and Immunology, 105 (1): 49-55.
Dean, D. M., Hugh, A. S., and Ronald, A. S., 1997. Food Allergy: Adverse Reactions to Foods and Food Additives. 2nd edition. Blackwell Science Inc., 583pp.
García-Orozco, K. D., Aispuro-Hernández, E., Yepiz-Plascencia, G, Calderón-de-la-Barca, A. M., and Sotelo-Mundo, R. R., 2007. Molecular characterization of arginine kinase, an allergen from the shrimp Litopenaeus vannamei. Internatioanl Archives Allergy and Immunology, 144 (1): 23-28.
Greaser, M. L., and Gergely, J., 1971. Reconstitution of troponin activity from three protein components. The Journal of Biological Chemistry, 246: 4226-4233.
Jona, R., and Fronda, A., 1997. Comparative histochemical analysis of cell wall polysaccharides by enzymatic and chemical extractions of two fruits. Biotechnology Histochemistry, 72 (1): 22-28.
Laemmli, U. K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680-685.
Leitermann, K., and Jr. Ohman, J. L., 1984. Cat allergen 1: Biochemical, antigenic, and allergenic properties. Journal of Allergy and Clinical Immunology, 74 (2): 147-153.
Leung, P. S., Chow, K. H., Ansari, A., Bandea, C. I., Kwan, H. S., Nagy, S. M., and Gershwin, M. E., 1994. Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. Journal of Allergy and Clinical Immunology, 94 (5): 882-890.
Leung, P. S., Chu, K. H., Chow, W. K., Ansari, A., Bandea, C. I., Kwan, H. S., Nagy, S. M., and Gershwin, M. E. 1994. Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. Journal of Allergy and Clinical Immunology, 94 (5): 882-890.
Liang, Y. L., Cao, M. J., Su, W. J., Zhang, L. J., Huang, Y. Y., and Liu, G. M., 2008. Identification and characterisation of the major allergen of Chinese mitten crab (Eriocheir sinensis). Food Chemistry, 111 (4): 998-1003.
Motoyama, K., Suma, Y., Ishizaki, S., Nagashima, Y., and Shiomi, K., 2007. Molecular cloning of tropomyosins identified as allergens in six species of crustaceans. Journal of Agriculture and Food Chemistry, 55 (3): 985-991.
Ruegg, U. T., and Rudinger, J., 1977. Reductive cleavage of cystine disulfides with tributylphosphine. Methods in Enzymology, 47: 111-116.
Shanti, K. N., Martin, B. M., Nagpal, S., Metcalfe, D. D., and Rao, P. V., 1993. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. The Journal of Immunology, 151 (10): 5354-5363.
Shanti, K. N., Martin, B. M., Nagpal, S., Metcalfe, D. D., and Rao, P. V., 1993. Identif i cation of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. The Journal of Immunology, 151 (10): 5354-5363.
Shiomi, K., Sato, Y., Hamamoto, S., Mita, H., and Shimakura, K., 2008. Sarcoplasmic calcium binding protein: identification as a new allergen of the black tiger shrimp Penaeus monodon. Internatioanl Archives Allergy and Immunology, 146 (2): 91-98.
Sicherer, S. H., and Sampson, H. A., 2006. Food allergy. Journal of Allergy and Clinical Immunology, 117 (2 Suppl Mini Primer): 470-475.
Smith, I., Cromie, R., and Stainsby, K., 1988. Seeing gel wells well. Analytical Biochemistry, 169 (2): 370-371.
Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J., and Klenk, D. C., 1985. Measurement of protein using bicinchoninic acid. Analytical Biochemistry, 150 (1): 76-85.
Steinhoff, M., Fischer, M., and Paschke-Kratzin, A., 2011. Comparison of extraction conditions for milk and hen’s egg allergens. Food Additives & Contaminants: Part A, 28 (4): 373-383.
Towbin, H., and Gordon, J., 1984. Immunoblotting and dot immunobinding: current status and outlook. Journal of Immunological Methods, 72 (2): 313-340.
Wessel, D., and Flügge, U. I., 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Analytical Biochemistry, 138 (1): 141-143.
Yu, C. J., Lin, Y. F., Chiang, B. L., and Chow, L. P., 2003. Proteomics and immunological analysis of anovel shrimp allergen, Pen m 2. The Journal of Immunology, 170: 445-453.
(Edited by Qiu Yantao)
* Corresponding author. Tel: 0086-532-82032389
E-mail: lizhenxing@ouc.edu.cn
(Received March 25, 2012; revised May 15, 2012; accepted May 10, 2013)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
 Journal of Ocean University of China2014年1期
Journal of Ocean University of China2014年1期
- Journal of Ocean University of China的其它文章
- Diagnosis of Physical and Biological Controls on Phytoplankton Distribution in the Sargasso Sea
- Seasonal Distribution of Bioaerosols in the Coastal Region of Qingdao
- Surface Heat Budget and Solar Radiation Allocation at a Melt Pond During Summer in the Central Arctic Ocean
- A Model Study on Dynamical Processes of Phytoplankton in Laizhou Bay
- Properties of Klebsiella Phage P13 and Associated Exopolysaccharide Depolymerase
- Study on Deformation Law of Circular Foundation Under Combined Loading
