Bone marrow mesenchymal stem cells with Nogo-66 receptor gene silencing for repair of spinal cord injury
Zhiyuan Li, Zhanxiu Zhang, Lili Zhao, Hui Li, Suxia Wang, Yong Shen
1 Department of Joint Orthopedics, Hebei Provincial Xingtai People’s Hospital, Xingtai, Hebei Province, China
2 Department of Spinal Orthopedics, Third Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, China
Bone marrow mesenchymal stem cells with Nogo-66 receptor gene silencing for repair of spinal cord injury
Zhiyuan Li1, Zhanxiu Zhang1, Lili Zhao1, Hui Li1, Suxia Wang1, Yong Shen2
1 Department of Joint Orthopedics, Hebei Provincial Xingtai People’s Hospital, Xingtai, Hebei Province, China
2 Department of Spinal Orthopedics, Third Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, China
We hypothesized that RNA interference to silence Nogo-66 receptor gene expression in bone marrow mesenchymal stem cells before transplantation might further improve neurological function in rats with spinal cord transection injury. After 2 weeks, the number of neurons and BrdU-positive cells in the Nogo-66 receptor gene silencing group was higher than in the bone marrow mesenchymal stem cell group, and significantly greater compared with the model group. After 4 weeks, behavioral performance was signi fi cantly enhanced in the model group. After 8 weeks, the number of horseradish peroxidase-labeled nerve fi bers was higher in the Nogo-66 receptor gene silencing group than in the bone marrow mesenchymal stem cell group, and signi fi cantly higher than in the model group. The newly formed nerve fi bers and myelinated ner ve fi bers were detectable in the central transverse plane section in the bone marrow mesenchymal stem cell group and in the Nogo-66 receptor gene silencing group.
nerve regeneration; spinal cord injury; bone marrow mesenchymal stem cells; Nogo-66 receptor; RNA interference; horseradish peroxidase; BrdU; gene silencing; neural regeneration
Li ZY, Zhang ZX, Zhao LL, Li H, Wang SX, Shen Y. Bone marrow mesenchymal stem cells with Nogo-66 receptor gene silencing for repair of spinal cord injury. Neural Regen Res. 2014;9(8):806-814.
Introduction
Spinal cord injuries result in complete or partial loss of sensation and/or mobility and affect the quality of life of patients[1-5]. Severe spinal cord injury often causes paralysis and loss of sensation and re fl ex function below the site of injury, as well as impairing autonomic activity, such as breathing, and other functions, such as bowel and bladder control. Stem cell therapy has considerable therapeutic potential in spinal cord injury[6-7]. A recent study has shown that canine bone marrow mesenchymal stem cells exhibit various features in vitro that make them suitable for spinal cord reconstruction, such as their ability to proliferate as undifferentiated spheres, and under appropriate stimuli, to differentiate into neurons, astrocytes and oligodendrocytes. Because these cells can form neurosphere-like clumps and differentiate into neuron-like cells expressing neuronal markers[8-9], they hold great potential for nerve repair. However, mesenchymal stem cell transplantation alone is not suf fi cient for spinal cord repair because the majority of the mesenchymal stem cells engrafted into the spinal cord phenotypically differentiate into glial lineages and rarely survive[10]. The microenvironment of the injured spinal cord is thought to play a crucial role in the differentiation and survival of engrafted mesenchymal stem cells[11]. The neurite growth inhibition mediated by the Nogo-66 receptor[12]is a major factor affecting the efficacy of mesenchymal stem cell transplantation. In this study, we used RNA interference to silence Nogo-66 receptor gene expression in mesenchymal stem cells. Our fi ndings demonstrate the effectiveness of this strategy for enhancing mesenchymal stem cell transplantation for spinal cord injury.
Results
Morphology of bone marrow mesenchymal stem cells
The numbers of bone marrow stromal cells and colonies were signi fi cantly increased at 5 days of culture. Cells at passages 1-3 proliferated actively, and the majority of cells were seen to adhere as a monolayer. These cells were either spindle-shaped, oval-shaped, fl at-shaped, triangular or irregular, and very strongly refractive, with more than two processes, some of which formed connections with each other. These cells had a visible nucleus and nucleolus, and when con fl uent, they grew in a parallel or spiral arrangement (Figure 1A-C). Flow cytometry showed that these cells were positive for CD29, CD44, CD105 and CD166, and negative for CD34, CD80 and CD86. The bone marrow mesenchymal stem cells were quite homogeneous, with a purity above 96%.
Nogo-66 receptor expression was reduced in siRNA-transfected bone marrow mesenchymal stem cellsRT-PCR and western blot assay showed that Nogo-66 receptor gene and protein expression in siRNA-transfected bone marrow mesenchymal stem cells were signi fi cantly decreased compared with cells transfected with a control siRNA (Figure 1D).
