Toxicity of Five Phenolic Compounds to Brine Shrimp Artemia sinica (Crustacea: Artemiidae)
Ali Shaukat, LIU Guangxing, LI Zhengyan XU Donghui HUANG Yousong and CHEN Hongju
1) Key Laboratory of Marine Environment and Ecology of Chinese Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
2) College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, P. R. China
3) Department of Environmental Sciences, Karakoram International University, Gilgit-Baltistan, 15100, Pakistan
Toxicity of Five Phenolic Compounds to Brine Shrimp Artemia sinica (Crustacea: Artemiidae)
Ali Shaukat1),2),3), LIU Guangxing1),2),*, LI Zhengyan1),2), XU Donghui2), HUANG Yousong2), and CHEN Hongju1),2)
1) Key Laboratory of Marine Environment and Ecology of Chinese Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
2) College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, P. R. China
3) Department of Environmental Sciences, Karakoram International University, Gilgit-Baltistan, 15100, Pakistan
The acute toxicity of five phenolic compounds each to 15 d oldArtemia sinicawas determined in this study. The brine pA.sinicawas hatched from the encysted dry eggs (Bohai Bay Brand) produced by Dongying OceanArtemiaCo., Ltd., China at ± 1℃ in pre-filtered (through pores of 0.45 µm in diameter) and autoclaved seawater (salinity 31, pH 7.5–8.0) in a cilindroconical beaker (2000 mL in volume) under continuous illumination (provided by a side set 20 W fluorescent lamp) with slight aeration.rtemiaindividuals from the same batch of the hatched were cultured in 10 mL toxicant solution prepared with seawater (salinity H 7.5–8.0) at room temperature (about 20℃) to determine 24 h, 48 h and 72 h medium lethal concentration (LC50) of 5 phenolic ounds each. It was found that the toxicity ofn-heptylphenol was the highest followed by nonylphenol,t-butylphenol, ichlorophenol and bisphenol A in order. The LC50values of the 5 compounds were calculated with regression analysis. The real entration (in µg L−1) of 5 phenolic compounds each in toxicant solutions was measured with GC/MS analysis. Significant loss of olic compounds caused by either adsorption or desorption was not found. The significant difference of LC50values was found g the five compounds 3 exposure times each. The range between the highest no-observed-effect concentration (NOEC) and 100% causing concentration of five phenolic compounds each was determined. The toxicity in term of 24 h LC50value ofn-HP was 9.10 higher than that of BPA, 1.71 times higher thant-BP, 1.53 times higher than 2,4-DCP and 1.36 times higher than NP, respectively.
Artemia sinica; bioassay; phenolic compound; toxicity shrim 27℃glass TenA31, p comp 2,4-d conc phen amon death times
1 Introduction
Phenolic compounds are commercially important surfactants with industrial, agricultural, and domestic applications. China annually produces 0.5 million tons of phenolic compounds, accounting for 10% of the world (Fuet al., 2007; Yanget al., 2011). These surfactants have been documented to be genotoxic (Jagetia and Aruna, 1997), carcinogenic (Tsutsuiet al., 1997), immunotoxic (Taysseet al., 1995) and endocrine disrupting (Liet al., 2004; Fuet al., 2007; Yanget al., 2011). Phenolic compounds are lipophilic, risking ultimately the environment and human health (Bradburyet al., 1989); they are bioaccumulative and either acutely or chronically toxic, irrespective of species (Mukherjeeet al., 1990). They have been frequently detected in marine environment and fish tissues.
