A pharmacobotanical study of two medicinal species of Fabaceae
Mubo A Sonibare, Tolulope A Oke, Mike O Soladoye
1Department of Pharmacognosy, Faculty of Pharmacy University of Ibadan, Ibadan Nigeria
2Department of Biological Sciences, Faculty of Science Bowen University, Osun State, Nigeria
A pharmacobotanical study of two medicinal species of Fabaceae
Mubo A Sonibare1*, Tolulope A Oke1, Mike O Soladoye2
1Department of Pharmacognosy, Faculty of Pharmacy University of Ibadan, Ibadan Nigeria
2Department of Biological Sciences, Faculty of Science Bowen University, Osun State, Nigeria
PEER REVIEW
Peer reviewer
Abiodun E Ayodele, Professor, Department of Botany, University of Ibadan, Ibadan, Nigeria.
Tel: +23408023255482
E-mail: bayodele@yahoo.com
Comments
It is a good paper reporting the anatomical and pharmacognostic features of two Nigerian medicinal plants, thus ensuring the correct identification of both plants when collected in fragmentary forms and screened for subsequent drug development.
Details on Page 135
Objective:To carry out a pharmacobotanical study of Lonchocarpus cyanescens (Schum & Thonn) Benth (L. cyanescens) and Leptoderris micrantha Dunn (L. micrantha) which are two key medicinal plants from the family Fabaceae.
Leaf epidermis, Trichomes, Stomata, Anatomy, Medicinal plants, Nigeria
1. Introduction
Medicinal plants have great applications in folk medicine within the African region; there is heavy reliance on them in alleviating various disease conditions with the consequent risk of gradual but imminent extinction[1-5]. Many plants are investigated for the development of phytocompounds useful in drug development[6]. There is no doubt that these natural compounds from plants had contributed positively to the health care delivery system in many rural communities in Africa[7]. However, a review of literature revealed that there are still seemingly insurmountable problems of misidentification in many medicinal plant groups. This has increased the quest for search for other efficient methods for the identification of medicinal plants and herbal medicines[8-10].
Thus, with the increasing trend in the use of medicinal plants, botanicals or herbal preparations particularly indeveloping countries where orthodox medical care is neither readily accessible nor affordable, it is only proper to ascertain quality control standards in the herbal products from these plants before they are subjected to human utilization[11]. The two medicinal plants treated in this study are important in African ethno medicine. The two plants have been mentioned in our recent ethnobotanical survey as part of the plants used in treating psychosis in Nigeria[12,13]. Other medicinal uses ofLeptoderris micrantha(L. micrantha) include its use for dropsy, swellings, oedema, gout, and pulmonary troubles[14]. The root ofLonchocarpus cyanescens(L. cyanescens) is used to teat arthritis and root and stem decoction is given to females after childbirth and also used to treat hernia[15].
Anatomical and micromorphological characteristics of leaves have played an important role in plant taxonomy, especially of particular groups at generic and specific levels. Studies in this field have attracted the attention of plant morphologists and systematists to resolve taxonomic conflicts in different groups of plant[16,17]. Although many studies have been conducted in this area in other plants for the purpose of correct identification of plant[18-22], little is known about the anatomy and micromorphology of the medicinal plants under study. Apart from a sketchy description ofL. micrantha, little or no work is available on its pharmacognostic features. The same is true forL. cyanescens.
Therefore, in view of the wide medicinal applications of the two plants under investigation, the present study was undertaken in order to document information on micromorphological features of the plants which would help in their identification and authentication as well as provide basic pharmacognostical data required for a herbal pharmacopoeial compilation.
Thus, this paper reports on the macro and micro characters ofL. cyanescensandL. micranthaleaves, their specific physical and chemical standards which may be useful as quality control parameters in the Nigerian herbal pharmacopoeia.
2. Materials and methods
2.1. Plant collection and authentication
L. cyanescenswas collected at the main site of Faculty of Veterinary Medicine, University of Ibadan, Nigeria. whileL. micranthawas collected at Iddo local Government along Eruwa road in Ibadan, Nigeria. The specimens were identified and authenticated by Mr. O. A. Osiyemi at the Forest Herbarium, Ibadan (FHI), Nigeria where vouchers were also deposited under FHI number 109689 forL. cyanescensand FHI number forL. micrantha, respectively.
