Anti-oxidative and anti-inflammatory effects of Tagetes minuta essential oil in activated macrophages
Parastoo Karimian, Gholamreza Kavoosi*, Zahra Amirghofran
1Biotechnology Institute, Shiraz University, Shiraz, 71441-65186, Iran
2Department of Immunology, Autoimmune Disease Research Center and Medicinal and Natural Products Chemistry Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Anti-oxidative and anti-inflammatory effects of Tagetes minuta essential oil in activated macrophages
Parastoo Karimian1, Gholamreza Kavoosi1*, Zahra Amirghofran2
1Biotechnology Institute, Shiraz University, Shiraz, 71441-65186, Iran
2Department of Immunology, Autoimmune Disease Research Center and Medicinal and Natural Products Chemistry Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
PEER REVIEW
Peer reviewer
Hasan Salehi, University of Shiraz, Shiraz, Iran.
E-mail: hsalehi@shirazu.ac.ir
Comments
TMO displayed an anti-oxidant property by scavenging superoxide, H2O2and NO radicals, and reduced oxidative stress. The decreased formation of ROS and NOS radicals in macrophages was possibly due to the radical scavenging activity of phenolic groups present in the oil and/or due to an inhibition of iNOS and NOX gene expressions. Furthermore, TMO decreased the expression of the genes for pro-inflammatory cytokine TNF-α. Details on Page 226
Objective:To investigate antioxidant and anti-inflammatory effects of Tagetes minuta (T. minuta) essential oil.
Tagetes minuta, Essential oil, Macrophages, Anti-inflammatory, Antioxidant
1. Introduction
Tagetes minuta(T. minuta) is a tall upright marigold plant in the sunflower (Asteraceae) family.Tagetesspecies originally has been used as a source of essential oil (extracted from leaves, stalks and flowers) for the flavoring in the food industries. The powders and extracts ofTagetesare rich in the orange-yellow carotenoid and are used as a food color for foods such as pasta, vegetable oil, margarine, mayonnaises, salad dressing, baked goods, confectionery, dairy products, ice cream, yogurt, citrus juice, mustard and as colorant in poultry feed[1-3].T. minutais also extensively used medicinally as a condiment and herbal tea in a wide variety of fields in its native region and as a popular traditional folk remedies and in the complementary and medical therapy.T. minutahas several medical benefits such as remedy for colds, respiratory inflammations, stomach problem, anti-spasmodic, anti-parasitic, anti-septic, insecticide and sedative. It is used for chest infections, coughsand catarrh, dilating the bronchi, facilitating the flow of mucus and dislodging congestion and can be used in cases of skin infections. It also has a healing effect on wounds, cuts, calluses and bunions[4-9]. However, such practices are largely based on folklore and train of traditional medicine rather than evidencebased research.
The most abundant components inT. minutaessential oil are dihydrotagetone (unsaturated acyclic monoterpene ketone), ocimene (unsaturated acyclic monoterpene hydrocarbon), tagetone (unsaturated acyclic monoterpene ketone) and limonene (unsaturated monocyclic monoterpene hydrocarbon[10-14].T. minutaessential oil has a significant antibacterial activity against both Gram-positive and Gram-negative bacteria[15-17]. Several studies have also described antifungal activities ofT. minutaessential oil againstCandida,PenicilliumandAspergilusspecies[18-20].T. minutaessential oil has been shown to possess anti-oxidant activity in 2, 2-diphenyl-1-picrylhydrazyl and 2, 2’-azino-di (3-ethylbenzthiazoline-6- sulphonate) (ABTS) assay[21,22].
Advances in chemical and pharmacological evaluations ofT. minutaessential oil have occurred in the past recent years; however, several useful features of this plant (e.g.the mechanisms underlying its antioxidant and anti-inflammatory effects) have remained unknown. Macrophages play a pivotal role in inflammatory responses. Overproduction of reactive oxygen species (ROS) and nitrogen (RNS) by macrophages is a classic indicator during inflammatory eventsin situ. The production of ROS and RNS radicals are under the control of nicotinamide adenine dinucleotide phosphate oxidase (NOX) and inducible nitric oxide synthase (iNOS), respectively. The aim of the present study was to investigate the level of potential modulating effects ofT. minutaessential oil on macrophages and their related functions including expression of NOX subunits [p22phox (phagocyte oxidase), p40phox, p47phox and p67phox], NOS and TNF-α mRNAs in lipopolysacharide (LPS)-stimulated macrophages. In addition,in vitroanti-oxidant capacity ofT. minutaessential oil was examined by assessments of ROS, RNS and hydrogen peroxide (H2O2) scavenging ability using ABTS, sodium nitrite, and H2O2scavenging, respectively. It was expected that these studies would reveal thatT. minutaessential oil exhibits radical scavenging activity (against superoxide anion, H2O2, and NO radicals) in macrophages, in part, due to an inhibition of iNOS and NOX gene expression. Furthermore, it was hypothesized for the first time thatT. minutaessential oil would decrease TNF-α mRNA expression as part of its known anti-inflammatory character and secondarily due to the ongoing quenching of radicals known to trigger formation of these pro-inflammatory cytokines.
2. Materials and methods
2.1. Chemicals and reagents
Sodium nitrite, sodium sulphate, ABTS, Griess reagent (naphthylethylenediamine, sulfanilamide, phosphoric acid), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), fetal calf serum, Dulbecco’s modified eagle medium, L-glutamine, dimethysulfoxide (DMSO) and LPS (fromEscherichia coli0111; B4) were purchased from Sigma-Aldrich (St, Louis, MO, USA) and Fluka (Heidelberg, Germany). RNXPlus buffer was obtained from Cinagen (Tehran, Iran). All other used chemicals and reagents were of the purest commercially available products.