Species in genusArtemiamostly inhabit natural salt lakes, coastal lagoons and saltworks scattering throughout the tropical, subtropical and temperate zones as well as inland shallow saline water bodies around agricultural and industrial areas (Stappen, 1996), It has been reported that adultArtemiainduce sexual maturation and increase fertilization rate of different invertebrates (Maldonado-Montiel and Rodríguez-Canché, 2005; Wouterset al., 2001; Naessenset al., 1997). Freshly hatchedArtemianauplii are very nutritive, which have been wide used as living feeds of a wide range of species in aquaculture.Artemiaaccumulates phenolic compounds, risking the aquaculture products; they may forward phenolic compounds to aquaculture products.Artemiamay serve as a model for assessing the toxicity of phenolic compounds to marine and estuarine invertebrates and crustaceans. Intensive biological investigations have been conducted on cysts, nauplii and juveniles of different species in genusArtemia(Persoone and Castritsi-Catharios, 1989; Goet al., 1990; Barahona-Gomarizet al., 1994; Barahona-Gomariz and Sánchez-Fortún, 1996; Sarabiaet al., 1998; Hadjispyrouet al., 2001; Koutsaftis and Aoyama, 2007); unfortunately investigations of the toxicity of phenolic compounds toArtimiawere scarce.
The purpose of present study was to determine the acutetoxicities of five phenolic compounds at different concentrations to 15-d-oldA.sinica. That was LC50, the concentration of a given phenolic compound causing 50% mortality of the testers after 24-, 48- and 72-h exposure.
2 Materials and Methods
2.1 Cysts Hatching
Dry encysted eggs of brine shrimpA. sinicaof Bohai Bay Brand were obtained from Dongying OceanArtemiaCo., Ltd., China. The eggs were hatched with a procedure reported by Barahona-Gomarizet al. (1994) with modifications. The dry cysts were hydrated in cold distilled water at 4℃ for more than 6 h with those eggs sinking to the bottom separated with a Buchner funnel, washed with cold water and hatching medium, hydrated in pre-filtered (through pores of 0.45 µm in diameter) and autoclaved seawater (salinity 31, acidity pH 7.5–8.0), and hatched at 27℃±1℃ (maintained with an Hopar heater, aquarium use for 24 h. The whole process was carried out in a cilindroconical glass beaker (2000 mL in volume) under a constant illumination (provided by a side posed 20 W fluorescent lamp) and with a slight aeration (with an airing stone at the bottom of beaker). To avoid salinity change due to evaporation, the beaker was covered by a masking tape.
The newly hatched neonates were transferred into a crystalline bowl (20 cm (diameter) ×10 cm (depth)) containing an appropriate volume of seawater with Pasteur pipettes, and fed twice a day (morning and evening, respectively) with living microalgaIsochrysis galbanaParke 8701 for 15 d during which nauplius will develop into adultArtemia. The culture was carried out at room temperature (about 20℃), under a constant illumination with a rhythm of 14-h light and 10-h dark and with aeration maintained by a stone at the bottom of the bowl and a connecting tube. At the end of culture, the average body length was measured from 30–60 animals (3–3.5 mm in body length) fixed in Lugol’s solution. The body length was the measurement from the top of head to the end of telson under a stereomicroscope (Nikon YS100, Japan) equipped with a camera and digital plan measurer (Naegel, 1999).
2.2 Chemicals
Five phenolic compounds including nonylphenol (NP, technical grade), bisphenol A (BPA, 97%), 2,4-dichlorophenol (2,4-DCP, 99%),tert-butylphenol (t-BP, >99.0%) andn-hexylphenol (n-HP) were purchased from Sigma-Aldrich, USA. Acetone and hexane (HPLC grade) were purchased from MREDA, USA. Internal standards naphthalene-d8, phenanthrene-d10 and pyrene-d10 and surrogate standard bisphenol A-d14 were obtained from Chem-Service. Stock solutions (100 mg L−1) of phenolic compounds were prepared with the mixture of hexane and acetone (1:1). Various working solutions were diluted from stock solutions with acetone.