2.2. Gross morphology
Morphological studies were carried out by observation of plant parts on the field with naked eyes and with the use of a hand lens where necessary.
2.3. Surface tissue preparation
For epidermal studies, Shultze’s method of maceration with improved technique[23] was followed. Leaves were taken in petri dishes, covered with 4 mL of concentrated nitric acid and kept under sun for 30 min. Adaxial and abaxial epidermal peels obtained with the use of camel hair brush were rinsed in distilled water severally, bleached with one to two drops of chloral hydrate for 30 seconds. To remove chlorophyll, stained in 1 % aqueous Safranin O solution for 3 min and mounted in dilute glycerol for observation.
2.4. Transverse section
Leaf transverse sections were made with free hand sectioning using surgical scapel. Briefly, scalpel was used to cut 3 cm×4 cm of the plants through the midrib region. The small portion was inserted into a 3 cm×4 cm section of unripe pawpaw to enhance easy cut. The transverse sections obtained were cleared using sodium hypochlorite 3.85% M/V and stained with Safranin O reagent and rinsed with 70% alcohol. Glycerol was added as mountant. The tissue distribution through the midrib was examined under low power magnification and was photographed with compound microscope fitted with a digital camera.
2.5. Phytochemical screening
Fresh plant leaves were air-dried, powered with a blender and stored in cellophane bags for phytochemical screening. Phytochemical screening on the powdered leaves for various constituents such as alkaloids, anthraquinone, cardiac glycoside, glycoside, tannins, terpenoids, saponins and steriods flavonoids, phlobatannins etc. was done using the standard procedure[24].
2.6. Moisture content
The moisture content of the drugs (air dried plants) was determined. Briefly, powdered drug (2 g) was transferred into a china dish and distributed evenly to a depth not exceeding 10 mm. The loaded plate was heated at 105 °C in hot air oven and weighed at different time intervals until a constant weight was obtained. The difference in weight after drying and initial weight is the moisture content. Same experiment was repeated six times for precision and percent moisture for the sample was calculated.
2.7. Total ash value
Powdered drug (2 g) was weighted accurately into a tarred silica crucible and incinerated at 450 °C in muffle furnace until free from carbon. The crucible was cooled to room temperature and weighed. Percentage of ash was calculated with reference to air dried substance.

2.8. Acid insoluble ash
Ash obtained from total ash was boiled with 25 mL of 2 N HCl for few minutes and filtered through an ashless filter paper. The filter paper was transferred into a tarred silica crucible and incinerated at 650 °C in muffle furnace until free from carbon. The crucible was cooled and weighted. Percentage of acid insoluble ash was calculated with reference to air dried substance as:

2.9. Water soluble ash
Ash obtained from total ash was boiled with 25 mL of distilled water for few minutes and filtered through an ashless filter paper. The filter paper was transferred into a tarred silica crucible and incinerated at 450 °C in muffle furnace until free from carbon. The crucible was cooled and weighed. Percentage of water soluble ash was calculated with reference to air dried substance.