Transplantation of Nogo-66 receptor-silenced bone marrow mesenchymal stem cells improved the morphology of the injured spinal cord
At 4 weeks after transection injury, spinal cord tissuebreakage, scars, and structural disorder were visible at the affected site in the model group, and a cavity was clearly visible (Figure 2A). In the bone marrow mesenchymal stem cell group, astrocytes aggregated at the edge of the affected site and formed scars at the junction of the intact and damaged spinal cord. The cavity was smaller than in the model group, but larger than in the Nogo-66 receptor gene silencing group (Figure 2B). In the Nogo-66 receptor gene silencing group, astrocytes exhibited reactive hypertrophy, aggregated and formed scars at the edge of the affected site. Some cells were spindle-shaped, forming a dense network with their processes, but the cavity disappeared (Figure 2C). Immunohistochemical staining showed that the number of BrdU-positive cells increased in rats transplanted with Nogo-66 receptor-silenced bone marrow mesenchymal stem cells (Figure 3), indicating improved survival of the transplanted bone marrow mesenchymal stem cells at the site of injury.
Transplantation of Nogo-66 receptor-silenced bone marrow mesenchymal stem cells promoted the growth of nerve fi bers after spinal cord injury
By horseradish peroxidase retrograde nerve tracing, only a few horseradish peroxidase-positive nerve fi bers were visible at the T8and higher segments in the model group (Figure 4A). The number of horseradish peroxidase-positive nerve fi bers in the bone marrow mesenchymal stem cell group was less than in the Nogo-66 receptor gene silencing group, but more than in the model group (Figure 4B). Te Nogo-66 receptor gene silencing group showed a large number of horseradish peroxidase-positive nerve fibers in the spinal cord (Figure 4C). Te number of horseradish peroxidase-positive nerve fiber bundles is shown in Figure 4D, demonstrating significant differences among the three groups at 8 weeks post-injury (P < 0.01).
Effects of Nogo-66 receptor-silenced bone marrow mesenchymal stem cell transplantation on tissue ultrastructure in the injured spinal cord
Transmission electron microscopy showed glial scarring and a small number of myelinated nerve fi bers, macrophage phagocytosis, degeneration and necrotic myelinated nerve fi bers in the model group (Figure 5A). Massive myelinated nerve fi bers and non-myelinated nerve fi bers could be seen in the Nogo-66 receptor gene silencing group, with more axons and intact myelin (Figure 5B). The numbers of myelinated nerve fi bers and non-myelinated nerve fi bers at the injury site in the bone marrow mesenchymal stem cell group were greater than in the model group, but less than in the Nogo-66 receptor gene silencing group (Figure 5C).
Nogo-66 receptor-silenced bone marrow mesenchymal stem cell transplantation improved behavioral performance in rats with spinal cord injury
Neurological function
After injury, rats manifested paraplegia, no activity of the hind limb or tail, and urinary dysfunction, but without defecatory dysfunction. Hind limb movement recovered at 2 and 4 weeks postinjury and became more coordinated at 6 weeks, and urinary function was partially restored, but residual urine was still visible in the bladder. The three groups exhibited similar changes after injury. Basso, Beattie and Bresnahan scores in the bone marrow mesenchymal stem cell and Nogo-66 receptor gene silencing groups were higher than in the model group. Moreover, Basso, Beattie and Bresnahan scores were higher in the Nogo-66 receptor gene silencing group than in the bone marrow mesenchymal stem cell group (P < 0.01, P < 0.05; Figure 6A).
Inclined plate test
At 4 weeks post-injury, scores in the inclined plate test were higher in the bone marrow mesenchymal stem cell and Nogo-66 receptor gene silencing groups than in the model group. Moreover, scores in the inclined plate test were higher in the Nogo-66 receptor gene silencing group than in the bone marrow mesenchymal stem cell group (P < 0.01, P <0.05; Figure 6B).
Nogo-66 receptor-silenced bone marrow mesenchymal stem cell transplantation reduced mortality in rats with spinal cord injury
At 8 weeks after injury, mortality was substantially lower in the bone marrow mesenchymal stem cell and Nogo-66 receptor gene silencing groups than in the model group (P < 0.05). Mortality was lower in the Nogo-66 receptor gene silencing group than in the bone marrow mesenchymal stem cell group (P < 0.05; Figure 6C).
Discussion
With the continued increase in vehicle use, the incidence of spinal cord injury has risen in tandem. Spinal cord injury has become one of the main causes of morbidity and mortality. Current treatment methods including surgery, medication and physiotherapy have limited ef fi cacy[13-29]. In recent years, mesenchymal stem cell transplantation for the treatment of neurological diseases has shown considerable therapeutic potential[30-32]. Mesenchymal stem cells have many advantages, including easy collection, advanced methods for separation, culture, amplification and exogenous gene transfection, feasibility of autologous implantation following in vitro ampli fi cation or genetic modi fi cation, low risk of immune rejection, and fewer ethical considerations. Mesenchymal stem cell transplantation has been shown in a variety of experimental studies to be able to treat nervous system injury. The mechanisms of action are complex. Mesenchymal stem cells show a high expansion potential, genetic stability, and stable phenotype, they can be easily collected and shipped from the laboratory to the bedside, and they are compatible with different delivery methods and formulations[33]. In addition, mesenchymal stem cells have two other extraordinary characteristics; they are able to migrate to sites of tissue injury and have strong immunosuppressive properties that can be exploited for successful autologous as well as heterologous transplantation without requiring pharmacological immunosuppression[34-35]. Furthermore, mesenchymal stem cells are capable of differentiating into neuronsand astrocytes in vitro and in vivo[36]. Recently, mesenchymal stem cell injection has shown promise for amyotrophic lateral sclerosis treatment in humans[37]. They are able to improve neurological de fi cits and promote the restoration of functional synaptic transmission when transplanted into animal models of neurological disorders[38]. Mesenchymal stem cells have been observed to migrate to the injured tissues and mediate functional recovery following brain, spinal cord and peripheral nerve lesions. However, bone marrow mesenchymal stem cell transplantation alone is not suf fi cient for spinal cord repair, because the majority of the mesenchymal stem cells implanted into the spinal cord have been shown to differentiate into a phenotype that is restricted to glial lineages and they rarely survive. The microenvironment of the injured spinal cord is believed to play a crucial role in inducing the differentiation and survival of the grafted mesenchymal stem cells.