2.3 Toxicity Assaying
The procedure of Hadjispyrouet al. (2001) was followed to assay the toxicity of five phenolic compounds each with modifications. The toxicant solutions (salinity 31, acidity pH 7.5–8) were prepared with filtered (through pores of 0.45 µm in diameter) and autoclaved seawater. TenArtemiaindividuals were cultured in 3 wells (each pre-filled with 10 mL of a toxicant solution) of 12 wells plates (flat bottom with lid, pre-sterilized, Costar, USA) at room temperature (about 20℃) without feeding to determine the critical concentration (LC50) and the selected concentration range (between the highest non-observedeffect concentration (NOEC) and the lowest observedeffect concentration) of a toxicant, with the first determined repeated three times. The random death was measured in control wells (pre-filled with 10 mL seawater), which was kept less than 10% of the total. A deadArtemiawas recorded under a stereomicroscope when its appendages did not move within 10–15 s even being gently touched by a prodder.
The used seawater each well was measured for the real concentration of a toxicant with the method described by Varóet al.(2006). Briefly, the used seawater was transferred into a Teflon bottle using a pasture pipette, and mixed with 5 mL dichloromethane (DCM, HPLC grade) and 1 g NaCl. The mixture was shaken vigorously till NaCl dissolved completely. After statically placed for 10 min or longer, DCM phase was separated with a pasture pipette and concentrated with a rotary evaporator to about 1mL with the trace water removed with anhydrous sodium sulfate. The concentrated DMC phase was further concentrated under a gentle flow of dry nitrogen to less than 0.5 mL. The DMC was replaced with acetone with the toxicant transformed with BSTFA (bis-trifluoro-acetamide). Internal standards (naphthalene-d8, phenanthrene-d10 and pyrene-d10) and surrogate standard (bisphenol A-d14) were added with the final volume adjusted to 1.0 mL before GC/MS analysis.
Gas chromatography-mass spectrometry (GC/MS) (Shimadzu QP-2010) was used to identify and quantify a phenolic compound following the procedure of Fuet al.(2007). Briefly, the phenolic compound was separated by gas chromatography (Shimadazu GC-17A) with a DB-5MS capillary column (length: 30 m; i.d.: 0.25 mm; coatedfilm thickness: 0.25 cm) and detected with a mass spectrometry (Shimadazu MS QP-2010). Selected ion monitoring mode was employed for mass scan with a sampling rate of 0.2 s. A phenolic compound was qualitatively and quantitatively determined according to the corresponding retention time and the relative intensity of quantifying and confirming ions, respectively.
2.4 Statistical Analysis
Mortality was recorded as mean percentage of deadArtemiaafter 24-, 48- and 72-h exposure. The median lethal concentration (LC50) was calculated by referring to a regression equation. One way analysis of variance (ANOVA) was employed to evaluate the significant differences of LC50values of five compounds with different exposing times, followed bypost hoctests with multiplecomparisons. Software SPSS version 13.0 was used and values were reported as means ± SD.
3 Results
The toxicity of five phenolic compounds was determined. According to the results of the definitive test, the following concentrations were tested: 3, 7, 8, 9, and 12 µg L−1forn-HP; 6, 7, 11, 12 and 16 µg L−1for NP; 8, 9, 11, 13 and 18 for 2,4-DCP; 9, 11, 13, 14 and 19 µg L−1, and 30, 63, 65, 70 and 122 µg L−1for t-BP and BPA, respectively. The highest NOEC (24 h exposure) forn-HP was 3 µg L−1, while that for NP was 6 µg L−1, and similarly for other compounds (Fig.1). It was also found that the concentration mortality line declined obviously, indicating that toxicity increased rapidly with concentration above the highest NOEC.
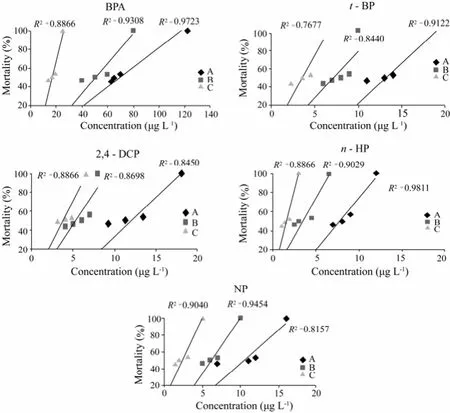
Fig.1 The range from the highest non observable to 100% death causing concentrations of five phenolic compounds. A, mortality rate for 24 h exposure; B, mortality rate for 48 h exposure; and C, mortality rate for 72 h exposure.
