Sexual Dimorphism in Mass of the Hindlimb Muscles of the Piebald Odorous Frog (Odorrana schmackeri)
*,
1Department of Zoology, College of Life Sciences, Henan Normal University, Xinxiang 453007, Henan, China
2Huarui College of Xinyang Normal University, Xinyang 464000, Henan, China
Sexual Dimorphism in Mass of the Hindlimb Muscles of the Piebald Odorous Frog (Odorrana schmackeri)
Lixia ZHANG1, Yunyun ZHAO1, Ling SHI, Xiaohong CHEN1*, Youqiang LU1,2and Liang QIAO1
1Department of Zoology, College of Life Sciences, Henan Normal University, Xinxiang 453007, Henan, China
2Huarui College of Xinyang Normal University, Xinyang 464000, Henan, China
Male-biased sexual dimorphism in hind limb muscles is widespread in anuran species where scramble competition is common among males. Such sexual difference is thought to result from sexual selection. In this view, we tested the differences in muscle mass between the sexes and between amplectant and non-amplectant males by quantifying the mass of four hindlimb muscles (triceps femoris, sartorius, gracilis and plantaris longus) of females and males of Odorrana schmackeri. The results showed that females signifi cantly exceeded males for muscle triceps femoris, gracilis, plantaris longus and total mass when controlled for body size. There are no signifi cant differences between amplectant and non-amplectant males. It is probable that the maintenance of the amplectant position in O. schmackeri may depend on the strength of hindlimb muscles in females to support the pair.
Amplexus, hindlimb muscle, Odorrana schmackeri, sexual dimorphism, sexual selection
1. Introduction
In anurans, as in many other organisms, sexes often differ in limb muscle mass as well as overall body size. Such sexual divergence is generally attributed to intra-sexual selection among males (Shine, 1989; Andersson, 1994). The robust forelimbs in males may result from adaptation for amplexus, during which males with robust forelimb muscles presumably are better able to maintain their grasp of the females and thus to reject rivals that attempt to take over his position (Oka et al., 1984, Yekta and Blackburn, 1992; Gaupp, 1896; Duellman, 1992; Peters and Aulner, 2000; Lee, 2001; Clark and Peters, 2006; Navas and James, 2007; Liao et al., 2012a; Mi, 2012). In some species the relatively larger hindlimbs in males may be linked to scramble competition in which amplectant males frequently kick attackers with their hindlimbs (Wells, 1979; Halliday, 1980; Wells, 2007). Moreover, maleswith robust hindlimbs may have a locomotor advantage in swimming or hopping, so that they can reach females fi rst (Lee and Corrales, 2002; Gillis and Biewener, 2000; Mi, 2013). Recently, Liao et al. (2012b) gave a counterexample to the general model that relative hindlimb muscle of females is signifi cantly larger than males in a toad (Bufo andrewsi). The current data suggest that there is no general pattern in hindlimb muscle dimorphism when different species are compared. Thus, the issue needs to be further researched among anurans.
The piebald odorous frog (Odorrana schmackeri) inhabits streams in mountainous forests, which is widely distributed in the southern and south-central areas of China (Fei et al., 2009). It is an explosive breeder with a spawning period of usually less than 2 weeks in late July. In addition, breeding males have swollen nuptial pads on their thumbs and sexually mature females have fully developed oocytes present prior to reproduction (our unpublished data). In recent years, information on population distribution, female-biased dispersal, auditory response characteristics of O. schmackeri have been reported (Ye and Fei, 2001; Fei et al., 2009; Yu et al., 2006; Wang et al., 2012), but little is known about the
evolution of sexual dimorphism in limb muscle mass of this species. The aims of this study were to investigate the intersexual difference in the hindlimb muscle mass and compare the masses of amplectant and non-amplectant males of this frog.
2. Materials and Methods
Specimens were collected during the breeding seasons of 2008 and 2009 at Baotianman Nature Reserve (33°31′N, 111°04′ E, 584 m–654 m elevation), southwest Henan province, China. The samples consisted of 19 females and 21 males, 7 of which were amplectant and 14 of which were calling non-amplectant males. These male frogs were adults which were identified by swollen nuptial pads on their thumbs. All frogs were sacrificed and stored in 10% neutral buffered formalin for dissections in experiment and their body size (snout to vent length, SVL) was measured to the nearest 0.1 mm with digital calipers. All individuals were dissected from 26th November to 2nd December 2012 in lab. Four hindlimb muscles (triceps femoris, sartorius, gracilis and plantaris longus) were dissected, then each muscle was dried to constant mass using a thermostat drier at 60°C and weighed using an electronic balance to the nearest 0.1 mg (Liao et al., 2012b). The action of these muscles as follows: the triceps femoris fl exes the thigh at the hip joint and extends the shank at the knee; the sartorius flexes the thigh at the hip joint and the shank at the knee joint; the gracilis extends the thigh at the hip joint and flexes the shank at the knee joint; the plantaris longus flexes the shank at the knee and the foot at the ankle (Wingerd, 1988). We chose these muscles because they may act in kicking rivals during scramble competition among anuran species engaging in amplexus (Lee and Corrales, 2002; Liao et al., 2012b).