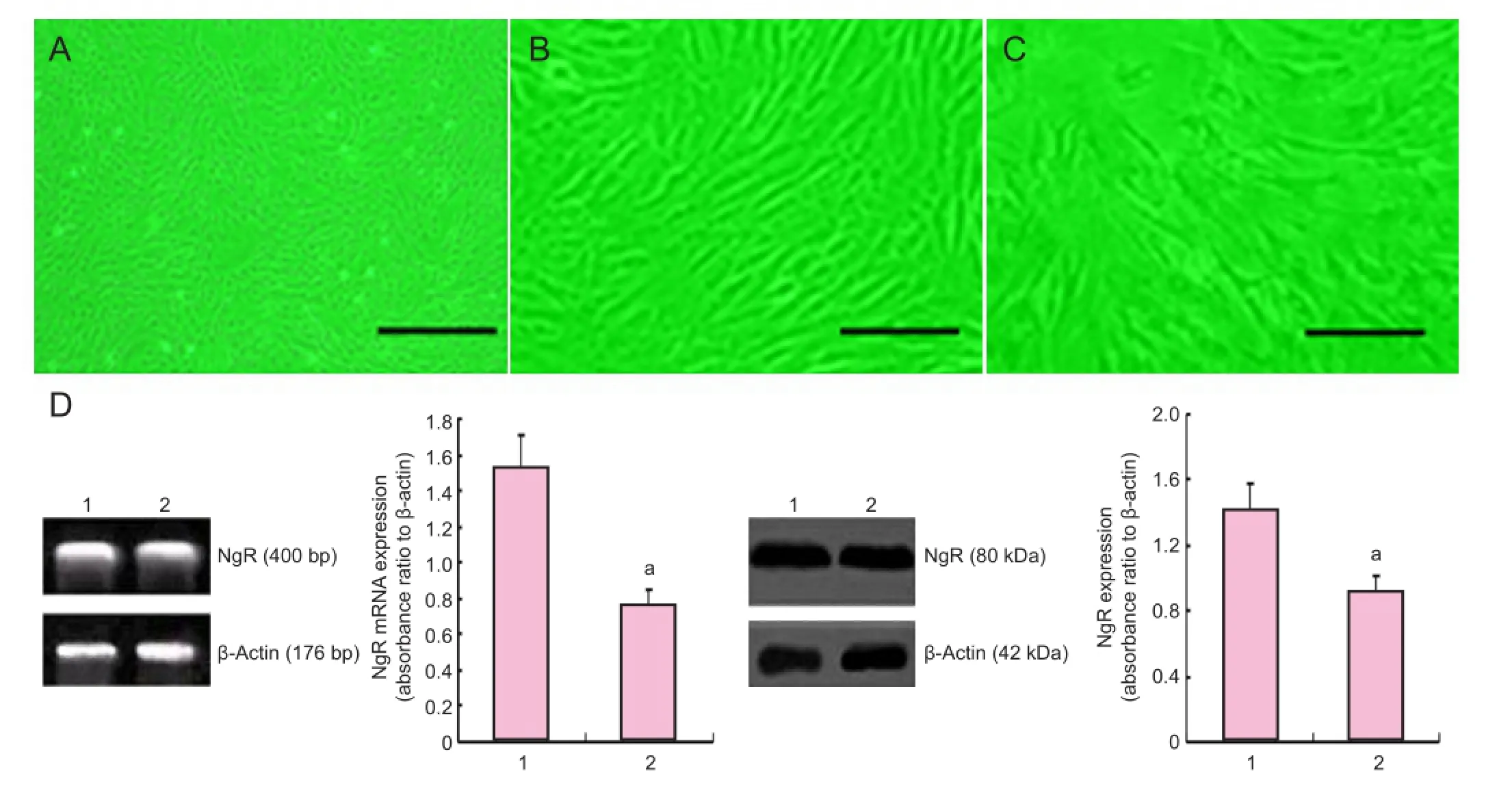
Figure 1 Morphology and transfection of bone marrow mesenchymal stem cells.

Figure 2 Effects of NgR-silenced bone marrow mesenchymal stem cells on tissue histology (T9-10) in rats with spinal cord injury (hematoxylin-eosin staining).

Figure 4 Effects of NgR-silenced BMSCs on the regeneration of nerve fi bers in the injured spinal cord of rats (HRP retrograde tracing).