As summarized in Table 1, the real and the selected concentrations of five phenolic compounds were comparable each other, which revealed that the loss caused by degradation, evaporation, absorption and among others were negligible. The mortality of 5 phenolic compounds each was plotted against concentration (Fig.1). A regression line was drawn for 3 exposure times of 5 compounds each, on which medium lethal concentration (LC50) was calculated. The LC50of the five toxicants toA.sinicaat different exposure times were listed in Table 2. It was found that LC50decreased with elongation of exposure time, and LC50of BPA was the highest among 5 toxicants while that of NP was the lowest in general. Such a trend indicated that BPA was less toxic toA.sinicathan others. For example, the toxicity in term of 24 h LC50ofn-HP was 9.10 folds higher than BPA, 1.71 folds higher thant-BP, 1.53 folds higher than 2,-4-DCP and 1.36 folds higher than NP. It was obvious that the toxicity of 5 phenolic compounds decreased fromn-HP to NP and further to 2, 4-DCP,t-BP and BPA.
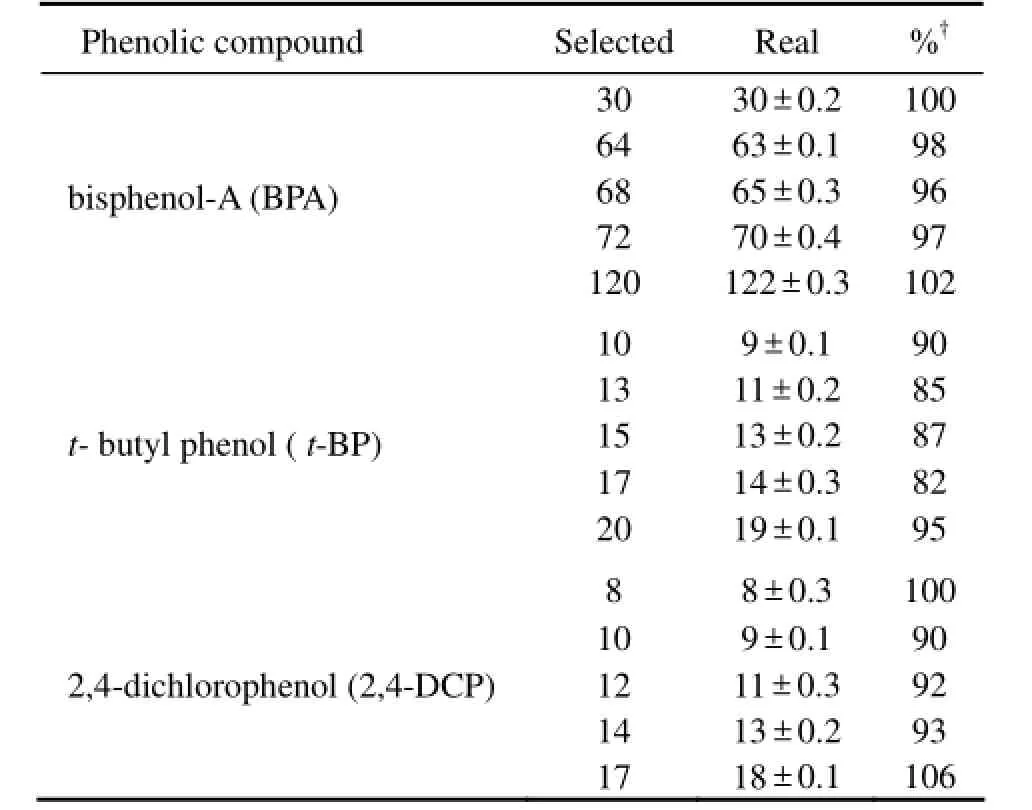
Table 1 The selected and real concentrations (µg L−1) of five phenolic compounds
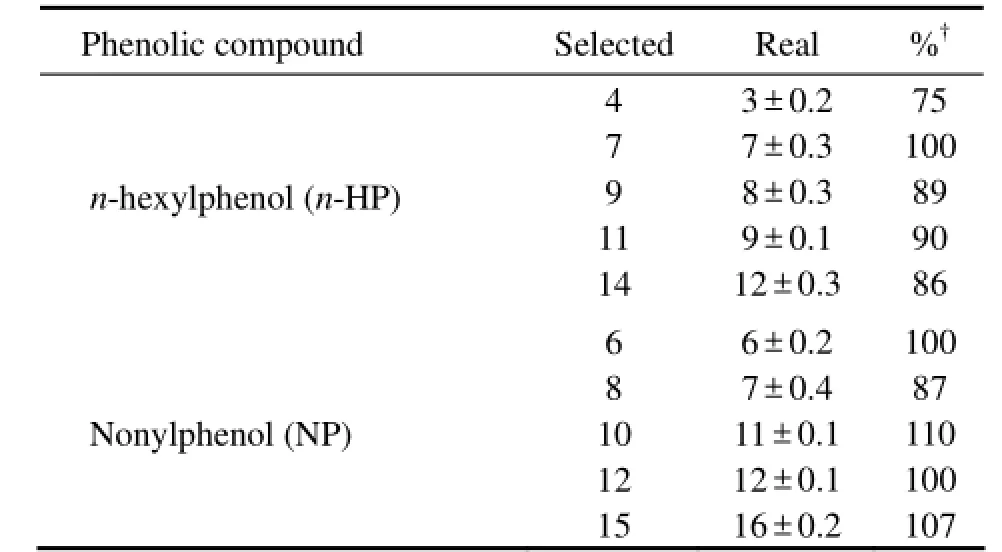
(continued)

Table 2 LC50values†of five phenolic compounds to A. sinica
Statistically significant difference (P<0.05) was detected among the toxicities of five phenolic compounds 3 exposure times each. However, no significant difference (P>0.05) was detected between 2, 4-DCP and NP 3 exposure times each and between 2, 4-DCP andn-HP 48 h and 72 h exposure each.
4 Discussion
