Body Size and Reproductive Tactics in Varanid lizards
1Hangzhou Key Laboratory for Animal Adaptation and Evolution, School of Life Sciences, Hangzhou Normal University, Hangzhou 310036, Zhejiang, China
2Hainan Key Laboratory for Herpetology, School of Life Sciences, Qiongzhou University, Sanya 572022, Hainan, China
3Jiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University, Nanjing 210046, Jiangsu, China
Body Size and Reproductive Tactics in Varanid lizards
Yu DU1,2, Longhui LIN1*, Yuntao YAO1, Chixian LIN2and Xiang JI3
1Hangzhou Key Laboratory for Animal Adaptation and Evolution, School of Life Sciences, Hangzhou Normal University, Hangzhou 310036, Zhejiang, China
2Hainan Key Laboratory for Herpetology, School of Life Sciences, Qiongzhou University, Sanya 572022, Hainan, China
3Jiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University, Nanjing 210046, Jiangsu, China
Body size and female reproduction in the water monitor lizard (Varanus salvator) were studied. Forty-two adult females larger than 500 mm SVL and 32 adult males larger than 400 mm SVL were donated by local people in Ledong, Hainan under permit to our laboratory in Hainan in 2013 and 2014. The largest male and female measured 745 and 755 mm SVL, respectively. The mean SVL was greater in adult females than in adult males. Males had larger heads (head width) than females of the same SVL. The smallest reproductive female in our sample was 565 mm SVL. Females produced a single clutch of 17.1 (10-23) pliable-shelled eggs per breeding season stretching from mid-June and mid-September. Clutch size and clutch mass were all positively related to female SVL. However, there was no signifi cant linear relationship between egg mass and female SVL. Larger females generally produced more eggs, and thus heavier clutches than did smaller ones. There was no signifi cant linear relationship between relative clutch mass and female SVL. Phylogenetic generalized least squares (PGLS) analysis, accounting for phylogenetic relationships, showed that clutch size was positively correlated with mean maternal SVL in varanid lizards. PGLS analysis showed that phylogenetic relationships did not affect clutch (or/and egg) mass and the SVL although there were significant linear relationship between clutch (or/and egg) mass and mean maternal SVL. Therefore, we could draw some general conclusions about the body size and reproductive tactics in varanid lizards that larger females generally produced more eggs, larger eggs and thus heavier clutches than did smaller ones.
body size, female reproduction, monitor lizard, Varanidae
1. Introduction
Fifty-three species of Varanus are now currently recognized wordwide (Pianka and King, 2004). They are morphologically conservative but vary widely in size (Pianka, 1994). Such a diverse monophyletic group can be exploited both to identify and to understand the actual course of evolution. Small body size has evolved three times among varanids, in the Australian Odatriaclade, and, in the Asian clade, in V. fl avescens and in the prasinus species complex; large body size also evolved in V. bengalensis and V. salvator in the Asian clade and independently in the common ancestor to V. salvadorii, V. komodoensis, and V. varius, as well as in the Australian perentie V. giganteus (Pianka, 1994).
Thopson and Pianka (2001) reviewed various aspects of the evolution of reproductive tactics among monitor lizards. Body size influences reproductive tactics more strongly than phylogeny; eggs of small species are laid in the spring and hatch in the summer; eggs of larger species are laid later, often overwinter, and the next year; smaller species have relatively larger hatchlings and larger clutch size compared with adult size than do larger
species. However, most of the conclusions are drawn by accounting for the maximum snout-vent length (SVL) rather than average maternal SVL, which is a better variable. Moreover, these data were collected more than ten years ago and new data are available. It is necessary to take a new review on these aspects.
Among the fifty-three species of Varanus, the water monitor lizard (V. salvator) is a relatively large-sized lizard. It has the largest distribution area of all recent varanids. It is recorded from Bangladesh, Brunei, Burma, southwestern China, northeastern India, Indonesia, Kampuchea, Laos, Malaysia, Singapore, Sri Lanka, Thailand, and Vietnam (Smith, 1932; Pianka and King, 2004). It has a wide range of variation in body size from hatchling to adulthood, and this feature makes the lizard well suited to the studies addressing the role of body size in influencing reproductive strategy. Here, we studied sexual dimorphism and female reproduction in V. salvator at the our labortatory in Hainan, China between 2013 and 2014 to evaluate sexual dimorphism in morphological characters such as body size and head size, and to investigate the relationships among clutch size, egg mass, clutch mass and female size in V. salvator. We also collected data from recently published references in female reproductive characteristics in varanid lizards, using the maternal SVL instead of maximum SVL, to examine different reproductive variables relationships while accounting for phylogenetic relationships.