Growth inhibitory factors associated with the myelin Figure 3 Effects of NgR-silenced BMSCs on the number of BrdU-positive cells in the injured spinal cord in rats (immunohistochemical staining). At 4 weeks after injury, BrdU-positive cells were not visible in the model group (A), but a large number were detected in the BMSC group (B) and in the NgR gene silencing group (C). Arrows show BrdU-positive cells. Scale bars: 50 μm. (D) Number of BrdU-positive cells in the injured spinal cord. Data are expressed as mean ± SD. Intergroup comparison was done using one-way analysis of variance. Paired comparison was performed using Dunnett’s t-test.aP < 0.01, vs. model group;bP < 0.05, vs. BMSC group. NgR: Nogo-66 receptor; BMSCs: bone marrow mesenchymal stem cells; BrdU: 5-bromodeoxyuridine. sheath limiting axonal regeneration are a major impediment to adult mammalian regeneration in the central nervous system. A variety of factors isolated from central nervous system myelin have been shown to inhibit nerve regeneration, including the protein Nogo, one of the most important growth inhibitory factors. Guo et al.[39]reported that Nogo protein gene expression was elevated after central nervous system injury in rats. Jiang et al.[40]showed that Nogo protein, Nogo-66 receptor and RhoA expression in the brain tissues of rats with focal cerebral infarction began to increase at 6 hours, reached its peak at 24 hours, and lowered back to normal levels at 96 hours. Nogo protein may leadto growth cone collapse and inhibit neurite extension. The Nogo monoclonal antibody can neutralize the inhibitory activity of the protein. In vitro cultured oligodendrocytes also exhibit an inhibitory effect on axonal extension. Similarly, Yang et al.[41]showed that Nogo neutralizing anti-body could promote recovery in rats with spinal cord injury. The inhibitory effect of Nogo protein is mediated by the No-go-66 receptor[42-47]. In this study, gene silencing was used to suppress Nogo-66 receptor expression in mesenchymal stem cells, thereby promoting neurite growth following mesenchymal stem cell differentiation, and improving the ef fi cacy of mesenchymal stem cell transplantation for repairing damage following central nervous system injury. Nogo-66 receptor gene knockout can result in permanent Nogo-66 receptor gene silencing, but the physiological function of the Nogo-66 receptor gene remains unclear, and permanent Nogo-66 receptor gene silencing may lead to unexpected consequences. RNAi is a very convenient and effective method of gene silencing, which is usually maintained for 3-5 days. This method is ideal for our study because high Nogo-66 receptor expression is observed following traumatic brain injury[41,48]. To avoid the drawbacks of permanent gene silencing, RNAi silencing combined with mesenchymal stem cell transplantation is the most promising treatment strategy for spinal cord injury.
Recent in vivo and in vitro studies in non-neuronal and neuronal tissues have shown that different pathways of macrophage activation result in cells with different properties. Interleukin-6 triggers the classically activated in fl ammatory macrophages (M1 phenotype), whereas the alternatively activated macrophages (M2 phenotype) are anti-in fl ammatory. In this study, mesenchymal stem cells were subjected to Nogo-66 receptor gene silencing before transplantation, which may result in better repair of the damaged brain tissue, and promote mesenchymal stem cell proliferation and differentiation in the grafted area after injury. Our results showed that mesenchymal stem cell transplantation, after Nogo-66 receptor gene silencing, is greatly superior to simple mesenchymal stem cell transplantation in the treatment of spinal cord injury in rats, in terms of histological and functional outcomes. Tissue repair was better in the Nogo-66 receptor gene silencing group compared with the model and bone marrow mesenchymal stem cell groups. Immunohistochemical staining demonstrated a signi fi cant difference in the number of BrdU-positive cells and horseradish peroxidase-positive nerve fi bers at the site of spinal cord injury, as follows: Nogo-66 receptor gene silencing group > bone marrow mesenchymal stem cell group > model group.
In summary, the Nogo-66 receptor gene in mesenchymal stem cells can be silenced using the RNAi approach. The mesenchymal stem cells can be transplanted into the site of spinal cord injury via the tail vein. The transplanted mesenchymal stem cells better survive, proliferate, differentiate and migrate at the site of injury, and promote the recovery of nerve function after spinal cord injury. Our fi ndings provide support for the use of this novel approach for the clinical treatment of spinal cord injury.
Materials and Methods
Design
A randomized, controlled, animal experiment.
Time and setting
Tis experiment was performed at Hebei Medical University in China from May 2010 to May 2011.
Materials
Sixty-four clean, healthy Wistar rats of both genders, aged 2 months and weighing 250-300 g, were purchased from the Chinese Academy of Medical Sciences Animal Laboratory (license No. SCXK20060008). All experimental procedures were performed in accordance with Chinese National Natural Science Foundation animal research regulations and the animal care guidelines of the National Institutes of Health.