2.10. Alcohol-soluble extractive value
About 5 g of the powdered drug was accurately weighed into 250 mL stoppered conical flask. A hundred milliliters of 90% ethanol was added and tightened firmly. The flask was shaken using a mechanical shaker for 6 h, and then allowed to stand for 18 h. The extract was filtered quickly (by suction filtration). The weight of a clean, heated and cooled flat-bottomed evaporating dish was accurately determined. Tweenty milliters of the filtrate was evaporated to dryness. The residue was dried to constant weight at 105 °C in an oven and the final weight determined. The alcohol soluble extractive value was calculated thus:

2.11. Water-soluble extractive values
About 5 g of powdered drug was treated with 100 mL water at in a stoppered flask with frequent shaking during first 6 h using electrical shaker and allowed to stand for 24 h. Temperature was maintained at 45 °C during entire process. Extract was filtered and 10 mL of filtrate was evaporated in a tarred dish at 105 °C and weighed. Water soluble extractive value was calculated as:
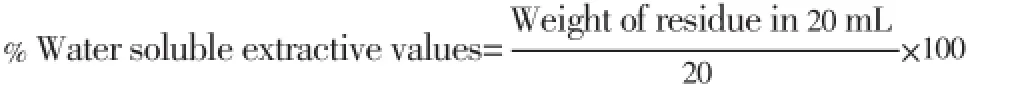
3. Results
3.1. Macroscopic characteristics
The two medicinal plants:L. cyanescensandL. micranthadiffer in their morphological features. InL. cyanescensleaf shape is lanceolate, margin is entire, surface is glabrous, midrib is prominent with papery texture. InL. micrantha, leaf is oblanceolate in shape, margin is entire, apex is acute, surface is glabrous, petiolate with sessile flower and flat, fruit is broadly elliptic.
3.2. Epidermis
Leaf epidermal studies ofL. micranthaandL. cyanescenswere carried out in search of anatomical characters of taxonomic value that may contribute to the proper identification of the plants. Qualitative and quantitative features of the epidermal morphology of the plants are recorded in Table 1. The epidermal cells on the adaxial surface ofL. micranthahave straight anticlinal walls and unicellular trichomes (Figures 1 A and B), while on the adaxial surface ofL. cyanescens, epidermal cells have wavy/undulating anticlinal walls (Figures 1 C and D).

Figure 1. Adaxial epidermis of L. micrantha and L. cyanescens. (400×) A&B: Adaxial epidermis of L. micrantha; C&D: Adaxial epidermis of L. cyanescens. trb: Trichome base; tr: Trichome; ec: Epidermal cell.
The abaxial surface ofL. micranthahas straight to curve walls and peltate trichomes. No stomata were observed on either of the surfaces in this species (Figures 2 A and B). Epidermal cells are smooth to slightly wavy on the abaxial surface ofL. cyanescens(Figure 2C). Both surfaces have many stomata, paracytic and anisocytic on the adaxial and abaxial surfaces, respectively (Figure 2D). However, fewer stomata and trichomes were seen on the adaxial surface onL. cyanescenscompared to the numerous seen on its abaxial surface. Multicellularuniseriate trichomes were observed on the abaxial surface. Mucilage was observed on the abaxial epidermal cells of both plants.

Table 1 Combined qualitative and quantitative features of the epidermal morphology of L. micrantha and L. cyanescens.

Figure 2. Abaxial epidermis of L. micrantha and L. cyanescens. (400×)A & B: Abaxial epidermis of L. micrantha; C & D: Abaxial epidermis of L. cyanescens showing smooth to slight wavy epidermal wall and anisocytic stomata. mu: Mucilage; tr: Trichome; ec: Epidermal cell; st: Stomata.
3.3. Leaf transverse section
Transverse sections of leaf blades ofL. cyanescensandL. micranthaare shown in Figures 3 A and B. The transverse section ofL. cyanescensappeared concave on the abaxial and convex on the adaxial surface. Cuticle is moderately thick and the vascular bundle is arc-shaped. The xylem vessel is lignified as it picks up stain with safranin reagent. The transverse section ofL. micranthahas numerous unicellular trichomes, covered with moderately thick cuticle. The vascular bundle is arc shaped with xylem inside and phloem outside. Variations were found in shapes and numbers of vascular bundles, trichomes and presence of mucilage. Also, numerous peltate trichomes were seen inL. micranthawhile these were absent inL. cyanescens.
3.4. Physicochemical constant and phytochemical data
The physicochemical constants recorded for the two medicinal plants:L. micranthaandL. cyanescensinclude: moisture content, total ash, acid insoluble ash, water insoluble ash, water extractive value, acid extractive value as presented in Table 2. Phytochemical data appeared in Table 3.

Table 2 The analytical values in respect of physicochemical constant for L. micrantha and L. cyanescens.

Table 3 Phytochemical screening results of the powdered leaves of L. micrantha and L. cyanescens.