2. Materials and methods
Forty-two adult females larger than 500 mm SVL and 32 adult males larger than 400 mm SVL were donated by local people in Ledong, Hainan under permit to our laboratory in Hainan in 2013 and 2014. All lizards were maintained in 30 m × 30 m × 2 m (1ength × width × height) enclosures, of which each was half-covered by a sun-shading net, had a 5 m × 5 m × 0.4 m pond, tree branches and bark hides, and housed 12-14 individuals. Chicken (Gallus gallus domestica) and fish (Tilapia mossambica) were provided daily so that excess food was always available. All enclosures were serviced weekly. This included cleaning or changing water in the pond, removing fecal matter, slough and dead food items, and checking animal well-being. The lizards were disturbed only if measuring, weighing or physical examinations were required.
Morphological measurements taken for each individual included body mass, snout-vent length (SVL), abdomen length (the distance between the points of insertion of the fore- and hind-limbs), tail length, forelimb length (humerus plus ulna), hindlimb length (femur plus tibia), head length, head width, interorbital distance, nostril diameter, internasal distance, 4thfinger length, 4thtoe length, eye diameter and tympanum diameter. All of these measurements were taken when the lizards calmed down, without anesthetics.
Egg-laying activities were monitored in real time using an infrared video camera with 16 probes, such that eggs could be always collected, measured and weighed soon after being laid. SVL and body mass were taken for each postpartum female. Eggs were measured for length and width and weighed. Relative clutch mass was calculated by dividing clutch mass by the female postpartum mass.
Prior to parametric analyses, all data were tested for normality using the Kolmogorov-Smirnov test and for homogeneity of variances using the Bartlett’s test. We used one-way analysis of variance (ANOVA) and oneway analysis of covariance (ANCOVA) to analyze the corresponding data. The homogeneity of slopes was checked prior to testing for differences in the adjusted means. Throughout this paper, values are presented as mean ± SE, and the signifi cance level is set at α = 0.05. Ordinary least squares (OLS) regression estimation was used to estimate slope for all conventional analyses. OLS regression was implemented on the R 2.15.3 (R Development Core Team, 2013), using the SMATR packages (Warton et al., 2012). We used phylogenetic generalized least squares (PGLS) regression methods (Martins and Hansen, 1997; Garland and Ives, 2000; Rohlf, 2001) to examine different variables relationships (e.g. SVL and clutch size / clutch mass / egg mass) while accounting for phylogenetic relationships among species. PGLS which is functionally equivalent to phylogenetically independent contrast method (Felsenstein, 1985; Garland and Ives, 2000) when assuming that residual variation between species is correlated through an evolutionary process along the specifi ed phylogenetic tree similar to a Brownian-motion model. PGLS incorporates phylogenetic information into generalized linear models offers a powerful method for analyzing continuous data that has been applied to estimation the evolutionary model and the relationships among life-history traits (Warne and Charnov, 2008; Barros et al., 2011). The PGLS method fi ts a linear model according to phylogenetic non-independence between data points. The strength and type of the phylogenetic signal in the data matrix can also be elucidated by adjusting branch length transformations, which can be optimized to fi nd the maximum likelihood transformation
given the data and the models (Orme et al., 2012). We used λ to analysis phylogenetic effects (λ = 0 indicates no phylogenetic effect, and λ = l indicates a strong phylogenetic effect equivalent to that expected under the Brownian motion model) and Akaike Information Criterion (AIC) to estimate merits and drawbacks of the models in the set used and the best model has the lowest AIC. PGLS regression analysis was implemented with the R package caper (Orme et al., 2012). The tests detailed previously were carried out using the topology including all collected species. This topology of species was based on proximate phylogenetic correlation assembled from Pyron et al. (2013). This tree were drawn using Mesquite (Maddison and Maddison, 2011). Because branch lengths were lacking divergence time, genetic distance or any other metric proportional to the expected variance for the evolution of each analyzed trait are unavailable, we arbitrarily set initial branch length to a value of 1, which is appropriate for a speciation model of evolution (Martins and Garland, 1991).