We used independent sample t tests to determine whether significant differences existed between the sexes and between amplectant and non-amplectant males in body size. To test for differences in hindlimb muscle mass between males and females, we ran general linear models (GLMs) with muscles mass as dependent variable, sexes as fi xed term, and body size as covariates. We ran GLMs treating male mating category as a fixed factor and body size as a covariate in order to assess differences in muscles mass between amplectant and nonamplectant males. In addition, we tested the slopes of the linear regression lines for homogeneity and tested the signifi cance of differences in adjusted means by analyses of covariance (ANCOVA). Prior to analyses raw data were transformed to their natural logarithm to correct for heterocedasticity and allometric effects. All probabilities were two-tailed, and the signifi cance level was set at P = 0.05. Data are presented as mean ± SD.
3. Results
The body size of females ranged from 72.2 mm to 84.0 mm (76.9 ± 2.8 mm; n = 19) and that of males from 40.6 to 55.7 mm (46.0 ± 4.5 mm; n = 21), with the former being significantly larger than the latter (t = 25.79, df = 38, P < 0.001). For all cases (males vs. females, amplectant vs. nonamplectant males), the slopes were homogeneous (P > 0.207 for all comparisons). The results of GLMs indicated that the three hindlimb muscles (triceps femoris, gracilis and plantaris longus) and the total muscle mass differed significantly between the sexes when the infl uence of SVL was controlled, but the mass of one muscle (sartorius) did not differ between the sexes (Table 1). Total hindlimb muscle mass regressed signifi cantly on SVL within each sex (Table 1, Figure 1). By ANCOVA analysis, the means of females adjusted for SVL signifi cantly exceeded males for the size of the three muscles and for total mass (all P < 0.007).
Average body size did not differ signifi cantly between amplectant (46.9 ± 4.4 mm) and non-amplectant (45.5 ± 4.6 mm) males (t = 0.89, df = 19, P = 0.491). When controlling for the influence of body size, mass of the muscles of amplectant males were not significantly exceeded that of non-amplectant males (Table 2), and the adjusted means did not differ significantly between the sexes (ANOVA: for all cases P > 0.304).
4. Discussion
Female-biased sexual size dimorphism is common among anuran species (Shine, 1979). A similar trend was also observed in O. schmackeri. Factors underlying the evolution of this pattern have been intensively studied. Fecundity selection is often considered as the ultimate cause because there is often a positive correlation between body size and fecundity (Halliday and Verrell, 1988; Andersson, 1994; Duellman and Trueb, 1994), but it also involves several proximate causes, including sexual differences in growth rate, age at maturity, and adult survivorship (Halliday and Tejedo, 1995; Monnet and Cherry, 2002; Zhang and Lu, 2012). Considering the information that females of this species mature later, thus live longer than males (our unpublished data), it may be reasonable to assume that the females grow to biggerbody size at maturity than males.