4. Discussion
The variations observed in the epidermal morphology ofL. micranthaandL. cyanescensfrom the family Fabaceae could be used to distinguish the species. Anatomical features are widely used in systematics for identification, for placing anomalous groups in satisfactory position in classification and for indicating patterns of relationship that may have been observed by superficial convergence in morphological features[25]. Nonglandular unicellular trichomes on both adaxial and abaxial surfaces are considered interesting and the density of hairs was more abundant on the abaxial surface for both plants. The high density of thick and coated hairs probably serves to reduce the rate of transpiration in plants and this buttresses the importance of trichomes in taxonomy as a diagnostic tool[26].
The adaxial and the abaxial surfaces of both plant species have comparable sizes of epidermal cells. Few stomata and trichomes were seen on the adaxial surface onL. cyanescenscompared to the numerous seen on its abaxial surface. Stomata were not observed on the adaxial and abaxial surfaces ofL. micranthaalthough there are numerous trichomes on both surfaces. Thus, stomata character and trichomes can aid classification and identification of these plants.
The two medicinal plants appear to be rich in secondary metabolite, widely used in traditional medicine to manage or cure various ailments. Phytochemical screening showed that both species contain metabolites such as alkaloids, anthraquinones, cardiac glycosides, tannins, saponins, steroids and flavonoids. The presence of the various secondary metabolites may support the folkloric claims on the medicinalpotential of these plants as anti-inflammatory, antispasmodic, analgesic and antidiuretic.
Physical constant parameters and quantitative evaluation such as moisture content, total ash value, acid insoluble ash, water insoluble ash, water and acid extractive values which form part of the parameters evaluated in standardizing herbal medicines, were also determined in this study. Low moisture content is reported to indicate less chances of microbial degradation of plant drugs during storage[27]. This is in line with our observations in this study, the moisture content value ofL. cyanescensandL. micranthaare respectively, (14.0±2.8) %, w/w and (6.0±6.5)%, w/w. The general requirement of moisture content in crude drug is that, it should not be more than 14%[28], thus the values obtained in this study are within the accepted range. A standard that is expressed as “not more than” value is recommended for total ash and acid insoluble ash values while water insoluble ash is expressed as “not less than”value. Based on the results obtained forL. cyanescensandL. micrantha, the total ash should not be more than 14.5±2.1 and 8.0±0.0 respectively with the acid insoluble ash values of not more than 7.5±0.7 and 4.5±0.7 respectively. The water insoluble ash should not be less than 7.75±1.1 and 10±1.4 respectively. For water extractive and acid extractive values which are also expressed as “not less than”, the results obtained indicate thatL. cyanescensandL. micranthashould not be less than 0.121±0.001 and 0.146±0.020 respectively for water extractive values and 0.55±0.10 and 0.45±0.10 respectively, for acid extractive values.
Anatomical studies on the two medicinal plants revealed sharp generic variations in sizes and types of stomata, shapes of epidermal cells on the adaxial and abaxial surfaces, sizes and types of trichomes, all of which could be employed in species identification. Correct identification of medicinally important species is necessary for their sustainable and effective utilization as well as for detecting adulteration of plant drugs[29,30]. Thus, micromorphological character variations observed in our study had provided the basis for the identification of these plants highlighting their taxonomic relationships in line with report on other plants[31-33]. Leaf epidermal and anatomical features such as stomata, trichomes and other markers have proved useful and of great taxonomic significance[34-36]. In our study of the micromorphological features and phytochemical screening of the leaves ofL. micranthaandL. cyanescens, we are able to draw the following conclusions: Firstly, stomata are absent on both adaxial and abaxial surfaces ofL. micrantha. Secondly, the leaves of both plants contain alkaloids, anthraquinones, cardiac glycosides, tannins, saponins, steroids, flavonoids which may be responsible for the therapeutic effects of the two plant species.