The model with better fit can be determined by a maximum-likelihood ratio test in which twice the difference in the natural log of the maximum likelihoods (LnL) of the OLS and PGLS models will be distributed approximately as a χ2with degrees of freedom equal to the difference in the number of parameters estimated in the two models (Warne and Charnov, 2008).
3. Results
Sexual dimorphismAll the other 13 morphometric variables were positively correlated with SVL (each P< 0.05).The largest male and female measured 745 and 755 mm SVL, respectively. The mean SVL was greater in adult females (641.0 ± 9.4 mm, N = 42) than in adult males (601.0 ± 13.5 mm, N = 32; ANOVA, F1,72= 6.314, P = 0.014); males had larger heads (HW) than females of the same SVL, whereas between-sex differences in the other 12 morphometric variables were not found (Table 1).
Female reproductionThe smallest reproductive female in our sample was 565 mm SVL. Females produced a single clutch of 17.1 (10-23) pliable-shelled eggs per breeding season stretching from mid-June and mid-September (Table 2). Clutch size (r = 0.82, F1,12= 24.798, P < 0.001) and clutch mass (r = 0.68, F1,12= 10.451, P = 0.007) were all positively related to female SVL (Figure 1). However, there was no signifi cant linear relationship between egg mass and female SVL (F1,12= 0.014, P >0.05). Larger females generally produced more eggs, and thus heavier clutches than did smaller ones. There was no signifi cant linear relationship between relative clutch mass and female SVL (F1,12= 0.008, P = 0.929).
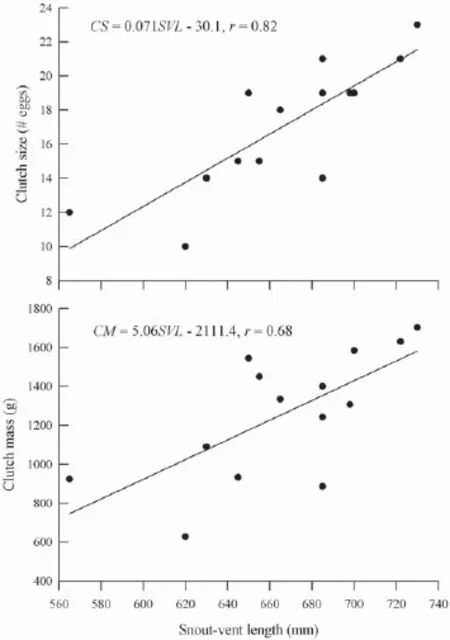
Figure 1 Linear regressions of clutch size and clutch mass on female SVL in Varanus salvator. Regression equations and coeffi cients are given in the fi gure.
Reproductive tactics in Varanid lizardsWe assembled published and our own research data on mean maternal SVL, clutch size, clutch mass, and egg mass for Varanid lizards (Table 3). Data from 30 species of Varanid lizards show that mean clutch size ranged from 3.4 eggs to 25.5 eggs and the size of gravid females ranged from 91 mm to 1340 mm. Table 4 summarizes the relationships among female reproductive traits in Varanid lizards according to OLS and PGLS analyses. Mean clutch size was positively correlated with mean SVL in both the OLS and PGLS model; and on the basis of likelihood ratio tests, PGLS model were better than OLS model (Figure 2, Table 4). PGLS analysis showed that phylogenetic relationships did not affect clutch (or/and egg) mass and the SVL (both λ = 0) although there were signifi cant linear relationship between clutch (or/and egg) mass and mean maternal SVL (Figure 2, Table 4).
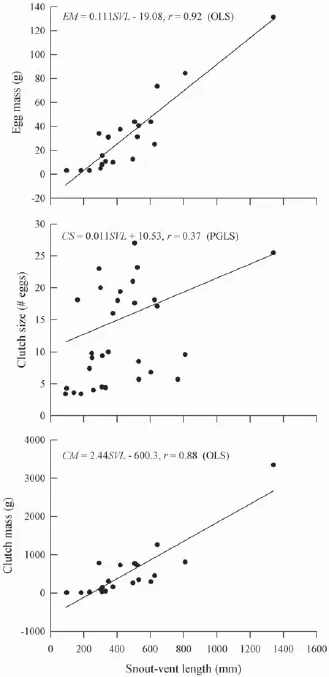
Figure 2 Ordinary least squares (OLS) regression of egg mass and clutch mass on female SVL, and phylogenetic generalized least squares (PGLS) regression of clutch size on female SVL in varanid lizards. Regression equations and coeffi cients are given in the fi gure.