Methods
Bone marrow mesenchymal stem cell isolation and culture
Bone marrow was harvested aseptically from the tibias of rats at the age of approximately 2 months. Nucleated cells were isolated by density gradient centrifugation using Percoll (1.073 g/mL) and were plated in growth medium consisting of Dulbecco’s modified Eagle’s medium/F12 (Hyclone, Logan, UT, USA) supplemented with 20% fetal bovine serum (Sigma, St. Louis, MO, USA) and benzylpenicillin (1 × 105U/mL)[10,49]. Te mesenchymal stem cells were isolated in the medium by their tendency to adhere to plastic[50-52]. After 3 days, the dishes were washed twice with PBS to remove nonadherent cells. Te remaining cells were fed every third day. Nonadherent cells were removed and adherent cells were expanded until subconfluence and processed through sequential passages. Most contaminating hematopoietic stem cells were progressively lost, and after the second passage, cultures contained a morphologically homogenous cell population designated bone marrow mesenchymal stem cells. Tis was con fi rmed by fl uorescence-activated cell-sorting analysis showing a lack of expression of the typical hematopoietic cell surface markers, including CD45, CD34 and CD14, and positivity for CD71, CD105 and CD44. Cells between passages 3 and 6 were used for our experiments. They were labeled using medium containing BrdU[10,52].
RNAi-transfected Nogo-66 receptor-silenced bone marrow mesenchymal stem cells
Te two target sequences of the rat Nogo-66 receptor mRNA were as follows: 5′-UGC AGU ACC UCU ACC UAC AAG ACA A-3′, 5′-UUG UCU UGU AGG UAG AGG UAC UGC A-3′. Te siRNA template was synthesized by Shanghai Sangon Biological Engineering Technology and Service Co., Ltd. in Shanghai, China. According to the in vitro transcription kit (SilencerTMsiRNA Construction Kit, Ambion) instructions, 1 mL of culture medium containing 1 × 109mesenchymal stem cells was added into a centrifuge tube and centrifuged at 800 r/min (radius = 16 cm) for 5 minutes. After discarding the supernatant, 900 μL culture medium withoutantibiotics was added, and cells were resuspended. Fifty pmol siRNA was diluted with 50 μL Opti-MEM, and 1 μL Lipofectamine 2000 was diluted with 50 μL Opti-MEM, mixed and incubated at room temperature for 15 minutes. Te two solutions were then gently mixed and incubated for 15 minutes at room temperature. Te mixture was added into the mesenchymal stem cell suspension, placed at 37°C in a 5% CO2saturated humidity incubator, and 72 hours later, Nogo-66 receptor expression was assessed.
We isolated total RNA from the injured spinal cord (4 mm long) using the RNeasy Kit (Qiagen), and obtained cDNA using reverse transcription. Primers were as follows: Nogo-66 receptor sense, 5′-GGG CAA CCT CAC GCG CAT CT-3′ and anti-sense, 5′-CGG GCA AAG TCC CAA AT-3′; β-actin sense, 5′-GTC CCT GTA TGC CTC TGG TC-3′ and anti-sense, 5′-GGT CTT TAC GGA TGT CAA CG-3′. All primers were synthesized by Sangon. Te β-actin gene was ampli fi ed for 24 cycles, and the others were ampli fi ed for 28 cycles. Te reverse transcription-PCR product was subjected to 1.3% agarose gel electrophoresis and processed with a gel imaging system (Beijing Seclaser Technology Co., Ltd., Beijing, China), and the ratio of Nogo-66 receptor absorbance to β-actin absorbance was used as an index of Nogo-66 receptor mRNA expression level.
Western blot assay for detecting the e ff ectiveness of Nogo-66 receptor transfection
Te undi ff erentiated mesenchymal stem cell suspension in the control group and the mesenchymal stem cell suspension at 72 hours after siRNA transfection were centrifuged at 800 r/min (radius = 16 cm) for 5 minutes. Te cells were collected. Supernatant was discarded. Four hundred μL protein extraction solution was added, and proteins were extracted. Protein concentration was determined using the Bradford method. Te samples were subjected to SDSPAGE, blotted onto a membrane, and incubated with rabbit anti-mouse Nogo-66 receptor gene antibody (1:800; Sigma) at 37°C on a shaker for 2 hours. The membrane was washed with Tris-bu ff ered saline containing Tween-20 for 5 minutes (four times). Te blots were incubated with goat anti-rabbit antibody (1:700; Sigma) at 37°C for 1.5 hours, washed with Tris-bu ff ered saline containing Tween-20 for 5 minutes (four times), and reacted with 3,3′-diaminobenzidine. Te experiment was repeated three times. Quantity one image (BioRad, Hercules, CA, USA) analysis was conducted. Te absorbance value ratios of the target and β-actin bands were assessed and expressed as protein expression levels.
Preparation of animal models of spinal cord injury
Transplantation of mesenchymal stem cells
Sixty-three model rats were equally and randomly divided into three groups. Six hours after injury, bone marrow mesenchymal stem cells pre-labeled with bromodeoxyuridine were transplanted via tail vein. Te model group was injected with 1 mL of stem cell-free culture medium. The bone marrow mesenchymal stem cell group was injected with 1 mL of bone marrow mesenchymal stem cells (5 × 109/L). The Nogo-66 receptor gene silencing group was injected with 1 mL (5 × 109/L) Nogo-66 receptor gene-silenced bone marrow mesenchymal stem cells. Intraperitoneal injection of gentamicin 2,000 U served as antibiotic treatment. Rats were fed in separate cages.