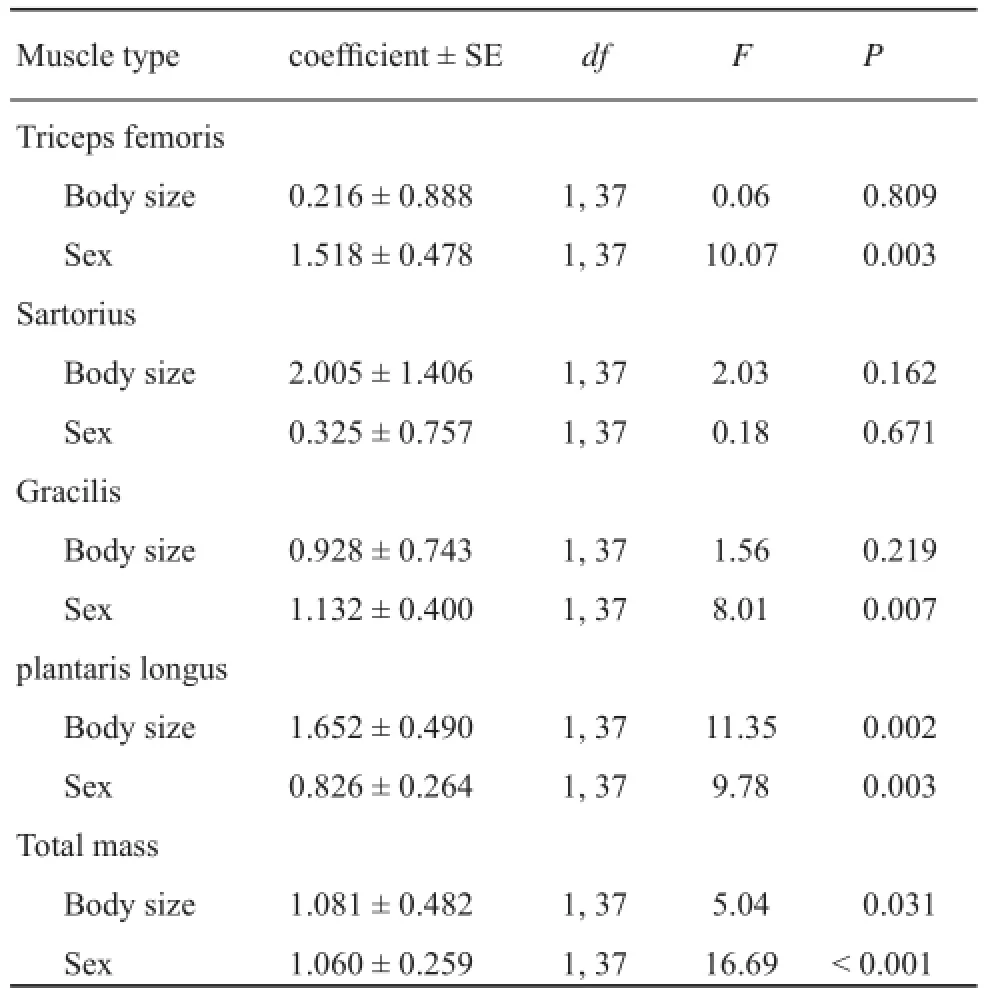
Table 1 Results of general linear models to test for differences in hindlimb muscle mass of Odorrana schmackeri between the sexes.
In anurans, mating success of male frogs is not only related to their ability to clasp a female (amplexus) but also related to their ability to prevent other males taking over their position by kicking before mating occurs (Wells, 1977; Arak, 1983; Elmberg, 1991). This may contribute to the evolution of male-biased sexual dimorphism in hindlimb muscles of anurans. As expected, many studies have found that the hindlimb muscle mass was greater in males than in females, even though females are larger in body size and mass (Lee and Corrales, 2002; Vargas, 2005; Mi, 2013). In O. schmackeri, however, we found the frog with female-biased sexual dimorphism in hindlimb muscles when removing the effect of body size. Why might this species show a reversed pattern of hindlimb dimorphism? O. schmackeri is a stream dweller which breeds in the rapid rocky-bedded streams of the forested uplands (Fei et al., 2009). In such adverse circumstances, it might be possible that the females with robust hindlimbs could maintain balance better during amplexus. The results were similar to those reported in B. andrewsi which lives in subtropical montane region where the conditions is harsh than flatlands, although their spawning sites are located in small, shallow pools near rivulets (Liao and Lu, 2009; Liao et al., 2012b). In addition, three hindlimb muscles mass (triceps femoris, gracilis and plantaris longus) in females was signifi cantly larger than in males, suggesting that these muscles may play an important role in maintenance the amplectant position. However, we did not fi nd a signifi cant difference in the muscle mass of sartorius between sexes. This may suggest that the muscle of females may play a minor role in pair-maintenance.