4. Discussion
External morphological characters are conventionally used to describe monitor lizard species and are categorized into meristic (quantified using numbers or counts) and morphometric (quantifi ed by measurements) features (Arida and Böhme, 2010). Among the 14 morphometric variables measured, only SVL and HW showed between-sex differences. Males had larger heads (HW) than females of the same SVL, where as female had larger SVL than males. Male water monitor lizards have larger heads than females, suggesting that sexual selection could have been a factor in the evolution of large heads of varanid lizards. Larger heads (hence large mouth) have an edge in the process of combat. Male-male ritual combat is pronounced in V. salvator, with males standing erect on their hind legs and tail, chests pressed together, grappling with their forelegs wrapped around each other (Pianka and King, 2004). The two contenders try to throw on another off balance during the “clinch phase”; sometimes the winner bites the loser (King and Green, 1999). Females having larger SVL is related to female reproduction. Females should be the larger sex in species where reproductive success is more tightly linked to body size in adult females. Selection acting to increase fecundity and litter volume is the main cause for increased female size in Gekko japonicus (Japanese gecko; Ji et al., 1991), Sphenomorphus indicus (brown forest skink; Ji and Du, 2000), and Phrynocephalus vlangalii (Qinghai toad-headed lizard; Zhang et al., 2005). Previous studies (Mertens, 1942; Shine et al., 1996) reported that tail length shows ontogenetic and sexual dimorphism (short in older ones and longer in males) in V. salvator, but we did not fi nd between-sex differences in tail length in this study.
Previous studies reported that V. salvator eggs are very variable in size, a length of 64 to 82.6 mm, a width of 32.3 to 45 mm, and a weight of 30 to 87.2 g is reported from different countries, with a high size variability occurring in one population and even in different clutches of one female (Schmidt, 1927; Meer Mohr, 1930; Kratzer, 1973; Anonymous, 1978; Vogel, 1979; Biswas and Kar, 1981; Moharana and Pati, 1983; Andrews and Gaulke, 1990). In this study, egg length, egg width and egg mass also show large variation (Table 2), however, none of them was signifi cantly related to female SVL. Maybe egg size is more associated with other factors such as food availability, water and even heat.
Our results indicated no signifi cant linear relationship between relative clutch mass and female SVL. In a previous study, relative clutch mass of V. tristis (with large clutch size of 10 eggs) is similar to that of other sympatric desert varanids with smaller clutch size (Pianka, 1994), suggesting that its larger clutch is achieved at the expense of relative neonate size (hatchling V. tristis are relatively small compare do adults). Thus, it seems that relative clutch mass remains relatively constant not only within but also between species.
Pianka and King (2004) reported that clutch size in V. salvator ranged from 5 to 22 eggs, with a mean of 13 eggs (data collected during different visits to a skinnery inSouth Sumatra). In a breeding group of V. salvator at the Madras Crocodile Bank, India (originating from Orissa, India), females laid one or two clutches per year, with a mean clutch size of 13.8 (Andrews and Gaulke, 1990). All mature males investigated in skinneries in North Sumatra were reproductively active, with clutch size ranging from 6 to 17 eggs (Shine et al., 1998). In this study, female monitor lizards produced a single clutch of 17.1 (10-23) pliable-shelled eggs per breeding season. Eggs were eaten by other neighbor monitor lizard during ovipositions. We observed the phenomenon of cannibalism by an infrared video camera installed in the enclosure. Thus, smaller clutch size reported in previous studies might be caused by the neglect of this cannibal behavior.