2.2. Plant materials and T. minuta essential oil preparation
Seed ofT. minutawas obtained from Institute of Medicinal Plants, Isfahan, Iran and was grown in green house conditions in Sadra near Shiraz, Iran. Seed of medicinal plant was grown in sterile soil. The Seeds ofT. minutawere sown in experimental greenhouse, in September 2011. One month later obtained seedlings were transferred to experimental field and distributed homogenously. The aerial parts of plants were harvested at the flowering stage. The leaves of the plants were separated from the stem and were dried in the shade for 72 h. The air-dried leaves (100 g) were hydro-distilled for 3 h using an all-glass Clevenger-type apparatus (Herbal Exir Co., Mashhad, Iran) according to the method outlined by the British Pharmacopeia[23]. The yield ofT. minutaessential oil from leaf material was near 1% (w/w). The obtained essential oil was dehydrated over anhydrous sodium sulphate and stored at 4 °C until analyzed by gas chromatography-mass spectrometry (GCMS) and was then used.
2.3. Identification of the T. minuta essential oil components
GC analysis was carried out using Agilent technology chromatograph with HP-5 column (30 m×0.32 mm, internal diameter 0.25 μm). Oven temperature was performed as follows: 60 °C to 210 °C at 3 °C/min; 210 °C to 240 °C at 20 °C/min and hold for 8.5 min, injector temperature 280 °C; detector temperature, 290 °C; carrier gas, N2(1 mL/min); split ratio of 1: 50. The OBO was analyzed using an Agilent model 7890-A series gas chromatography and Agilent model 5975-C mass spectrometry. The HP-5 MS capillary column (phenyl methyl siloxane, 30 m ×0.25 mm, internal diameter× 25 μm) was used with helium at 1 mL/min as the carrier gas. GC oven temperature was programmed from 60 °C to 210 °C at a rate of 3 °C/min and was then increased from 210 °C to 240 °C at rate of 20 °C/min and was kept constant at 240 °C for 8.5 min. The split ratio was adjusted to 1: 50 and the injection volume was 1 mL. The injector temperature was 280 °C. The quadrupole mass spectrometer was scanned over 40-550 amu with an ionizing voltage of 70 eV. Retention indices were determined using retention times ofn-alkanes (C8-C25) that were injected after theT. minutaessential oil under the same chromatographic conditions. The retention indices for all components were determined according to the method that usesn-alkanes as standard. The compounds were identified by comparison of retention indices with those reported in the literature and by comparison of their mass spectra with the Wiley GC-MS Library, Adams Library, Mass Finder 2.1 Library data published mass spectra data[24].
2.4.ROSscavenging assay
The ROS scavenging activity of theT. minutaessential oil was determined as previously described[25]. Briefly,10 μL of theT. minutaessential oil (0-500 μg/mL in DMSO) was added to 1.0 mL of diluted ABTS radical solution (7 mmol/L ABTS and 2.54 mmol/L potassium persulfate). After mixing, the absorbance (A) was read at 734 nm using an Ultrospec 2000 spectrophotometer (Pharmacia, Uppsala, Sweden). The percentage of ROS scavenging was calculated as [(A734blank-A734sample)/A734blank]×100. The concentrations that could provide 50% inhibition (IC50) were calculated from the graph that plotted the inhibition percentage against differentT. minutaessential oil concentrations.
2.5.H2O2scavenging assay
H2O2scavenging activity of theT. minutaessential oil was determined as previously described[26]. Briefly, 10 μL of theT. minutaessential oil (0-500 μg/mL in DMSO) was incubated with 1.0 mL of H2O2(50 mmol/L in 100 mmol/L phosphate buffer pH 7.4) at 37 °C for 60 min. After incubation, the absorbance (A) was read at 230 nm against a blank solution containing phosphate buffer without H2O2using a spectrophotometer. The percentage of H2O2scavenging was calculated as [(A230blank-A230test)/ A230blank]×100. IC50was calculated from the graph that plotted the inhibition percentage against differentT. minutaessential oil concentrations.
2.6.RNSscavenging assay
RNS scavenging activity of theT. minutaessential oil was determined as previously described[26]. Briefly, 10 μL of theT. minutaessential oil (0-500 μg/mL in DMSO) was incubated with 0.5 mL of sodium nitrite (10 μg/mL in 100 mmol/L sodium citrate pH 5) at 37 °C for 2 h. After incubation, 0.5 mL of Griess reagent was added and the absorbance (A) was read at 540 nm using a spectrophotometer. The percentage of RNS scavenging was calculated as follows: [(A540blank-A540sample)/A540blank×100. IC50was calculated from the graph that plotted the inhibition percentage against differentT. minutaessential oil concentrations.
2.7. Macrophages cell culture
The J774.1A murine macrophage cell line was obtained from the cell bank of the Pasteur Institute of Iran, Tehran. Cells were cultured in Dulbecco’s modified eagle medium containing 2 mmol/L L-glutamine, 100 IU/mL penicillin, 100 μg/ mL streptomycin and 10% heat-inactivated fetal calf serum at 37 °C in a humidified CO2incubator. Cultures were allowed to grow until confluence at which point adherent macrophages were scraped from the flask and were washed with warm medium (25 °C). Cells were counted and their viability was determined by trypan blue dye exclusion. The cells were seeded at concentration of 2×106cells per millilitre in 24-well tissue culture plates in triplicate (Jet Biofil, Kyoto, Japan). After culturing for 18 h to allow cells to adhere, non-adherent cells were removed by gentle rinsing with medium. Remaining adherent cells were then cultured in the presence or absence of medium bearing LPS (1 μg/mL). After 2 h,T. minutaessential oil was added at a final concentration of 0-200 μg/mL. Two sets of wells withoutT. minutaessential oil but containing LPS and DMSO solvent (0.1%) were used as negative controls. After 24 h of incubation at 37 °C, the culture supernatants in each well were removed and the cells harvested were used for RNA extraction and real-time PCR analysis.