Histological examination
At 4 weeks after injury, the rats were randomly selected from each group and killed under anesthesia. Specimens were harvested for histological examination to determine the degree of recovery. Te dissected spinal cord tissues were postfi xed for 3 hours in 4% paraformaldehyde, soaked overnight in 10% followed by 30% sucrose, and cut into 15-mm-thick sagittal and parasagittal sections using a cryostat. Hematoxylin-eosin staining was carried out for general histological examination.
Immunocytochemistry for BrdU expression in cells
Four weeks after operation, two rats were randomly taken from each group. Immunocytochemistry for the detection of BrdU requires a pretreatment of tissue sections to denature DNA. All staining was done on free- fl oating 40-μm sections. A mouseanti-BrdU monoclonal antibody (1:100; Boehringer Mannheim, Ingelheim am Rhein, Germany) was used in combination with avidin biotin complex and a horse anti-mouse IgG antibody conjugated with biotin (1:167; Vector Laboratories, Burlingame, CA, USA). Primary and secondary antibodies were incubated at 37°C. Ten fi elds were randomly selected from each slice under the light microscope at a magni fi cation of × 200 (Sigma). Te number of BrdU-positive cells was calculated in each fi eld of vision, and the mean value was obtained.
Retrograde tracing with horseradish peroxidase
Eight weeks after operation, two rats were randomly taken from each group. After surgery, the spinal cord was exposed at T12and 1 μL aqueous suspension of 30% horseradish peroxidase (Sigma) was injected bilaterally 1 mm into the spinal dorsal vein. After injection, the incision was closed, and the animals were maintained for 36 hours before being perfused with bu ff ered 1% paraformaldehyde and 1.25% glutaraldehyde. The spinal cord was removed and stored in 20% sucrose in 0.1 mol/L PBS at 4°C overnight. The spinal cord was dissected, and 10 fields were randomly selected from each slice under the light microscope at × 200. Te number of horseradish peroxidase-la-beled nerve fibers was calculated in each field, and the mean value was obtained.

Figure 6 Effects of NgR-silenced BMSC transplantation on behavioral performance and survival in rats with spinal cord injury.
Electron microscopy
华安说,你跑后,欧阳橘红在保卫科反省了一个月,写了五万多字检讨。她怕过不了关,把思想深处点点滴滴的活思想都挖了出来。包括和雷志雄不和谐的性生活也和盘托出,把与你的关系上升到世界观的高度加以批判。在保卫科反省一个月后就接受群众批斗。批斗大会上,欧阳橘红胸前挂一串破鞋,两小时的批斗,她的眼睛都盯在自己的脚尖上,眼神不敢朝台下瞄。厂门前,有个大批判专栏,题目是《把欧阳橘红的腐朽思想批深批臭》。大批判办命令她,每天上班前二十分钟,站在批判栏前,把每一篇批臭她的文章,读二遍以上,不少于四十分钟。那时正是上班高峰,又是上班的必由之路,大家看猴似的,看她读批臭自己的文章。
Eight weeks after operation, two rats were randomly selected from those subjected to labeling from each group, and perfused intracardially with saline[17-20], followed by 2% glutaraldehyde and 4% paraformaldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.4. Immediately after perfusion, the spinal cords were removed and post fi xed in the same fi xative overnight at 4°C. Te spinal cord segment at the injury site was sliced into 1-mm pieces, postfixed for 2 hours in 1% OsO4in 0.1 mol/L cacodylate buffer, dehydrated in graded ethanol solutions, and embedded in Epon-812. Semi-thin plastic sections (1 μm) were cut and stained with 1% toluidine blue before examination with a Nikon Eclipse TE300 microscope equipped with a Spot RT Color CCD camera. For electron microscopy, blocks were trimmed and sections were cut at 100-nm thickness, mounted on copper grids, stained with uranyl acetate and lead citrate, and viewed with a JEOL Jem 1200 EX transmission electron microscope (JEOL Ltd., Tokyo, Japan).
Evaluation of functional recovery
Two types of functional tests were used to assess functional recovery. Each test was observed by two independent investigators. Te test was performed at 1, 2, 4, 6 and 8 weeks post-operation.
Basso, Beattie and Bresnahan test: the open- fi eld locomotion test assesses movement, weight support and coordination. It was scored using the standardized Basso, Beattie and Bresnahan locomotor scoring system. Basso, Beattie and Bresnahan scores range from 0 ( fl accid paralysis) to 21 (normal gait). Rats were acclimated to the testing environment (90-cm diameter plastic wading pool; 4-cm height) prior to testing. Basso, Beattie and Bresnahan scores were averaged for each group.
Inclined plane test: this test evaluates the maximum angle on the inclined plane upon which each animal can maintain a stable position for 5 seconds. Rats were placed on a board that was incrementally raised to increasing angles[17-20]. Angle scores were averaged for each group.
Mortality: the mortality of each group was evaluated at 8 weeks post-injury.
Statistical analysis
Data were expressed as mean ± SD, and analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Intergroup comparison was done using one-way analysis of variance. Paired comparison was done using Dunnett’s t-test. A value of P <0.05 was considered statistically signi fi cant.
Author contributions:All authors designed, implemented, evaluated the study, and approved the final version of the paper.
Con fl icts of interest:None declared.