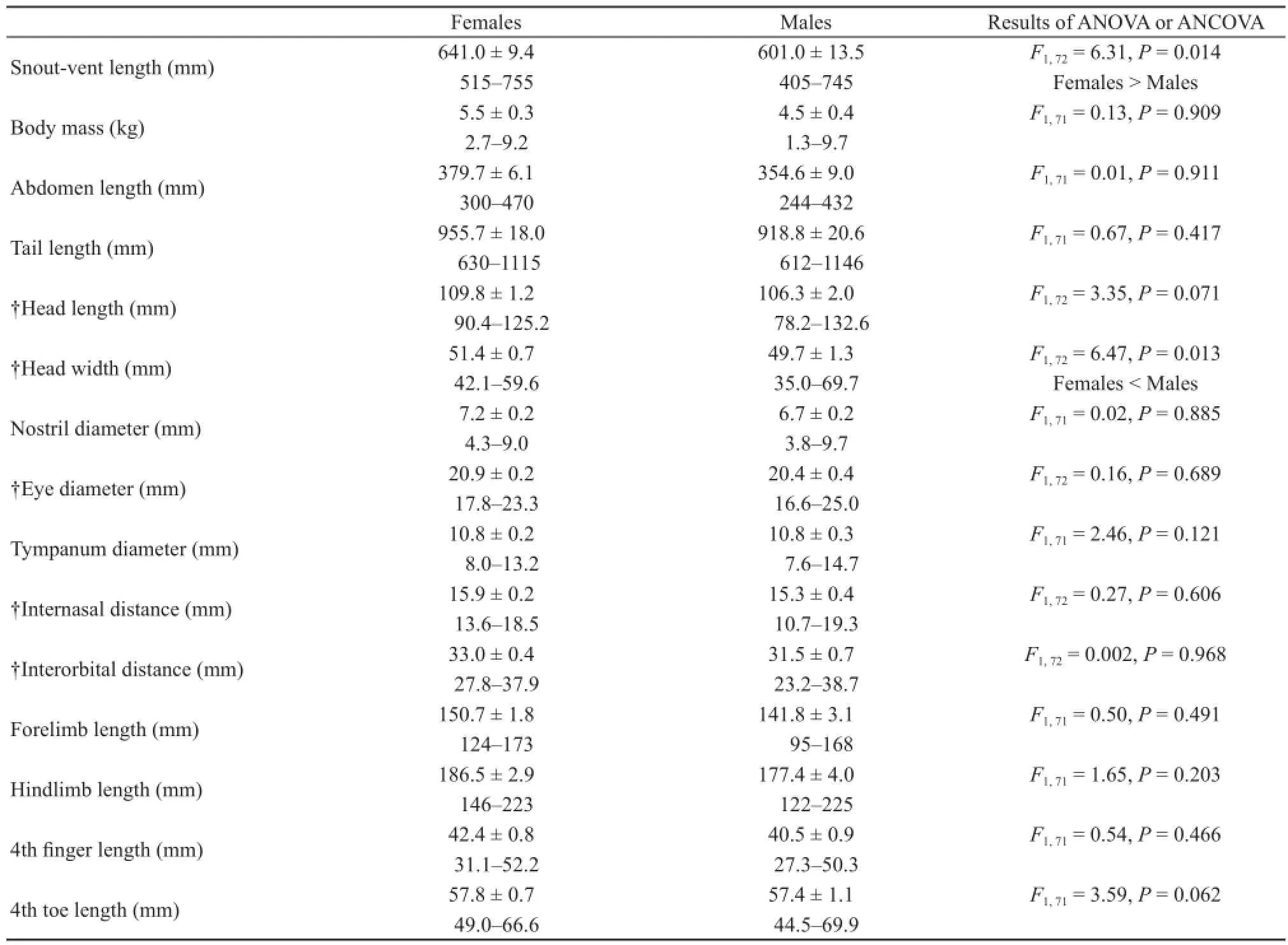
Table 1 Descriptive statistics, expressed as mean ± SE and range, for size and morphology of 74 water monitor lizards (42 females, and 32 male). Results of one-way ANOVA (for SVL and variables with a mark of †) or ANCOVA (for the remaining variables) with SVL as the covariate are given in the table. Variables with a mark of † were analyzed using unequal slopes models.