The present study was conducted to determine the acute toxicity of five phenolic compounds (BPA,t-BP, 2,-4-DCP,n-HP and NP) to fifteen-days-oldA. sinica. The NOEC and 100% death causing concentration of 5 phenolic compounds each was determined (Fig.1). There were substantial variations in relative order of toxicity among 5 phenolic compounds assayed. However,n-HP and NP were found to be the first two most toxic compounds toA. sinicaat very low concentrations (Table 1).
The relative toxicity of phenolic exposures has been recorded, for instance, chlorophenols greatly influences the organoleptic property of fish and shellfish at ppb range of concentrations (Ruberta, 2001). Walker (1988) listed several most sensitive species to phenolic compounds, which included bluegills,Daphnias, fathead minnows, featherbacks, giant gourami, grass shrimp, guppies andPhotobacterium phosporeum. Hooftman and Vink (1980) documented 96 h LC50values of pentachlorophenol (PCP) to marine polychaete wormOphryotrocha diadema, which ranged from 0.6 to 1.2 mg L−1. The documented included also the LC50of alkylphenol to Atlantic salmonSalmo salar(0.15 to 1.23 mg L−1) and to shrimpCrangon septemspinos(0.15 to 2.7 mg L−1) (McLeeseet al., 1981). Nevertheless, our results withA. sinicacorroborated the findings of above authors that the toxicity of the tested compounds was strongly dependent on the chemical species of the phenolic exposure as well as the test animals.
In the present study, the signif icantly greater sensitivity ofA.sinicaat 72 h of exposure was observed for all phenolic compounds. These results suggested that this stage (72 h) was a better indicator of phenolic compounds potency than either the 24 or 48 h of exposure. The influence of age ofA.sinicaon the toxicity of different chemicals may be related to the fact that growth of test species is rapid.