2.8. Cell viability assay
The effect ofT. minutaessential oil on the viability of J774A.1 cells was determined by MTT assay as described previously[27]. Cells (2×104cells per well) were incubated for 24 h (at 37 °C in 5% CO2) with different concentrations (0-200 μg/ mL) ofT. minutaessential oil. Thereafter, 10 μL of MTT (5 mg/ mL) was added to each well and incubated for an additional 4 h at 37 °C followed by treatment with 100 μL of lysis buffer (10% SDS in 10 mmol/L HCl). The absorbance of each well was determined by spectrophotometer at dual wavelengths of 570 and 630 nm on a microplate ELISA reader (BioTek Elx 808, Winooski, VT, 05403, USA). Viability percentage was calculated by the following formula (Absorbance of treated cells/Absorbance of corresponding control)×100. The control wasT. minutaessential oil-untreated cells containing DMSO at the highest concentration used (0.1%). The concentration that provided a 50% inhibition (IC50) was calculated from a graph, plotting the inhibition percentage against differentT. minutaessential oil concentrations.
2.9.RNAextraction and cDNAsynthesis
Total RNA was extracted using RNX-plus buffer from Cinagen, Tehran, Iran. Briefly, about 2×106cells were transferred to 1 mL of RNX-plus buffer in an RNase-free microtube, mixed thoroughly and left at room temperature for 5 min. A volume of 200 μL of chloroform was added to the slurry and was mixed gently. The mixture was centrifuged at 13 200gat 4 °C for 15 min, the supernatant was transferred to a new tube and was precipitated with an equal volume of isopropanol for 15 min on ice. The RNA pellet was washed using 75% ethanol, briefly dried and resuspended in 15 μL of RNase free water. The purified total RNA was quantified by NanoDrop ND 1000 spectrophotometer (Wilmington, DE). A sample (0.005 mg) of RNA was used for first strand cDNA synthesis, using 100 pmoL oligo-dT (18 mer), 15 pmoL dNTPs, 20 U RNase inhibitor, and 200 U M-Mulv reverse transcriptase (all from Fermentas, Hanover, MD) in a 0.02 mL final volume.
2.10. Quantitative real-timePCR
Primer design, in the form of exon junction was carried out using AlleleID 7 software (Premier Biosoft Intl., Palo Alto, CA) for the internal controls glyceraldehydes-3-phosphate dehydrogenase (GAPDH) (NM-010927) and β-actin (NM-007393.3) and tested genes NOX p22phox (NM-007806), NOX p40phox (NM-008677), NOX p47phox (NM-010876), NOX p67phox (NM-010877), iNOS (NM-008084) and TNF-α (NM-013693) (Table 1). The GAPDH and β-actin were used as internal control (whose expression proved not to be influenced by LPS) for data normalization[28]. Relative real-time PCR was performed in a 20 μL volume containing 1 μL cDNA, 1×Syber Green buffer (Qiagen, Hilden,Germany) and 4 pmol of each primer. The amplification reactions were carried out in a line Gene k thermal cycler (Bioer Technology Co., Hangzhou, China) with initial denaturing of 94 °C for 2 min, followed by 40 cycles of 94 °C for 10 seconds, annealing temperature of each primer pair was done for 15 seconds and 30 seconds for extension to occur at 72 °C. After 40 cycles, the specificity of the amplifications was checked based on the melting curves resulting from heating the amplicons from 50 °C to 95 °C. All amplification reactions were repeated twice under identical conditions beside a negative control and 5 standard samples. To ensure that the PCR was generated from cDNA and not genomic DNA, proper control reactions were carried out without the reverse transcriptase treatment. For quantitative real time PCR data, relative expression of NOXs, iNOS and TNF-α gene were calculated based on the threshold cycle (CT) method. The CT for each sample was calculated using the Line-gene K software and the method of Larionovet al[29]. Accordingly, fold-expression of target mRNAs over the reference values were calculated by equation 2-ΔΔCT, where ΔCT was determined by subtracting the corresponding internal control CT value from the specific CT of targets, and ΔΔCT was obtained by subtracting the ΔCT of each experimental sample from that of the control sample[30].
2.11. Statistical analysis
All data are expressed as means plus standard deviations of at least three independent experiments. The significant differences between treatments were analyzed by One-way analyses of variance (ANOVA) test atP<0.05 using statistical package for the social sciences (SPSS, Abaus Concepts, Berkeley, CA) and Prism 5 (Graph Phad, San Diego, USA) software.

Table 1 Primer used for real-time analysis.
3. Results
3.1. Plant materials
TheT. minutaessential oil was prepared by water-distillation, and its chemical composition was determined by GC-MS. As shown in Table 2, GC-MS analysis indicated that the main components were dihydrotagetone (33.86%), E-ocimene (19.92%), tagetone (16.15%), cis-β-ocimene (7.94%), Z-ocimene (5.27%), limonene (3.1%) and epoxyocimene (2.03%). GC-MS analysis of the essential oil indicated the main componentsT. minutaessential oil were dihydrotagetone, E-ocimene, tagetone, cis-β-ocimene, Z-ocimene, limonene and epoxyocimene.
3.2. Antioxidant activity of T. minuta essential oil
T. minutaessential oil displayed a concentration dependent ROS, RNS and H2O2scavenging activity. IC50for ROS, RNS and H2O2scavenging were (12.0±3.0), (15.0±2.5) and (13.0±4.0) μg/mL ofT. minutaessential oil, respectively. At concentrations >30 μg/ mL, theT. minutaessential oil significantly scavenges ROS, RNS, and H2O2by 100%. TheT. minutaessential oil analyzed here possessed potentin vitroROS, RNS and H2O2scavenging activity. TheT. minutaessential oil at >30 μg/mL had the ability to scavenge all ROS, RNS and H2O2radicals, an indicator of its potency as a radical scavenger.

Table 2 Chemical composition of T. minuta essential oil.