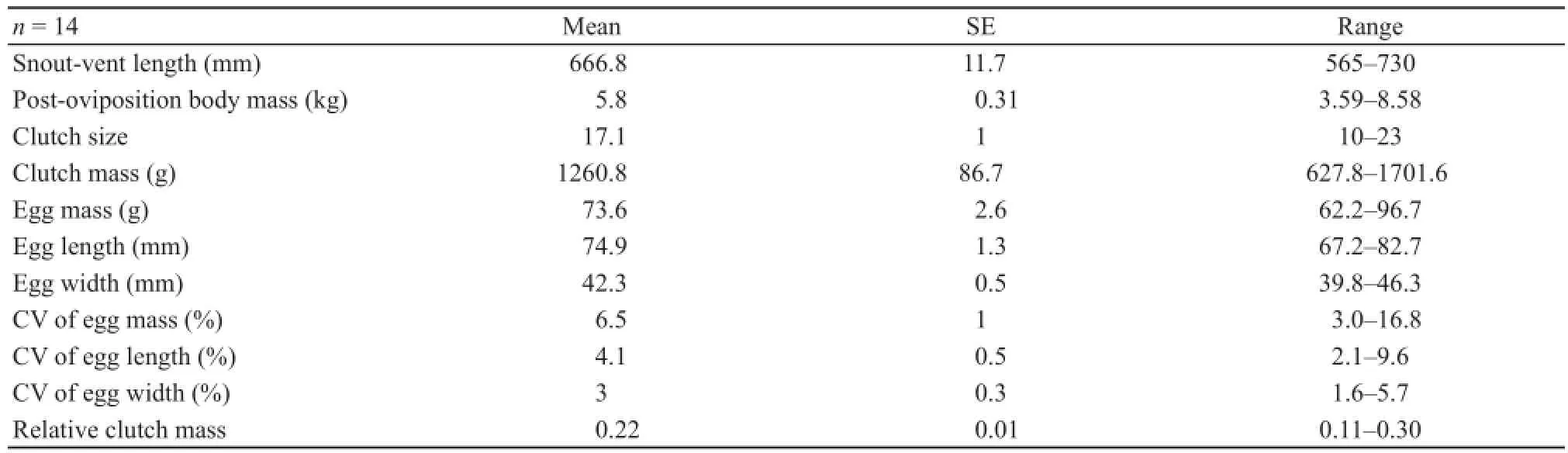
Table 2 Descriptive statistics, expressed as mean ± SE and range, for female reproductive characteristics of Varanus salvator.

Table 3 Descriptive statistics for female reproductive characteristics in varanid lizards. Data were collected from 6 references (Pianka, 1995; Thompson and Pianka, 2001; Pianka et al., 2004; Gaikhorst et al., 2010; Xu et al., 2010; Mendyk, 2011).
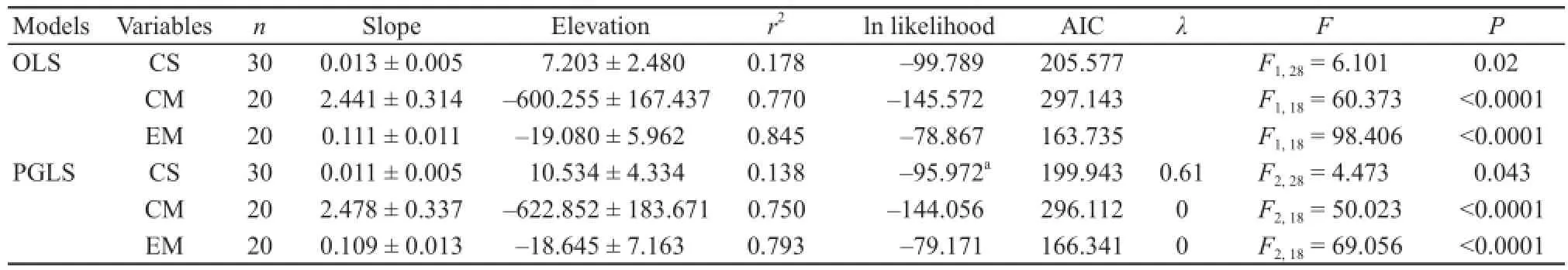
Table 4 Regressions of clutch size (CS), clutch mass (CM) and egg mass (EM) on snout-vent length (SVL) in varanid lizards based on ordinary least squares (OLS) regression and phylogenetic generalized least squares (PGLS) regression.aOn the basis of likelihood ratio tests, the models which are labeled statistically significantly are better than the corresponding regression models between same variables. Signifi cant associations between variables are shown in bold.
There are two basic consensuses on clutch size: (1) Clutch sizes are larger and more variable among larger species [e.g., V. spenceri has much larger clutch size (20 eggs) than does its similar-sized sister species, V. mertensi, which lays only about 8 eggs; V. salvator are considerably larger than their sister species, V. rudicollis, and the former species lay 17 eggs (in this study, Table 2), where as the latter lays 8 eggs]; (2) Maternal SVL influences clutch size much more strongly within a species than it does between species (Purvis and Rambaut, 1995; Thompson and Pianka, 1999, 2001; Pianka and King, 2004). In this study, clutch size and clutch mass were all positively related to female SVL (Figure 1). Larger females generally produced more eggs, and thus heavier clutches than did smaller ones in V. salvator.