Several studies have compared the relative sensitivity of crustaceans at different developing stages to phenolic compounds. Ferrandoet al.(1992) found that PCP is more toxic to 48 h and 168 h oldA.salinathan to rotifers (e.g.,Brachionus calyciflorus). Barahona-Gomariz and Sánchez-Fortún (1996) found that 168 h oldA.salinalarvae is 4.76 and 24.30 folds more sensitive to 2,6-dimethylphenol than 24 h and 48 h oldA. salinalarvae, respectively. To freshwater crustaceanDaphnia magna, Adema (1978) found that pure PCP is equally or slightly less toxic to 7 d and 1 d old daphnids, respectively. Barahona-Gomariz and Sánchez-Fortún (1996) also found that 168 h and 48 h oldA. salinawere equally sensitive to PCP, 2,4-dinitrophenol (2,4-DNP), o-nitrophenol, p-nitrophenol, diaminophenol and diamidophenol, while 168 hA. salinais more sensitive to 2,6-dichloroindophenol and 2,6-dimethylphenol than 48 h, and 24 h oldA. salina. So, the sensitivity ofA. sinicain relation to other tested organisms presently used in aquatic toxicology varies with the compound and species.
AgedArtemiais less sensitive to cadmium and mercury than developingArtemia; however the aged is equally sensitive to azide to developingArtemia(Sleet and Brendel, 1985). Ruberta (2001) reported that PCP inhibited the reproduction ofO. diademaand affected the embryo ofCrassostrea gigasandMytilus edulis. Barahona-Gomariz and Sánchez-Fortún (1996) found that 48 h and 168 h oldA. salinaare more sensitive to 2,4-DNP than fish. Vermaet al. (1981) found that 96 h LC50of 2,4-DNP was 1.34 mg L−1forNotopterus notopterus, 0.70 mg L−1for juvenile Atlantic salmon and 18 mg L−1for rainbow trout eggs.
The 24 h LC50of 0.39 mg L−1was recorded forDaphnia magnaexposed to PCP by Ferrandoet al. (1992), while 48 h LC50of 0.33 mg L−1was obtained by Keller (1993) for this species. It seems thatA. salinaandDaphnia magnawere equally sensitive to PCP. However, Stephensonet al. (1991) determined 48 h LC50of pure PCP to adult daphnids,D. magna,D. pulex and D. galeatawere 1.78, 4.59 and 0.51 mg L−1, respectively. In order to compare the toxicity of 2, 4-DCP with that of 2,6-dichloroindophenol (PCP), our findings were compared with the previously documented that chlorophenols are a group of toxic phenolic compounds (Ruberta, 2001). In comparison with other species,A.sinicais sensitive to the toxicants in a concentration dependent manner.
Obviously, the present study emphasized the need to accomplish chronic tests in turn to look at the effects of longer exposures of phenolic compounds to sub-acute concentrations of the organism test on maturation, growth, reproduction and survival, and frequently have more ecological relevance than acute toxicity data.
Acknowledgements
We are very grateful to the reviewers for their valuablecomments. The authors also thank Mr. Yuezhi Yang for his assistance during the GC/MS operation. This study was financially supported by the National Science Foundation of China (Grant Nos. 40876066 and 41106095).
Adema, D. M. M., 1978. Daphnia magna as a test animal in acute and chronic toxicity tests. Hydrobiologia, 59: 125-134.
Barahona-Gomariz, M. V., and Sánchez-Fortún, S., 1996. Comparative sensitivity of three age classes of Artemia salina larvae to several phenolic compounds. Bulletin Environmental Contamination and Toxicology, 56: 271-278.
Barahona-Gomariz, M. V., Sanz-Barrera, F., and Sanchez-Fortun, S., 1994. Acute toxicity of organic solvents on Artemia salina. Bulletin Environmental Contamination and Toxicology, 52: 766-771.