The morpho-anatomical features observed in the two medicinal plants are sufficiently distinctive and may be used at specific level for delimitation of plants. The different features peculiar to each of the medicinal plants provide the basis for its detailed assessment and form part of the catalogue of characters that may be employed in proper identification of these plants and as quality control standards which can be used for the compilation of herbal pharmacopoeial.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are grateful to Mr. OA Osiyemi for plant identification and to Mr. AA Adeniran for technical assistance in slide preparation. This study is supported by the University of Ibadan Senate Research Grant (Grant No. SRG/FP/2010/4A).
Comments
Background
A major problem in traditional medicine is the misidentification of drug plants particularly when these are bought directly from the local markets. There is therefore the need to use other characters apart from the macrocharacters to ascertain the authenticity of such crude drug samples.
Research frontiers
The study provides information on the microcharacters and pharmacognostic features ofL. cyanescens(Schum & Thonn.) Benth. andL. micranthaDunn.
Related reports
Some related researches are Taxonomic importance of anatomical and micromorphological features in plants (Kahraman and Celep, 2010, Saheed and Illoh, 2010), and Plants are investigated for the development of phytocompounds useful in drug development (Cornaraet al., 2013).
Innovations and breakthroughs
Additional information on useful characters for the authentication of samples of the two important medicinal plants.
Applications
It provided some means of authentication of crude drug samples for new drug development.
Peer review
It is a good paper reporting the anatomical and pharmacognostic features of two Nigerian medicinal plants thus ensuring the correct identification of both plants when collected in fragmentary forms and screened for subsequent drug development.
[1] Mavundza EJ, Maharaj R, Finnie JF, Kabera G, Staden JV. An ethnobotanical survey of mosquito repellent plants in uMkhanyakude district, KwaZulu-Natal province, South Africa. J Ethnopharmacol 2011; 137(3): 1516-1520.
[2] Sonibare MA, Moody JO, Adesanya EO. Use of medicinalplants for the treatment of measles in Nigeria. J Ethnopharmacol 2009; 122(2): 268-272.
[3] Sonibare MA, Gbile ZO. Ethnobotanical survey of antiasthmatic in South Western Nigeria. Afr J Trad Compl Altern Med 2008; 5(4): 340-345.
[4] Elujoba AA, Odeleye OM, Ogunyemi CM. Traditional medicine development for medical and dental primary health care delivery system in Africa. Afr J Trad Compl Altern Med 2005; 2(1): 46-61.
[5] Sayeed Hassan AKM, Afroz F, Bari LS, Munshi JL, Jahan MAA, Khatun R. Callus induction and high frequency regeneration of plantlets of Scoparia dulcis L., a perennial medicinal herb, through auxiliary shoot proliferation. Plant Tiss Cult Biotechnol 2008; 18(1): 75-83.
[6] Cornara L, D’Arrigo C, Pioli F, Borghesi B, Bottino C, Patrone E, et al. Micromorphological investigation on the leaves of the rock samphire (Crithmum maritimum L.): Occurrence of hesperidin and diosmin crystals. Plant Biosyst 2009; 143(2): 283-292.
[7] Semenya SS, Potgieter MJ, Tshisikhawe MP. Use, conservation and present availability status of ethnomedicinal plants of Matebele-village in the Limpopo province, South Africa. Afr J Biotechnol 2013; 12 (18): 2392-2405.
[8] Howard C, Socratous E, Williams S, Graham E, Fowler MR, Scott NW, et al. PlantID-DNA-based identification of multiple medicinal plants in complex mixtures. Chin Med 2012; 7: 18.
[9] Brinckmann J. Reproducible efficacy and safety depend on reproducible quality: matching the various quality standards that have been established for botanical ingredients with their intended uses in cosmetics, dietary supplements, foods, and medicines. HerbalGram 2011; 91: 40-55.
[10] Li M, Cao H, But PPH, Shaw PC. Identification of herbal medicinal materials using DNA barcodes. J Syst Evol 2011; 49(3): 271-283.
[11] Hossan MS, Agarwala B, Sarwar S, Karim M, Jahan R, Rahmatullah M. Traditional use of medicinal plants in Bangladesh to treat urinary tract infections and sexually transmitted diseases. Ethnobot Res Appl 2010; 8: 61-74.