3.3. T. minuta essential oil reduced cell viability at high concentrations
The MTT assay results indicated that low concentrations (1-50 μg/mL) ofT. minutaessential oil had no effect on J774A.1 cell viability. However, at higher concentrations (100-200 μg/mL), cell viability was significantly reduced in a concentration-related manner, with the maximum effect (100% cell death) at concentrations >200 μg/mL (Figure 1). Non-cytotoxic concentrations (<50 μg/mL) were thus used for the subsequent studies including expression of genes.
3.4. T. minuta essential oil reducedNOXp22phox mRNAexpression inLPS-stimulated macrophages
The un-stimulated (control) cells showed low level of NOX p22phox mRNA expression. LPS stimulation of macrophages resulted in an increase in NOX p22phox mRNA expression (26.5±1.7) fold of LPS-untreated control cells (P<0.001). The addition ofT. minutaessential oil at 1 to 50 μg/mL significantly decreased the NOX p22phox mRNA expression in LPS-treated cells from (21.0±2.7) to (4.5±0.8) fold of the control (P<0.001) dose-dependently, indicating the inhibitory effect ofT. minutaessential oil on p22phox mRNA induction/ formation (Figure 2).

Figure 1. Effect of the T. minuta essential oil on the viability of J774 cell lines.TMO: T. minuta essential oil. The cells were treated with various concentrations of essential oil (0-200 μg/mL) and incubated for 24 h. Control (0 μg/mL) was cells treated only with the solvent (DMSO) at concentration of 0.1%. Data represent mean±SD from three sets of independent experiments.

Figure 3. Effects of T. minuta essential oil on NOX p40phox mRNA expression in LPS-stimulated macrophages.TMO: T. minuta essential oil. The cells were cultured in 24-well plates and treated with and without LPS. Various concentrations of essential oil (1-50 μg/mL) were added. After 24 h, the expression of NOX p47phox mRNA was analyzed by real-time PCR. Cells treated with DMSO as the solvent (control) and cells treated with the solvent and LPS was considered as positive controls.
3.5. T. minuta essential oil reduced NOX p40phox mRNA Expression in LPS-Stimulated Macrophages
The un-stimulated (control) cells showed low level of NOX p40phox mRNA expression while, the expression of NOX p40phox mRNA in LPS-treated cells was (6.3±0.4) fold of the control (P<0.001). The addition ofT. minutaessential oil at 1 to 50 μg/mL significantly decreased the NOX p40phox mRNA expression in LPS-treated cells from (5.1±0.7) to (2.4±0.2) fold of the control, dose-dependently (P<0.05), indicating the inhibitory effect ofT. minutaessential oil on p40phox mRNA induction/formation (Figure 3).

Figure 2. Effects of T. minuta essential oil on NOX p22phox mRNA expression in LPS-stimulated macrophages.TMO: T. minuta essential oil. The cells were cultured in 24-well plates and treated with and without LPS. Various concentrations of essential oil (1-50 μg/mL) were added. After 24 h, the expression of NOX p22phox mRNA was analyzed by real-time PCR. Cells treated with DMSO as the solvent (control) and cells treated with the solvent and LPS was considered as positive controls.
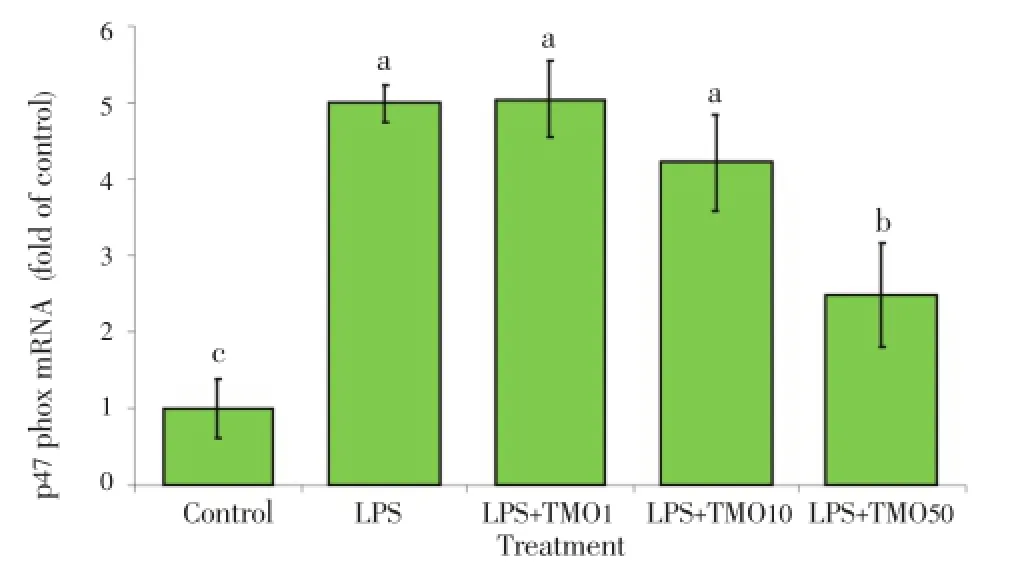
Figure 4. Effects of T. minuta essential oil on NOX p47phox mRNA expression in LPS-stimulated macrophages.TMO: T. minuta essential oil. The cells were cultured in 24-well plates and treated with and without LPS. Various concentrations of TMO (1-50 μg/mL) were added. After 24 h, the expression of NOX p47phox mRNA was analyzed by real-time PCR. Cells treated with DMSO as the solvent (control) and cells treated with the solvent and LPS was considered as positive controls.