PGLS analysis, accounting for phylogenetic relationships, showed that clutch size was positively correlated with mean maternal SVL (Figure 2, Table 4). PGLS analysis showed that phylogenetic relationships did not affect clutch (or/and egg) mass and the SVL
although there were significant linear relationship between clutch (or/and egg) mass and mean maternal SVL (Figure 2, Table 4). Similar results were found in some Phrynocephalus lizards, which indicated that ecological processes play a more important role than phylogeny in shaping patterns of reproductive variation (Jin et al., 2003). Therefore, we could draw some general conclusions about the body size and reproductive tactics in varanid lizards that larger females generally produced more eggs, larger eggs and thus heavier clutches than did smaller ones.
AcknowledgementsFinancial supports were provided by grants from Natural Science Foundation of China (31270571) and Hainan Key Program of Science and Technology (ZDXM20110008) and 131 Talent Project of Hangzhou City. We are grateful to Yanfu QU for assistance in the laboratory.
Andrews H. V., Gaulke M.1990. Observations on the reproductive biology and growth of the water monitor (Varanus salvator) at the Madras Crocodile Bank. Hamadryad, 15: 1–5
Anonymous.1978. Varanus salvator breeding at Madras Snake Park. Hamadryad, 3: 4
Arida E., Böhme W.2010. The origin of Varanus: When fossils, morphology, and molecules alone are never enough. Biawak, 4: 117–124
Barros F. C., Herrel A., Kohlsdorf T.2011. Head shape evolution in Gymnophthalmidae: Does habitat use constrain the evolution of cranial design in fossorial lizards? J Evol Biol, 24: 2423–2433
Biswas S., Kar S.1981. Some observations on nesting habits and biology of Varanus salvator (Laurenti) of Bhitarkanika Sanctuary, Orissa. Rec Zool Surv India, 73: 95–109
Felsenstein J.1985. Phylogenies and the comparative method. Am Nat, 125: 1–15
Gaikhorst G., McLaughlin J., Larkin B., McPharlin M.2010. Successful captive breeding of Mitchell’s Water Monitor, Varanus mitchelli (Mertens 1958), at Perth Zoo. Zoo Biol, 29: 615–625
Garland Jr. T., Ives A. R.2000. Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am Nat, 155: 346–364
Ji X., Du W. G.2000. Sexual dimorphism in body size and head size and female reproduction in a viviparous skink Sphenomorphus indicus. Zool Res, 21: 349–354
Ji X., Wang P. C., Hong W. X.1991. The reproductive ecology of the gecko Gekko japonicus. Acta Zool Sinica, 37: 185–192
Jin Y. T., Li J. Q., Liu N. F.2003. Elevation-related variation in life history traits among Phrynocephalus lineages on the Tibetan Plateau: do they follow typical squamate ecogrographic patterns? J Zool, 290: 293–301
King D., Green B.1999. Monitors: The biology of varanid lizards. Krieger Malabar Fla
Kratzer H.1973. Beobachtungen über die Zeitigungsdauer eines Eigeleges von Varanus salvator. Salamandra, 9: 27–33
Maddison W. P., Maddison D. R.2011. Mesquite: A modular system for evolutionary analysis. Version 2.75. http:// mesquiteproject.org
Martins E., Garland T.1991. Phylogenetic analyses of the correlated evolution of continuous characters: A simulation study. Evolution, 45: 534–557
Martins E. P., Hansen T. F.1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecifi c data. Am Nat, 149
Meer Mohr J. C.1930. Over eieren van Varanus salvator en van Python curtus. Trop Nat, 19: 156–157
Mendyk R. W.2011. Reproduction of Varanid Lizards (Reptilia: Squamata: Varanidae) at the Bronx Zoo. Zoo Biol, 30: 1-16
Mertens R.1942. Die familie der warane (Varanidae). Abb Senck Naturf Ges, 462, 465, 466: 1–391
Moharana S., Pati S.1983. Het eierleggen van Varanus salvator in het Nandan Kanan Zoological Park in India. Lacrta, 41: 67–68