[12] Sonibare MA, Soladoye MO, Subuloye TO. Ethnobotanical survey of antipsychotic plants in Lagos and Ogun states of Nigeria. Eur J Sci Res 2008; 19: 634-644.
[13] Sonibare MA, Umukoro S, Shonibare ET. Antipsychotic property of aqueous and ethanolic extracts of Lonchocarpus cyanescens (Schumach and Thonn.) Benth. (Fabaceae) in rodents. J Nat Med 2012; 66(1): 127-132.
[14] Burkill HM. The useful plants of west tropical Africa. Vol. 4. 2nd ed. Kew, UK: Royal Botanical Gardens; 1985.
[15] Okujagu JI, Etatuvie SO, Eze I, Jimoh B, Nwokeke C, Mbaoji C, et al. Medicinal plants of Nigeria: South-West Nigeria. Volume 1. Lagos, Nigeria: Nigeria Natural Medicine Development Agency; 2005, p. 133.
[16] Kahraman A, Celep F. Anatomical properties of Colchicum kurdicum (Bornm.) Stef. (Colchicaceae). Aust J Crop Sci 2010; 4(5): 369-371.
[17] Saheed SA, Illoh HC. A taxonomic study of some species in Cassiinae (Leguminosae) using leaf epidermal characters. Not Bot Hort Agrobot Cluj 2010; 38(1): 21-27.
[18] Ajayi GO, Kadiri AB, Egbedi ME, Oyeyemi OO. Pharmacognostic study of two medicinal species of Rytigynia (Rubiaceae) from Nigeria. Phytol Balcanica 2011; 17(3): 355-359.
[19] Sonibare MA, Jayeola AA, Egunyomi A. A survey of epidermal morphology of the genus Ficus Linn. (Moraceae). Bot Bull Acad Sin 2005; 46: 231-238.
[20] Yasmin G, Khan MA, Shaheen N, Hayat MQ. Micromorphological investigation of foliar anatomy of genera Aconogonon and Bistorta of family Polygonaceae. Int J Agric Biol 2009; 11(3): 285-289.
[21] Paopun Y, Umrung P, Thanomchat P, Kongpakdee C. Epidermal surface and leaf anatomy of Thunbergia laurifolia Lindl. J Microsc Soc Thailand 2011; 4 (2): 61-64.
[22] Nurit-Silva K, Costa-Silva R, Coelho VPM, Agra MF. A pharmacobotanical study of vegetative organs of Solanum torvum. Rev Bras Farmacogn 2011; 21(4): 568-574.
[23] Subrahmanyam NS. Labortary manual of plant taxonomy. New Delhi: Vikas publishing house PVT. Ltd; 1996.
[24] Sofowora A. Medicinal plants and traditional medicine in Africa. 3rd ed. Ibadan, Nigeria: Spectrum Books Limited; 2008, p. 199-202.
[25] Essiett UA, Illoh HC, Udoh UE. Leaf epidermal studies of three species of Euphorbia in Akwa Ibom State. Adv Appl Sci Res 2012; 3(4): 2481-2491.
[26] Adeniji KA, Ariwaodo JO. Comparative foliar epidermal studies of genus pericopsis (Papilionaceae) in Nigeria. Phytol Balcanica 2012; 18(1): 37-41.
[27] Kunle OF, Egharevba HO, Ahmadu PO. Standardization of herbal medicines-A review. Int J Biodivers Conserv 2012; 4: 101-112.
[28] British Pharmacopoeia Commission. British pharmacopoeia. London, UK: Her Majesty’s Stationery Office; 1980, p. 1196.
[29] Yousaf Z, Shinwari ZK, Asghar R, Parveen A. Leaf epidermal anatomy of selected Allium species, family Alliacaeae from Pakistan. Pak J Bot 2008; 40(1): 77-90.
[30] Khan F, Yousaf Z, Rani S, Khan F. Taxonomic treatment of medicinally important arboreal flora of tropical and subtropical region based on leaf epidermal anatomical markers. J Med Plant Res 2011; 5(28): 6439-6454.