Some researchers reported that hindlimb muscles differ between amplectant and non-amplectant males and speculated that males with more robust hindlimbs may produce the vigorous kicking motions during amplexus and thus resist attacks (Gillis and Biewener, 2000; Lee and Corrales, 2002; Vargas, 2005; Mi, 2013), but other researchers have failed to find such a trend (e.g., B.andrewsi: Liao et al., 2012b; O. schmackeri: this study). The lack of muscle size difference between amplectant and non-amplectant males may be suggest that there is little intrasexual selection on the hindlimbs in this species. Scramble-competition polygyny is currently recognized as common among anurans (Wells, 1977), especially in explosive breeding species where scramble competition (many males attempt to dislodge a male that is already
grasping a female) is more extensive (Wells, 1977; Arak, 1983; Elmberg, 1991). Based on over three years of fi eld research, however, we did not fi nd that males of O. schmackeri exhibit scramble competition for mates. This may be why there is no difference in muscle size between amplectant and non-amplectant males. In addition, most research studies have found that linear regression of the total hindlimb muscle mass on SVL is highly signifi cant (Lee and Corrales, 2002; Liao et al., 2012b; Mi, 2013). The hindlimb muscle mass of the piebald odorous frog followed a similar pattern in each sex. This may suggest that the evolution of hindlimb muscles in O. schmackeri is not independent of body size under natural or sexual selection pressure.
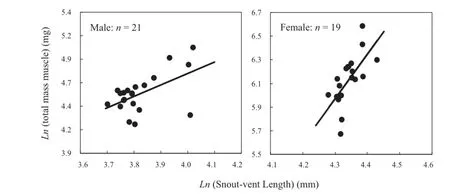
Figure 1 Total hindlimb muscle mass (triceps femoris + sartorius + gracilis + plantaris longus) of Odorrana schmackeri as a function of body size. Ln indicates natural lognrithms.

Table 2 Results of general linear models to test for differences in hindlimb muscle mass of Odorrana schmackeri between amplectant and non-amplectant males.
Though O. schmackeri is an explosive breeder, it is very difficult to find amplectant individuals in the field. The accuracy of these results may be affected by small sample size, so further studies are needed to avoid the pitfalls of them. In conclusion, the current research reveals that sexual dimorphism in hindlimb muscles is present in the piebald odorous frog, with females showing more robust hindlimb muscles than those of males. This may suggest a stronger effect of natural selection on females to maintain pair stability and oviposition sites.
AcknowledgementsWe thank Lei LI, Jie YANG and the staff of Baotianman Nature Reserve for field assistance and Yunyun ZHAO and Panpan JIA for lab assistance, as well as two anonymous referees for their comments on an earlier draft of the manuscript. This study was supported by National Sciences Foundation of China (No. 31372164, 30870277) and the Scientific Research Foundation of Henan Normal University (No. 01046500145), as well as Joint Funds for Fostering Talents of NSFC and the People’s Government of Henan Province (Grant No. U1304309).
Andersson M.1994. Sexual selection. Princeton, NJ: Princeton University Press
Arak A.1983. Male–male competition and mate choice in anuran amphibians. In: Bateson P. (ed.), Mate Choice. pp. 181-210. Cambridge, Cambridge University Press
Clark D. L., Peters S. E.2006. Isometric contractile properties of sexually dimorphic forelimb muscles in the marine toad Bufo marinus Linnaeus 1758: Functional analysis and implications for amplexus. J Exp Biol, 209: 3448–3456
Duellman W. E., Trueb L.1994. Biology of Amphibians. Baltimore, Johns Hopkins University Press
Duellman W. E.1992. Reproductive strategies of frogs. Scient Am, 267: 80–87
Elmberg J.1991. Factors affecting male yearly mating success in the common frog, Rana temporaria. Behav Ecol Sociobiol, 28: 125–131
Fei L., Hu S. Q., Ye C. Y., Huang Y. Z.2009. Fauna Sinica, Amphibia, Anura Ranidae. Beijing, Science Press
Gaupp E.1896. Anatomie des Frosches. 1. Abt. Lehre vom Skelet und vom Muskelsystem. Braunschweig, Vieweg und Sohn
Gillis G. B., Biewener A. A.2000. Musculoskeletal mechanisms for accommodating locomotion in different environments: Hind limb extensor muscle function during hopping and swimming in the toad (Bufo marinus). J Exp Biol, 203: 3547–3563
Halliday T.1980. Sexual Strategy. Chicago, University of Chicago Press
Halliday T. R., Verrell P. A.1988. Body size and age in amphibians and reptiles. J Herpetol, 22: 253–265
Halliday T., Tejedo M.1995. Intrasexual selection and alternative mating behaviour. In: Heat-wole H., Sullivan B. K. (Eds.) Amphibian Biology. Vol. 2. Social Behavior, pp. 419-468. New South Wales, Surrey Beatty and Sons, Chipping Norton
Lee J. C.2001. Evolution of a secondary sexual dimorphism in the toad, Bufo marinus. Copeia, 2001: 928–935
Lee J. C., Corrales A. D.2002. Sexual dimorphism in hind-limb muscle mass is associated with male reproductive success in Bufo marinus. J Herpetol, 36: 502–505
Liao W. B., Lu X.2009. Sex recognition by male Andrew’s toad Bufo andrewsi in a subtropical montane region. Behav Process, 82:100–103
Liao W. B., Wu Q. G., Barrett K.2012a. Evolution of sexual dimorphism in the forelimb muscles of Andrew’s toad (Bufo andrewsi) in response to putative sexual selection. Anim Biol, 62: 83–93
Liao W. B., Liao Y. M., Xiao W. M., Chen W., Mi Z. P., Li C.2012b. Sexual dimorphism in hind limb muscle mass of the Andrew’s toad (Bufo andrewsi) in relation to sexual selection. NW J Zool, 8: 252–256
Mi Z. P.2012. Sexual dimorphism in the forelimb muscles of the Asiatic toad Bufo gargarizans. Herpetol J, 22: 219–224
Mi Z. P.2013. Sexual dimorphism in the hindlimb muscles of the Asiatic toad (Bufo gargarizans) in relation to male reproductive success. Asian Herpetol Res, 4: 56–61.