3.6. T. minuta essential oil reducedNOXp47phox mRNAexpression inLPS-stimulated macrophages
The un-stimulated (control) cells showed low level of NOX p47phox mRNA expression while, the expression of NOX p47phox mRNA in LPS-treated cells was (5.00±0.25) fold of the control induction/formation (P<0.001). The addition ofT. minutaessential oil at 1 to 50 μg/mL significantly decreased this gene expression in LPS-treated cells from (5.00±0.50) and (1.60 ±0.28) fold of control, dose-dependently (P<0.01) indicating the inhibitory effect ofT. minutaessential oil on p47phox mRNA induction/formation (Figure 4).
3.7. T. minuta essential oil reducedNOXp67phox mRNAexpression inLPS-stimulated macrophages
With respect to NOX p67phox, a decrease in the gene expression was detected in LPS-stimulated macrophages which were treated withT. minutaessential oil. The relative NOX p67phox mRNA expression in cells treated with LPS alone was (5.00±0.25) fold of LPS-untreated the control cells (P<0.001). The addition ofT. minutaessential oil at 1 to 50 μg/mL significantly decreased the NOX p67phox mRNA expression in LPS-treated cells from (4.0±0.5) and (1.0±0.4) fold of the control, dosedependently (P<0.01), indicating the inhibitory effect ofT. minutaessential oil on p67phox mRNA induction/formation (Figure 5).

Figure 5. Effects of T. minuta essential oil on NOX p67phox mRNA expression in LPS-stimulated macrophages.TMO: T. minuta essential oil. The cells were cultured in 24-well plates and treated with and without LPS. Various concentrations of TMO (1-50 μg/mL) were added. After 24 h, the expression of NOX p67phox mRNA was analyzed by real-time PCR. Cells treated with DMSO as the solvent (control) and cells treated with the solvent and LPS was considered as positive controls.
3.8. T. minuta essential oil reduced iNOSmRNAexpression inLPS-stimulated macrophages
LPS stimulation of macrophages resulted in an increase in iNOS mRNA expression (27.5 ±1.4) fold of LPS-untreated cells (P<0.001). The addition ofT. minutaessential oil at concentrations 1 to 50 μg/mL significantly decreased the iNOS mRNA expression in LPS-treated cells from (20.4±1.4) to (3.8±1.0) fold of untreated cells, dose-dependently (P>0.05) indicating the inhibitory effect ofT. minutaessential oil on iNOS mRNA induction/formation (Figure 6).

Figure 6. Effects of T. minuta essential oil on iNOS mRNA expression inLPS-stimulated macrophages.TMO: T. minuta essential oil. LPS-stimulated macrophages. The cells were cultured in 24-well plates and treated with and without LPS. Various concentrations of TMO (1-50 μg/mL) were added. After 24 h, the expression of NOS mRNA was analyzed by real-time PCR. Cells treated with DMSO as the solvent (control) and cells treated with the solvent and LPS was considered as positive controls.
3.9. T. minuta essential oil reducedTNF-αmRNAexpression inLPS-stimulated macrophages
LPS stimulation of macrophages resulted in an increase in TNF-α mRNA expression compared to the conditions in LPS-untreated cells (9.8±0.5) fold (P<0.001). The addition ofT. minutaessential oil at 1 to 50 μg/mL significantly decreased the TNF-α mRNA expression from (9.7±0.4) and (3.3±0.6) fold of the control, dose-dependently (Figure 7). These data indicated the inhibitory effect ofT. minutaessential oil on TNF-α mRNA induction/formation.
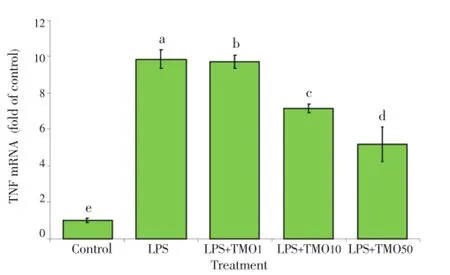
Figure 7. Effects of T. minuta essential oil on TNF mRNA expression in LPS-stimulated macrophages.TMO: T. minuta essential oil. The cells were cultured in 24-well plates and treated with and without LPS. Various concentrations of TMO (1-50 μg/mL) were added. After 24 h, the expression of TNF mRNA was analyzed by realtime PCR. Cells treated with DMSO as the solvent (control) and cells treated with the solvent and LPS was considered as positive controls.
4. Discussion
The antioxidant and anti-inflammatory effects ofT. minutaessential oil were investigated in the present study. GC-MS analysis of the essential oil indicated the main componentsinT. minutaessential oil were dihydrotagetone, E-ocimene, tagetone, cis-β-ocimene, Z-ocimene, limonene and epoxyocimene. Previous study reported the main components ofT. minutaessential oil were β-ocimene, dihydrotagetone, tagetone, Z-ocimene and E-ocimene[11]. Another study reported thiophenes and polyacetylenic compounds in theTagetesspecies andT. minutahad the highest total thiophene yield[12]. Accordingly, the main components ofT. minutaessential oil could be tagetone (cis/trans, ketone/alcohol, aldehyde/alcohol), ocimene (cis/trans, ketone/alcohol, aldehyde/alcohol) and thiophene derivatives[11-14]. For the reasons that, essential oils composition depend on the species, climate, altitude, time of collection and growth stage, thus the plants analyzed in this research had roughly same components with other previously analyzedT. minutaessential oil however, showed important differences in their quality and quantity of components.