Peer review:This study used gene-silenced bone marrow mesenchymal stem cell transplantation in animal models of spinal cord injury, provided new ideas and experimental evidence for the treatment of spinal cord injury in the clinic. This method can restore neurological function in patients with spinal cord injury and improve their quality of life.
[1] Fukuda S, Nakamura T, Kishigami Y, et al. New canine spinal cord injury model free from laminectomy. Brain Res Brain Res Protoc. 2005;14(3):171-180.
[2] Kang SK, Shin MJ, Jung JS, et al. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006;15(4):583-594.
[3] Lepore AC, Bakshi A, Swanger SA, et al. Neural precursor cells can be delivered into the injured cervical spinal cord by intrathecal injection at the lumbar cord. Brain Res. 2005;1045(1-2):206-216.
[4] Lim JH, Byeon YE, Ryu HH, et al. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007;8(3):275-282.
[5] Nishio Y, Koda M, Kamada T, et al. Te use of hemopoietic stem cells derived from human umbilical cord blood to promote restoration of spinal cord tissue and recovery of hindlimb function in adult rats. J Neurosurg Spine. 2006;5(5):424-433.
[6] Akiyama Y, Honmou O, Kato T, et al. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167(1):27-39.
[7] Ogawa Y, Sawamoto K, Miyata T, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69(6):925-933.
[8] Kamishina H, Deng J, Oji T, et al. Expression of neural markers on bone marrow-derived canine mesenchymal stem cells. Am J Vet Res. 2006;67(11):1921-1928.
[9] Siegel G, Krause P, Wöhrle S, et al. Bone marrow-derived human mesenchymal stem cells express cardiomyogenic proteins but do not exhibit functional cardiomyogenic differentiation potential. Stem Cells Dev. 2012;21(13):2457-2470.
[10] Shi E, Kazui T, Jiang X, et al. Intrathecal injection of bone marrow stromal cells attenuates neurologic injury after spinal cord ischemia. Ann Torac Surg. 2006;81(6):2227-2234.
[11] Zhang W, Yan Q, Zeng YS, et al. Implantation of adult bone marrow-derived mesenchymal stem cells transfected with the neurotrophin-3 gene and pretreated with retinoic acid in completely transected spinal cord. Brain Res. 2010;1359:256-271.
[12] Wang YT, Lu XM, Zhu F, et al. The use of a gold nanoparticle-based adjuvant to improve the therapeutic e ffi cacy of hNgR-Fc protein immunization in spinal cord-injured rats. Biomaterials. 2011;32(31):7988-7998.
[13] Kose EA, Bakar B, Ayva SK, et al. Neuroprotective e ff ects of racemic ketamine and (S)-ketamine on spinal cord injury in rat. Injury. 2012;43(7):1124-1130.
[14] Harkema SJ, Hillyer J, Schmidt-Read M, et al. Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil. 2012;93(9):1588-1597.
[15]Cameron AP, Rodriguez GM, Schomer KG. Systematic review of urological followup after spinal cord injury. J Urol. 2012;187(2):391-397.
[16] Austin JW, Kang CE, Baumann MD, et al. Te e ff ects of intrathecal injection of a hyaluronan-based hydrogel on inflammation, scarring and neurobehavioural outcomes in a rat model of severe spinal cord injury associated with arachnoiditis. Biomaterials. 2012;33(18):4555-4564.
[17] Post MW, van Leeuwen CM, van Koppenhagen CF, et al. Validity of the life satisfaction questions, the life satisfaction questionnaire, and the satisfaction with life scale in persons with spinal cord injury. Arch Phys Med Rehabil. 2012;93(10):1832-1837.
[18] Pershouse KJ, Barker RN, Kendall MB, et al. Investigating changes in quality of life and function along the lifespan for people with spinal cord injury. Arch Phys Med Rehabil. 2012;93(3):413-419.
[19] Ottomanelli L, Goetz LL, Suris A, et al. E ff ectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747.
[20] Cao QL, Howard RM, Dennison JB, et al. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol. 2002;177(2):349-359.
[21] Carvalho KA, Vialle EN, Moreira GH, et al. Functional outcome of bone marrow stem cells (CD45(+)/CD34(-)) after cell therapy in chronic spinal cord injury in Wistar rats. Transplant Proc. 2008;40(3):845-846.
[22] Sun Z, Wen Y, Mao Q, et al. Adenosine-triphosphate promoting repair of spinal cord injury by activating mammalian target of rapamycin/signal transducers and activators of transcription 3 signal pathway in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24(2):165-171.
[23] Gwak YS, Kang J, Unabia GC, et al. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2012;234(2):362-372.
[24] Zariffa J, Curt A, EMSCI Study Group, et al. Functional motor preservation below the level of injury in subjects with American Spinal Injury Association Impairment Scale grade A spinal cord injuries. Arch Phys Med Rehabil. 2012;93(5):905-907.
[25] Behrman AL, Ardolino E, Vanhiel LR, et al. Assessment of functional improvement without compensation reduces variability of outcome measures after human spinal cord injury. Arch Phys Med Rehabil. 2012;93(9):1518-1529.