Bradbury, S. P., Henry, T. R., Niemi, G. J., Carlson, R. W., and Snarski, V. M., 1989. Use of respiratory cardiovascular response of rainbow trout (Salmo gairdneri) in identifying acute toxicity syndromes in fi sh. Part 3: Polar narcosis. Environmental Toxicology and Chemistry, 8: 247-262.
Ferrando, M. D., Andreu-Moliner, E., and Fernández-Casalderrey, A., 1992. Relative sensitivity of Daphnia magna and Brachionus calyciflorus to five pesticides. Journal of Environmental Science and Health, Part B, 27: 511-522
Fu, M., Li, Z., and Gao, H., 2007. Distribution characteristics of nonylphenol in Jiaozhou Bay of Qingdao and its adjacent rivers. Chemosphere, 69: 1009-1016.
Go, E. C., Pandey, A. S., and MacRae, T. H., 1990. Effect of inorganic mercury on the emergence and hatching of the brine shrimp Artemia franciscana. Marine Biology, 107: 93-102.
Hadjispyrou, S., Kungolos, A., and Anagnostopoulos, A., 2001. Toxicity, bioaccumulation, and interactive effects of organotin, cadmium, and chromium on Artemia franciscana. Ecotoxicology and Environmental Safety, 49: 179-186.
Hooftman, R. N., and Vink, G. J., 1980. The determination of toxic effects of pollutants with the marine polychaete worm Ophryotrocha diadema. Ecotoxicology and Environmental Safety, 4: 252-262.
Jagetia, G. C., and Aruna, R., 1997. Hydroquinone increases the frequency of micronuclei in a dose-dependent manner in mouse bone marrow. Toxicology Letters, 93: 205-213.
Keller, A. E., 1993. Acute toxicity of several pesticides, organic compounds and a wastewater effluent to the freshwater mussel, Anodonta imbecilis, Ceriodaphnia dubia, and Pimephales promelas. Bulletin of Environmental Contamination and Toxicology, 51: 696-702.
Koutsaftis, A., and Aoyama, I., 2007. Toxicity of four antifouling biocides and their mixtures on the brine shrimp Artemia salina. Science of Total Environment, 387: 166-174.
Li , Z., Li, D., Oh, J. R., and Je, J. G., 2004. Seasonal and spatial distribution of nonylphenol in Shihwa Lake, Korea. Chemosphere, 56: 611-618.
Maldonado-Montiel, T. D. N. J., and Rodríguez-Canché, L. G., 2005. Biomass production and nutritional value of Artemia sp. (Anostraca: Artemiidae) in Campeche, México. Revista de Biologia Tropical/International Journal, 53 (3-4): 447-455.
McLeese, D. W., Zitko, V., Sergeant, D. B., Burridge, L., and Metcalfe, C. D., 1981. Lethality and accumulation of alkylphenol in aquatic fauna. Chemosphere, 10 (7): 723-730.
Mukherjee, D., Bhattacharya, S., Kumar, V., and Moitra, J., 1990. Biological signi fi cance of [14C] phenol accumulation in different organs of a murrel, Channa punctatus, and the common carp, Cyprinus carpio. Biomedical and Environmental Sciences, 3: 337-342.
Naegel, L. C. A., 1999. Controlled production of Artemia biomass using an inert commercial diet, compared with the microalgae Chaetoceros. Aquacultural Engineering, 21: 49-59.
Naessens, E., Lavens, P., Gómez, L., Browdy, C. L., McGovern-Hopkins, K., Spencer, A. W., Kawahigashi, K., and Sorgeloos, P., 1997. Maturation performance of Penaeus vannamei co-fed Artemia biomass preparations. Aquaculture, 155: 87-101.
Persoone, G., and Castritsi-Catharios, M., 1989. A simple bioassay with Artemia larvae to determine the acute toxicity of antifouling paints. Water Research, 23: 893-897.