[31] Sharma NK, Thanki YJ, Shaily Bhardwaj NV. Anatomical studies of Lagenaria siceraria (Mol.) Standl and Cucumis sativa L. (Cucurbits). Asian J Plant Sci 2011; 10(2): 158-161.
[32] Stefanescu C, Tamas M, Barbu-Tudoran L. Anatomical studies on Scopolia carniolica Jacq. vegetative organs. Not Bot Hort Agrobot Cluj 2006; 34: 12-17.
[33] Panahi P, Jamzad Z, Pourmajidian MR, Fallah A, Pourhashemi M. Taxonomic implications of micromorphological features for taxon delimitation within the Quercus libani complex (Fagaceae) in Iran. Phytol Balcanica 2012; 18(3): 263-276.
[34] Adedeji O, Jewoola OA. Importance of leaf epidermal characters in the Asteraceae family. Not Bot Hort Agrobot Cluj 2008; 36(2): 7-16.
[35] Gostin AN. Anatomical and micromorphological peculiarities of Adonis vernalis L. (Ranunculaceae). Pak J Bot 2011; 43(2): 811-820.
[36] Ullah Z, Khan MR, Ahmad M, Zafar M, Ullah K. Systematic implications of foliar epidermis in Andropogoneae (Poaceae) from Hindukush-himalayas Pakistan. J Med Plant Res 2011; 5(6): 949-957.
10.1016/S2221-1691(14)60221-5
*Corresponding author: Mubo A Sonibare, Senior Lecturer, PhD, Department of Pharmacognosy, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria.
Tel: 00234-8134901273
E-mail: sonibaredeola@yahoo.com
Foundation Project: Supported by the University of Ibadan Senate Research Grant (Grant No. SRG/FP/2010/4A).
Article history:
Received 17 Oct 2013
Received in revised form 29 Oct , 2nd revised form 12 Nov, 3rd revised form 5 Dec 2013
Accepted 20 Jan 2014
Available online 28 Feb 2014
Methods:The epidermal peel was obtained by soaking the leaf in concentrated nitric acid (HNO3) in a petri dish. Both surfaces were carefully mounted on clean glass slides and dehydrated by ethyl alcohol, and stained with safaranin O for 2 min. Transverse sections of plant leaf were obtained by free hand sectioning. Phytochemical screening for various constituents was carried out on the powdered leaves. Other parameters such as, moisture content, ash value, acid insoluble ash, water-soluble ash, water and alcohol extractive values were obtained by standard techniques.
Results:The distinctive features of the species include: the presence of stomata on both surfaces of L. cyanescens and the absence in L. micrantha. Presence of larger epidermal cells in both upper and lower surfaces of L. cyanescens [(35.25±1.64)×(31.25±2.36), (43.0±2.63)×(39.5±5.11)] respectively compared to L. micrantha. Glandular multicellular trichomes are present in L. micrantha but absent in L. cyanescens. Numerous trichomes surround the transverse section of the leaf of L. micrantha but absent in L. cyanescens. Preliminary phytochemical screening showed that both species contain secondary metabolites such as alkaloids, anthraquinones, cardiac glycosides, tannins, saponins, steroids and flavonoids.
Conclusions:The microscopic and phytochemical data provided in this study are useful for the standardization of the medicinal plants.
 Asian Pacific Journal of Tropical Biomedicine2014年2期
Asian Pacific Journal of Tropical Biomedicine2014年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Comparative assessment of physicochemical properties of unripe peach (Prunus persica) and Japanese apricot (Prunus mume)
- Hibiscus sabdariffa extract inhibits in vitro biofilm formation capacity of Candida albicans isolated from recurrent urinary tract infections
- Lupeol: An antioxidant triterpene in Ficus pseudopalma Blanco (Moraceae)
- Quantitative analysis of γ-oryzanol content in cold pressed rice bran oil by TLC-image analysis method
- Pancreatic islet regeneration and some liver biochemical parameters of leaf extracts of Vitex doniana in normal and streptozotocin-induced diabetic albino rats
- Investigation of in vivo neuropharmacological effect of Alpinia nigra leaf extract