Orme D., Freckleton R., Thomas G., Petzoldt T., Fritz S., Isaac N.2012. Comparative analyses of phylogenetics and evolution in R. R package version 0.5. http://CRANR-projectorg/ package=caper
Pianka E. R.1994. Comparative ecology of Varanus in the Great Victoria desert. Aust J Ecol, 19: 395–408
Pianka E. R.1995. Evolution of body size: Varanid lizards as a model system. Am Nat, 146: 398-414
Pianka E. R., King D. R., King R. A.2004. Varanoid Lizards of the World. Bloomington: Indiana University Press
Purvis A., Rambaut A.1995. Comparative analysis by independent contrasts (CAIC): An apple macintosh application for analysing comparative data. Comput Appl Biosci, 11: 247–251
Pyron R., Burbrink F., Wiens J.2013. A phylogeny and revised classifi cation of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol, 13: 93
R Development Core Team2013. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria, http://www.R-project.org
Rohlf F.2001. Comparative methods for the analysis of continuous variables: Geometric interpretations. Evolution, 55: 2143–2160
Schmidt K. P.1927. The reptiles of Haian. B Am Mus Nat Hist, 54: 395–465
Shine R., Ambariyanto, Harlow P. S., Mumpuni.1998. Ecological traits of commercially harvested water monitors, Varanus salvator, in northern Sumatra. Wildlife Res, 25: 437–447
Shine R., Harlow P., Keogh J. S., Boeadi.1996. Commercial harvesting of giant lizards: The biology of water monitors Varanus salvator in Southern Sumatra. Biol Conserv,77: 125–134
Smith M. A.1932. Some notes on monitors. J Bombay Nat Hist Soc, 35: 615–619
Thompson G. G., Pianka E. R.1999. Reproductive ecology of the black-headed goanna Varanus tristis (Squamata: Varanidae). J R Soc W Austr, 62: 27–31
Thompson G. G., Pianka E. R.2001. Allometry of clutch and neonate sizes in monitor lizards (Varanidae: Varanus). Copeia, 2001: 443–458
Vogel P.1979. Innerartliche Auseinandersetzungen bei freilebenden Bindenwaranen (Varanus salvator). Salamandra, 15: 65–83
Warne R.W., Charnov E.L.2008. Reproductive allometry and the size-number trade-off for lizards. Am Nat, 172: E80–E98
Warton D., Duursma R., Falster D., Taskinen S.2012. (Standardised) Major Axis Estimation and Testing Routines R package version 3.2.6. http://web.maths.unsw.edu.au/~dwarton
Xu Z. Q., Yuan Y. H., Chen Z. B., Zheng W., Chen J., Wu W. C., Shen Y. X.2010. Some reproductive characteristics of Varanus bengalensis in captivity. Sichuan J Zool, 29: 70-72
Zhang X.D., Ji X., Luo L.G., Gao J.F., Zhang L.2005. Sexual dimorphism and female reproduction in the Qinghai toad-headed lizard Phrynocephalus vlangalii. Acta Zool Sin, 51: 1006-1012
*Corresponding author: Dr. Longhui LIN, from School of Life Sciences, Hangzhou Normal University, Zhejiang, China, with his research focusing on physiological and evolutionary ecology of reptiles.
E-mail: linlh@outlook.com
Received: 26 October 2014 Accepted: 9 December 2014
 Asian Herpetological Research2014年4期
Asian Herpetological Research2014年4期
- Asian Herpetological Research的其它文章
- Population Dynamics Following the Last Glacial Maximum in Two Sympatric Lizards in Northern China
- A New Species of the Genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a Proposal for an Eclectic Rule for Species Delimitation
- Group-spawning and Simultanous Polyandry of a Stream-dwelling Frog Feirana kangxianensis
- Can an Invasive Prey Species Induce Morphological and Behavioral Changes in an Endemic Predator? Evidence from a South Korean Snake (Oocatochus rufodorsatus)
- Food Habits and Distribution of the Lake Taal Sea Snake (Hydrophis semperi Garman 1881) and the Sympatric Little File Snake (Acrochordus granulatus Schneider 1799) in Lake Taal, Philippines
- Sexual Dimorphism in Mass of the Hindlimb Muscles of the Piebald Odorous Frog (Odorrana schmackeri)