Ruberta, G., 2001. Ecotoxicology and chemical evaluation of phenolic compounds in industrial effluents. Chemosphere, 44: 1737-1747.
Sarabia, R., Torreblanca, A., Del Ramo, J. J., and Dýaz-Mayans, J., 1998. Effects of low mercury concentration exposure on hatching, growth and survival in the Artemia strain La Mata parthenogenetic diploid. Comparative Biochemistry and Physiology Part A, 120: 93-97.
Sleet, R. B., and Brendel, K., 1985. Homogeneous populations of Artemia nauplii and their potential use in vitro testing in developmental toxicology. Teratogenesis, Carcinogenesis, and Mutagenesis, 5: 41-54.
Stappen, V. G., 1996. Introduction, biology and ecology of Artemia. In: Manual on the Production and Use of Live Food for Aquaculture. Lavens, P., and Sorgeloos, P., eds., FAO Fisheries Technical Paper, 361pp.
Stephenson, G. L., Kaushik, N. K., and Solomon, K. R., 1991. Acute toxicity of pure pentachlorophenol and a technical formulation to three species of Daphnia. Archives of Environmental Contamination and Toxicology, 20: 73-80.
Taysse, L., Troutaud, D., Khan, N. A., and Deschaux, P., 1995. Structure-activity relationship of phenolic compounds (phenol, pyrocatechol and hydroquinone) on natural lymphocytotoxicity of carp (Cyprinus carpio). Toxicology, 98: 207-214.
Tsutsui, T., Hayashi, N., Maizumi, H., Huff, J., and Barrett, J. C., 1997. Benzene-, catechol-, hydroquinone- and phenol-induced cell transformation, gene mutations, chromosome aberrations, aneuploidy, sisterchromatid exchanges and unscheduled DNA synthesis in Syrian hamster embryo cells. Mutation Research, 373: 113-123.
Varó, I., Amat, F., Navarro, J. C., Barred, E., Pitarch, M., and Serrano, R., 2006. Assessment of the efficacy of Artemia sp. (Crustacea) cysts chorion as barrier to chlorpyrifos (organophosphorus pesticide) exposure. Effect on hatching and survival. Science of the Total Environment, 366: 148-153.
Verma, S. R., Rani, S., and Dalela, R. C., 1981. Synergism, antagonism, and additivity of phenol, pentachlorophenol, and dinitrophenol to a fish (Notopterus notopterus). Archives of Environmental Contamination and Toxicology, 10: 365-370.
Walker, J. D., 1988. Relative sensitivity of algae, bacteria, invertebrates, and fish to phenol: analysis of 234 tests conducted for more than 149 species. Toxicity Assessment, 3: 415-447.
Wouters, R., Molina, C., Lavens, P., and Calderón, J., 2001. Lipid composition and vitamin content of wild female Litopenaeus vannamei in different stages of sexual maturation. Aquaculture, 198 (3-4): 307-323.
Yang, L., Li, Z., Li, Z., and Gao, H., 2011. Removal capacity and pathways of phenolic endocrine disruptors in an estuarine wetland of natural reed bed. Chemosphere, 83: 233-239.
(Edited by Qiu Yantao)
* Corresponding author. Tel: 0086-532-66782672
E-mail: gxliu@ouc.edu.cn
(Received March 25, 2012; revised April 24, 2012; accepted September 25, 2013)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
 Journal of Ocean University of China2014年1期
Journal of Ocean University of China2014年1期
- Journal of Ocean University of China的其它文章
- Diagnosis of Physical and Biological Controls on Phytoplankton Distribution in the Sargasso Sea
- Seasonal Distribution of Bioaerosols in the Coastal Region of Qingdao
- Surface Heat Budget and Solar Radiation Allocation at a Melt Pond During Summer in the Central Arctic Ocean
- A Model Study on Dynamical Processes of Phytoplankton in Laizhou Bay
- Properties of Klebsiella Phage P13 and Associated Exopolysaccharide Depolymerase
- Study on Deformation Law of Circular Foundation Under Combined Loading