TheT. minutaessential oil analyzed here possessed potentin vitroROS, RNS and H2O2scavenging activity. TheT. minutaessential oil at >30 μg/mL had the ability to scavenge all ROS, RNS and H2O2radicals, an indicator of its potency as a radical scavenger. ROS are oxygen-derived small molecules, including oxygen radicals such as superoxide, hydroxyl and peroxyl and some non-radicals that are easily converted into radicals, such as hydrogen peroxide. ROS, once produced, can interact with various molecules including other small inorganic molecules as well as macromolecules such as proteins and lipids. During these interactions, ROS may destroy or change the function of the target molecule[31]. The ROS reducing activity ofT. minutaessential oil observed in our study imply the beneficial role of this product for reducing damages in biological tissues. The radical scavenging activity of compounds is mainly due to their oxidation-reduction potential, which can play an important role in neutralizing free radicals. This activity is related to phenolic hydroxyl groups[32].T. minutaessential oil mainly contains dihydrotagetone, ocimene, tagetone and limonene which all are monoterpenes. This antioxidant activity was confirmed by previous research with IC50between 35-344 μg/mL[21,22]. Thus,T. minutaessential oil analyzed in this research showed stronger antioxidant activity rather than previously analyzed one.In vitroinhibition of the NO radical is a measure of antioxidant activity of plant extracts. As results of the present study show, theT. minutaessential oil used here have the ability to scavenge total RNS at concentration >30 μg/mL.
The MTT assay results indicated that low concentrations (1-50 μg/mL) ofT. minutaessential oil had no effect on J774A.1 cell viability (IC50=95 μg/mL). In order to determine the antioxidant and anti-inflammatory effects ofT. minutaessential oil on macrophages, the concentrations of >50 μg/mLT. minutaessential oil which were overtly cytotoxic to the cells were not used. Although the constituents of essential oils can act as antioxidants, they may also act as pro-oxidants and affect inner cell membranes and organelles such as mitochondria in eukaryotic cells. Depending on the type and concentration, this effect may result in cellular cytotoxicity.
In macrophages ROS production is under the control of NOX. This multi-component enzyme consists of several cytosolic components including p91phox, p67phox, p40phox, p47phox and the small Rho G protein (Rac 1 or Rac 2, Rac: Rho-related C3 botulinum toxin substrate), which assemble on the cellular membrane to activate the enzyme[33]. Studies have shown that phosphorylation of p47phox leads to conformational changes, allowing its translocation and interaction with p22phox. Translocation of p47phox brings with it the other subunits, p67phox and p40phox to the membrane[34]. Activation of this enzyme complex leads to fusion of the vesicles containing NOX with the plasma membrane or the phagosomal membrane. The active enzyme converts molecular oxygen to superoxide anion through a one-electron transfer[35]. As our study showed,T. minutaessential oil was able to decrease the expression of key components of NOX. It has been shown that the assembly of p47phox, p67phox and p22phox at the membrane is necessary for oxidase function[36]. Thus, it can be assumed that reduced ROS generation by stimulated macrophages in the presence ofT. minutaessential oil might be, in part, due to the modulation of the expression of NOX subunits.
In addition to ROS, the overproduction of RNS by activated macrophages seems to play an important role in different steps of many inflammatory processes[37]. RNS are nitrogencontaining oxidants, mainly NO which is a free radical playing a key role in the pathogenesis of pain and inflammation. NO in macrophages is generated by activation of iNOS. This enzyme has the ability to produce high concentrations of NO after stimulation with bacterial endotoxins or a variety of proinflammatory cytokines such as TNF-α, IL-1 and IL-6[38]. NO generation involves several steps including the activation of nuclear transcription factor (NF)-κB and subsequent iNOS gene expression[39]. NF-κB regulates the expression of various genes involved in inflammatory responses. Its activation can also be regulated by various cytokines, among which TNF-α is the most important. In response to inflammatory stimuli such as LPS, macrophages secrete a variety of inflammatory mediators such as TNF-α and IL-1β. The production of TNF-α cytokine is important for the induction of NO synthesis in LPS-stimulated macrophages[40]. As results of this study showed thatT. minutaessential oil was able to reduce inducible expression of TNF-α gene, which indicated that the reduced NO production seen in the macrophage cultures might be partly related to the suppression of TNF-α expression. TNF-α is known to play a crucial role in inflammatory responses and is involved in the pathogenesis of inflammatory diseases[41]. As results of our study showed,T. minutaessential oil significantly reduced iNOS mRNA expression in stimulated macrophages. Suppression of TNF-α expression in macrophages as well as reduced iNOS gene expression, due to theT. minutaessential oil indicates the ability of this product to diminish immune reactions and provides further evidence that this plant may have potent immuno-modulatory properties.
Considering all these finding,T. minutaessential oil displayed an anti-oxidant property by scavenging superoxide, H2O2and NO radicals, and reduced oxidative stress. This suggested that there was a potential for use of this product in the therapy of oxidative damage, a process that usually accompanies inflammatory conditions. The decreased formation of ROS and NOS radicals in macrophages was possibly due to theradical scavenging activity of phenolic groups present in the oil and/or due to an inhibition of iNOS and NOX gene expressions. Furthermore,T. minutaessential oil decreased the expression of the genes for pro-inflammatory cytokine TNF-α. Therefore, a reduced expression of the above-noted inflammatory enzymes and cytokines could be attributed to a suppression of the NF-kB pathway in the treated cells. These data suggest a potential therapeutic usefulness forT. minutain the modulation of macrophages and provides evidence to support the use ofT. minutaas a tea/additive/traditional remedy for treatment of inflammatory diseases. Furtherin vivostudies are recommended to more fully understand the therapeutic potential ofT. minutaessential oil in a multitude of inflammatory disorders.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This work was supported by the funding from Shiraz University (Grant no. 88-GR-AGRST-108) and Shiraz University of Medical Science (Grant No. 3937).
Comments
Background
T. minutais an aromatic plant that has several medical benefits such as a remedy for the colds, respiratory inflammations, stomach problems, anti-spasmodic, antiparasitic, antiseptic and sedative. This work aimed to investigate antioxidant and anti-inflammatory effects ofT. minutaessential oil.
Research frontiers
The aim of the present study was to investigate the level of potential modulating effects ofT. minutaessential oil on macrophages and their related functions including expression of NOX subunits, NOS and TNF-α mRNAs in LPS-stimulated macrophages. In addition,in vitroantioxidant capacity ofT. minutaessential oil was examined by assessments of ROS, RNS and H2O2scavenging ability.