[26] Wijesuriya N, Tran Y, Middleton J, et al. Impact of fatigue on the health-related quality of life in persons with spinal cord injury. Arch Phys Med Rehabil. 2012;93(2):319-324.
[27] Triolo RJ, Bailey SN, Miller ME, et al. Longitudinal performance of a surgically implanted neuroprosthesis for lower-extremity exercise, standing, and transfers after spinal cord injury. Arch Phys Med Rehabil. 2012;93(5):896-904.
[28] van Leeuwen CM, Hoekstra T, van Koppenhagen CF, et al. Trajectories and predictors of the course of mental health after spinal cord injury. Arch Phys Med Rehabil. 2012;93(12):2170-2176.
[29] Sale P, Mazzarella F, Pagliacci MC, et al. Predictors of changes in sentimental and sexual life after traumatic spinal cord injury. Arch Phys Med Rehabil. 2012;93(11):1944-1949.
[30] Bhang SH, Lee YE, Cho SW, et al. Basic fi broblast growth factor promotes bone marrow stromal cell transplantation-mediated neural regeneration in traumatic brain injury. Biochem Biophys Res Commun. 2007;359(1):40-45.
[31] Teus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional bene fi ts after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656-670.
[32] Shen LH, Li Y, Gao Q, et al. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative e ff ects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56(16):1747-1754.
[33] Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211(1):27-35.
[34] Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7(1):36-45.
[35] Beggs KJ, Lyubimov A, Borneman JN, et al. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15(8-9):711-721.
[36] Jori FP, Napolitano MA, Melone MA, et al. Molecular pathways involved in neural in vitro di ff erentiation of marrow stromal stem cells. J Cell Biochem. 2005;94(4):645-655.
[37] Mazzini L, Mareschi K, Ferrero I, et al. Stem cell treatment in Amyotrophic Lateral Sclerosis. J Neurol Sci. 2008;265(1-2):78-83.
[38] Bae JS, Han HS, Youn DH, et al. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells. 2007;25(5):1307-1316.
[39] Guo XG, Guo Y, Huang T. Expression of Nogo-A mRNA after injury of the rat central nervous system. Neural Regen Res. 2008;3(12):1368-1371.
[40] Jiang W, Xia F, Han J, et al. Patterns of Nogo-A, NgR, and RhoA expression in the brain tissues of rats with focal cerebral infarction. Transl Res. 2009;154(1):40-48.
[41] Yang HL, Wu JB, Tang TS, et al. Expressions of Nogo-66 receptor at mRNA and protein levels after spinal cord injury in rats. Spine J. 2012;12(9):S153.
[42] Xiao F, Luo HM. Effects of Nogo-66 receptor on both neuronal neurite regeneration and amyloid-β protein production. Alzheimers Dement. 2011;7(4):S657-S658.
[43] Zhao X, Wu J, Kuang F, et al. Silencing of Nogo-A in rat oligodendrocyte cultures enhances process branching. Neurosci Lett. 2011;499(1):32-36.
[44] Wang F, Xing S, He M, et al. Nogo-A is associated with secondary degeneration of substantia nigra in hypertensive rats with focal cortical infarction. Brain Res. 2012;1469:153-163.
[45] Zhang L, Kuang X, Zhang J. Nogo receptor 3, a paralog of Nogo-66 receptor 1 (NgR1), may function as a NgR1 co-receptor for Nogo-66. J Genet Genomics. 2011;38(11):515-523.
[46] Bongiorno D, Petratos S. Molecular regulation of Nogo-A in neural cells: Novel insights into structure and function. Int J Biochem Cell Biol. 2010;42(7):1072-1075.
[47] Shen JY, Yi XX, Xiong NX, et al. GSK-3β activation mediates Nogo-66-induced inhibition of neurite outgrowth in N2a cells. Neurosci Lett. 2011;505(2):165-170.
[48] Zhan RS, Chen SJ, Wang WG, et al. E ff ects of Nogo-neutralizing antibody and neurotrophin-3 on axonal regeneration following spinal cord injury in rat. Neural Regen Res. 2008;3(12):1319-1323.
[49] Tompson RB, Emani SM, Davis BH, et al. Comparison of intracardiac cell transplantation: autologous skeletal myoblasts versus bone marrow cells. Circulation. 2003;108 Suppl 1:II264-271.
[50] Azizi SA, Stokes D, Augelli BJ, et al. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95(7):3908-3913.
[51] Colter DC, Class R, DiGirolamo CM, et al. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97(7):3213-3218.
[52] Chen J, Li Y, Wang L, et al. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189(1-2):49-57.
Copyedited by Patel B, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.131595
Zhiyuan Li, Department of Joint
Orthopedics, Hebei Provincial Xingtai
People’s Hospital, Xingtai, Hebei Province, China, myarcher@126.com.
http://www.nrronline.org/
Accepted: 2014-02-08
- 中国神经再生研究(英文版)的其它文章
- Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases
- Regulatory effects of anandamide on intracellular Ca2+concentration increase in trigeminal ganglion neurons
- Nasal mucosal inhalation of amyloid-beta peptide 3-10 defective adenovirus attenuates cytotoxicity induced by beta-amyloid (1-42)
- The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice
- Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division
- Fusion protein of single-chain variable domain fragments for treatment of myasthenia gravis