Monnet J. M., Cherry M. I.2002. Sexual size dimorphism in anurans. Proc Roy Soc B, 269: 2301–2307
Navas C. A., James R. S.2007. Sexual dimorphism of extensor carpi radialis muscle size, isometric force, relaxation rate and stamina during the breeding season of the frog Rana temporaria Linnaeus 1758. J Exp Biol, 210: 715–721
Oka Y, Ohtani R, Satou M., Ueda K.1984. Sexually dimorphic muscles in the forelimb of the Japanese toad, Bufo japonicus. J Morph, 180: 297–308
Peters S. E., Aulner D. A.2000. Sexual dimorphism in forelimb muscles of the bullfrog, Rana catesbeiana: A functional analysis of isometric contractile properties. J Exp Biol, 203: 3639–3654
Shine R.1979. Sexual selection and sexual dimorphism in the Amphibia. Copeia, 1979: 297–306
Shine R.1989. Ecological causes for the evolution of sexual dimorphism: A review of the evidence. Q Rev Biol, 64: 419–461
Vargas-Salinas F.2005. Bufo marinus: amplexus displacement. Herpetol Rev, 3: 431–432
Wang Y., Lane A., Ding P.2012. Sex-biased dispersal of a frog (Odorrana schmackeri) is affected by patch isolation and resource limitation in a fragmented landscape. Plos One, 7: e47683
Wells K. D.1977. The social behaviour of anuran amphibians. Anim Behav, 25: 666–693
Wells K. D.1979. Reproductive behavior and male mating success in a Neotropical toad, Bufo typhonius. Biotropica, 11: 301–307
Wells K. D.2007. The ecology and behavior of amphibians. Chicago, University of Chicago Press
Wingerd B. D.1988. Frog Dissection Manual. JHU Press
Ye C. Y., Fei L.2001. Phylogeny of genus Odorrana (Amphibia: Ranidae) in China. Acta Zool Sin, 47: 528–534
Yu Z. L. Qiu Q., Xu Z. M., Shen J. X.2006. Auditory response characteristics of the piebald odorous frog and their implications. J Comp Physiol A, 192: 801–806
Zhang L. X., Lu X.2013. Sexual size dimorphism in anurans: Ontogenetic determination revealed by an across-species comparison. Evol Biol, 40: 84–91
*Corresponding author: Prof. Xiaohong CHEN, from Henan Normal University, Xinxiang, Henan, China, with her research focusing on the evolution of anurans.
E-mail: xhchen-xx@sohu.com
Received: 21 March 2014 Accepted: 1 December 2014
 Asian Herpetological Research2014年4期
Asian Herpetological Research2014年4期
- Asian Herpetological Research的其它文章
- Population Dynamics Following the Last Glacial Maximum in Two Sympatric Lizards in Northern China
- A New Species of the Genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a Proposal for an Eclectic Rule for Species Delimitation
- Group-spawning and Simultanous Polyandry of a Stream-dwelling Frog Feirana kangxianensis
- Can an Invasive Prey Species Induce Morphological and Behavioral Changes in an Endemic Predator? Evidence from a South Korean Snake (Oocatochus rufodorsatus)
- Food Habits and Distribution of the Lake Taal Sea Snake (Hydrophis semperi Garman 1881) and the Sympatric Little File Snake (Acrochordus granulatus Schneider 1799) in Lake Taal, Philippines
- Body Size and Reproductive Tactics in Varanid lizards