Related reports
Advances in chemical and pharmacological evaluations ofT. minutaessential oil such as antioxidant and antimicrobial have occurred in the past recent years. However, several useful features of this plant (e.g.the mechanisms underlying its antioxidant and anti-inflammatory effects) have remain unknown.
Innovations and breakthroughs
These studies would reveal thatT. minutaessential oil exhibits radical scavenging activity (against superoxide anion, H2O2, and NO radicals) in macrophages, in part, due to an inhibition of iNOS and NOX gene expression.
Applications
These data suggest a potential therapeutic usefulness forT. minutain the modulation of macrophages and provides evidence to support the use ofT. minutaas a tea/additive/ traditional remedy for treatment of inflammatory diseases.
Peer review
T. minutaessential oil displayed an anti-oxidant property by scavenging superoxide, H2O2and NO radicals, and reduced oxidative stress. The decreased formation of ROS and NOS radicals in macrophages was possibly due to the radical scavenging activity of phenolic groups present in the oil and/or due to an inhibition of iNOS and NOX gene expressions. Furthermore,T. minutaessential oil decreased the expression of the genes for pro-inflammatory cytokine TNF-α.
[1] Iranian Herbal Pharmacopoeia. Tehran: Iranian Ministry of Health & Medical Education Publications; 2002.
[2] Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol 2010; 101: 372-378.
[3] Rahimi R, Shams-Ardekani MR, Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol 2010; 16: 4504-4514.
[4] Nikkon F, Habib MR, Saud ZA, Karim MR. Tagetes erecta and its mosquitocidal potency against Culex quinquefasciatus. Asian Pac J Trop Biomed 2011; 1: 186-188.
[5] Kamaraj C, Rahuman AA, Bagavan A, Elango G, Zahir AA, Santhoshkumar T. Larvicidal and repellent activity of medicinal plant extracts from Eastern Ghats of South India against malaria and filariasis vectors. Asian Pac J Trop Med 2011; 4: 698-705.
[6] Govindarajan M. Larvicidal and repellent properties of some essential oils against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera: Culicidae). Asian Pac J Trop Med 2011; 4: 106-111.
[7] Aristatile B, Al-Assaf AH, Pugalendi KV. Carvacrol suppresses the expression of inflammatory marker genes in D-galactosamine-hepatotoxic rats. Asian Pac J Trop Med 2013; 6: 205-211.
[8] Maity N, Nema NK, Abedy MK, Sarkar BK, Mukherjee PK. Exploring Tagetes erecta Linn flower for the elastase, hyaluronidase and MMP-1 inhibitory activity. J Ethnopharmacol 2011; 137: 1300-1305.
[9] Wang M, Tsao R, Zhang S, Dong Z, Yang R, Gong J, et al. Antioxidant activity, mutagenicity/anti-mutagenicity, and clastogenicity/anti-clastogenicity of lutein from marigold flowers. Food Chem Toxicol 2006; 44: 1522-1529.
[10] Breme K, Tournayre P, Fernandez X, Meierhenrich UJ, Brevard H, Joulain D, et al. Identification of odor impact compounds of Tagetes minuta essential oil: comparison of two GC-olfactometry methods. J Agric Food Chem 2009; 57: 8572-8580.
[11] Lopez SB, Lopez ML, Aragon LM, Tereschuk ML, Slanis AC,Feresin GE, et al. Composition and anti-insect activity of essential oils from Tagetes species on Ceratitis capitata Wiedemann and Triatoma infestans Klug. J Agric Food Chem 2011; 59: 5286-5292.
[12] Marotti I, Marotti M, Piccaglia R, Nastri A, Grandi S, Dinelli G. Thiophene occurrence in different Tagetes species: agricultural biomasses as sources of biocidal substances. J Sci Food Agric 2010; 90: 1210-1217.
[13] Mohamed MA, Harris PJ, Henderson J, Senatore F. Effect of drought stress on the yield and composition of volatile oils of drought-tolerant and non-drought-tolerant clones of Tagetes minuta. Planta Med 2002; 68: 472-484.
[14] Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol 2010; 101: 4676-4689.
[15] Céspedes CL, Avila JG, Martínez A, Serrato B, Calderón-Mugica JC, Salgado-Garciglia R. Antifungal and antibacterial activities of Mexican tarragon (Tagetes lucida). J Agric Food Chem 2006; 54: 3521-3527.
[16] Tereschuk ML, Baigori MD, Abdala LR. Antibacterial activity of Tagetes terniflora. Fitoterapia 2003; 74: 404-416.
[17] Héthélyi E, Dános B, Tétényi P, Koczka I. GC-MS analysis of the esssential oils of four Tagetes species and the anti-microbial activity of Tagetes minuta. Flavor Fragr J 1986; 1: 169-173.
[18] Dunkel FV, Jaronski ST, Sedlak CW, Meiler SU, Veo KD. Effects of steam-distilled shoot extract of Tagetes minuta and entomopathogenic fungi on larval Tetanops myopaeformis. Environ Entomol 2010; 39: 979-988.
[19] Mares D, Tosi B, Poli F, Andreotti E, Romagnoli C. Antifungal activity of Tagetes patula extracts on some phytopathogenic fungi: ultrastructural evidence on Pythium ultimum. Microbiol Res 2004; 159: 295-304.
[20] Thembo KM, Vismer HF, Nyazema NZ, Gelderblom WC, Katerere DR. Antifungal activity of four weedy plant extracts against selected mycotoxigenic fungi. J Appl Microbiol 2010; 109: 1479-1486.
[21] Gong Y, Liu X, He WH, Xu HG, Yuan F, Gao YX. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold residue. Fitoterapia 2012; 83: 481-489.
[22] Parejo I, Bastida J, Viladomat F, Codina C. Acylated quercetagetin glycosides with antioxidant activity from Tagetes maxima. Phytochemistry 2005; 66: 2356-2362.
[23] British Pharmacopeia. London: The British Pharmacopoeia Commission; 1998, p. 137-138.
[24] Adams RP. Identification of essential oil components by gas chromatography/mass spectrometery. 4th ed. Illinois, USA: Allured Publishing Corporation; 2007, p. 456-494.
[25] Kavoosi G, Rowshan V. Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assa-foetida oleo-gum-resin: effect of collection time. Food Chem 2013; 138: 2180-2187.
[26] Kavoosi G, Teixeira da Silva JA, Saharkhiz MJ. Inhibitory effects of Zataria multiflora essential oil and its main components on nitric oxide and hydrogen peroxide production in lipopolysaccharidestimulated macrophages. J Pharm Pharmacol 2012; 64: 1492-1500.
[27] Nouri AM, Thompson C, Cannell H, Symes M, Purkiss S, Amirghofran Z. Profile of epidermal growth factor receptor (EGFr) expression in human malignancies: effects of exposure to EGF and its biological influence on established human tumor cell lines. Inter J Mol Med 2000; 6: 495-500.
[28] Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics 2005; 21: 389-395.
[29] Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 2005; 6: 62.
[30] Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod. Methods 2001; 25: 402-408.
[31] Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 2010; 48; 749-762.
[32] Huang CC, Wang HF, Chen CH, Chen YJ, Yih KH. A study of four antioxidant activities and major chemical component analyses of twenty-five commonly used essential oils. J Cosmet Sci 2011; 62: 393-404.
[33] Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J 2005; 386: 401-416.
[34] Minakami R, Sumimotoa H. Phagocytosis-coupled activation of the superoxide-producing phagocyte oxidase, a member of the NADPH oxidase (NOX) family. Int J Hematol 2006; 84: 193-198.
[35] Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87: 245-313.
[36] Kleniewska P, Piechota A, Skibska B, Gorąca A. The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp (Warsz) 2012; 60: 277-294.
[37] Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, et al. Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses. J Biol Chem 2012; 287: 2984-2995.
[38] Sellers SL, Iwasaki A, Payne GW. Nitric oxide and TNFα are critical regulators of reversible lymph node vascular remodeling and adaptive immune response. PLoS One 2013; 8: 60741.
[39] Lorenzo O, Picatoste B, Ares-Carrasco S, Ramírez E, Egido J, Tuñón J. Potential role of nuclear factor kB in diabetic cardiomyopathy. Mediators Inflamm 2011; doi: 10.1155/2011/652097.
[40] Zha LY, Mao LM, Lu XC, Deng H, Ye JF, Chu XW, et al. Antiinflammatory effect of soyasaponins through suppressing nitric oxide production in LPS-stimulated RAW 264.7 cells by attenuation of NF-κB-mediated nitric oxide synthase expression. Bioorg Med Chem Lett 2011; 21: 2415-2418.
[41] Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012; 36: 764-785.
10.1016/S2221-1691(14)60235-5
*Corresponding author: Gholamreza Kavoosi, Biotechnology Institute, Shiraz University, P.O.71441-65186, Shiraz, 71441-65186, Iran.
Tel: +98-711-2272805
E-mail: ghkavoosi@shirazu.ac.ir
Foundation Project: Supported by the funding from Shiraz University (Grant no. 88-GR-AGRST-108) and Shiraz University of Medical Science (Grant No. 3937).
Article history:
Received 1 Jan 2014
Received in revised form 10 Jan, 2nd revised form 16 Jan, 3rd revised form 22 Jan 2014
Accepted 26 Feb 2014
Available online 28 Mar 2014
Methods:In the present study T. minuta essential oil was obtained from leaves of T. minuta via hydro-distillation and then was analyzed by gas chromatography-mass spectrometry. The antioxidant capacity of T. minuta essential oil was examined by measuring reactive oxygen, reactive nitrogen species and hydrogen peroxide scavenging. The anti-inflammatory activity of T. minuta essential oil was determined through measuring NADH oxidase, inducible nitric oxide synthase and TNF-α mRNA expression in lipopolysacharide-stimulated murine macrophages using realtime PCR.
Results:Gas chromatography-mass spectrometry analysis indicated that the main components in the T. minuta essential oil were dihydrotagetone (33.86%), E-ocimene (19.92%), tagetone (16.15%), cis-β-ocimene (7.94%), Z-ocimene (5.27%), limonene (3.1%) and epoxyocimene (2.03%). The T. minuta essential oil had the ability to scavenge all reactive oxygen/reactive nitrogen species radicals with IC5012-15 μg/mL, which indicated a potent radical scavenging activity. In addition, T. minuta essential oil significantly reduced NADH oxidase, inducible nitric oxide synthaseand TNF-α mRNA expression in the cells at concentrations of 50 μg/mL, indicating a capacity of this product to potentially modulate/diminish immune responses.
Conclusions:T. minuta essential oil has radical scavenging and anti-inflammatory activities and could potentially be used as a safe effective source of natural anti-oxidants in therapy against oxidative damage and stress associated with some inflammatory conditions.
 Asian Pacific Journal of Tropical Biomedicine2014年3期
Asian Pacific Journal of Tropical Biomedicine2014年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- An update on Ayurvedic herb Convolvulus pluricaulis Choisy
- Evidence of increasing L1014F kdr mutation frequency in Anopheles gambiae s.l. pyrethroid resistant following a nationwide distribution of LLINs by the Beninese National Malaria Control Programme
- Prevalence and antimicrobial resistance of Salmonella spp. in raw retail frozen imported freshwater fish to Eastern Province of Saudi Arabia
- Hesperidin as a preventive resistance agent in MCF-7 breast cancer cells line resistance to doxorubicin
- Long-term spatial memory and morphological changes in hippocampus of Wistar rats exposed to smoke from Carica papaya leaves
- Survey on cattle ticks in Nur, north of Iran
