Chemical composition and larvicidal activity of essential oil of Origanum majorana (Lamiaceae) cultivated in Morocco against Culex pipiens (Diptera: Culicidae)
Fouad El-Akhal, Abdelhakim El Ouali Lalami, Yassine Ez Zoubi, Hassane Greche, Raja Guemmouh
1Regional Diagnostic Laboratory Epidemiological and Environmental Hygiene, Regional Health Directorate, EL Ghassani Hospital, Fez, Morocco.
2Sidi Mohamed Ben Abdellah University, Faculty of Sciences Dhar El Mahraz, Laboratory Analysis and Modelling of Ecosystems Continentals, Fez, Morocco.
3National Institute of Medicinal and Aromatic Plants, Sidi Mohamed Ben Abdellah University, Fez, Morocco.
Chemical composition and larvicidal activity of essential oil of Origanum majorana (Lamiaceae) cultivated in Morocco against Culex pipiens (Diptera: Culicidae)
Fouad El-Akhal1,2, Abdelhakim El Ouali Lalami1*, Yassine Ez Zoubi3, Hassane Greche3, Raja Guemmouh2
1Regional Diagnostic Laboratory Epidemiological and Environmental Hygiene, Regional Health Directorate, EL Ghassani Hospital, Fez, Morocco.
2Sidi Mohamed Ben Abdellah University, Faculty of Sciences Dhar El Mahraz, Laboratory Analysis and Modelling of Ecosystems Continentals, Fez, Morocco.
3National Institute of Medicinal and Aromatic Plants, Sidi Mohamed Ben Abdellah University, Fez, Morocco.
ARTICLE INFO
Article history:
Received 28 Jul 2014
Received in revised form 29 Aug 2014
Accepted 20 Sep 2014
Available online 23 Sep 2014
Essential oil
Origanum majorana
Culex pipiens
Larvicidal activity
Morocco
Fez
Objective:To evaluate the larvicidal activity of essential oil of Origanum majorana (Lamiaceae) cultivated in Morocco against Culex pipiens (Diptera: Culicidae).
1. Introduction
The species of the genusCulexare incumbent vector for several pathogens such as West Nile virus, affecting humans and/or animals[1-3]. The outbreaks of West Nile virus infection have been reported in Morocco in 1996, 2003 and 2010[4,5].Culex pipiens(Cx. pipiens) has been strongly suspected as the vector responsible for transmission[4-7].
These mosquito species usually breed in stagnant water with high levels of organic matter, such as artificial containers[8,9], and blocked drainages or the ditches in urban and suburban areas[10]. In the region of Fez, the species ofCulexgenus are found all the year[11]. Their presences may pose a threat to the population of Fez region, which is considered as crossroads and place of residence of several travelers and nationals of sub saharienns countries affectedby vector-borne diseases[12].
To fight against these vectors of disease, insecticides are the most widely used products, but they have several disadvantages since they can be the source of various environmental problems[13-15].
Indeed, most chemical insecticides utilized caused a major problem in development of resistance by certain mosquitoes[16-19]. In addition, researchers and scientists are currently trying to find effective and accessible alternative from natural products, which are of renewed interest and growing popularity[20].
In Morocco, studies on the insecticidal activity of vegetable extracts against the mosquito larvae are very limited[21,22], at least to our knowledge the essential oil ofOriganum majorana(O. majorana) has not been the subject of previous studies. Furthermore, the comparison of natural products with synthetic chemicals helps to better exploit these natural bio-insecticides.
The literature reports that the genusOriganum(Lamiaceae) is characterised by a large number of biological activities, including antioxidant, antiinflammatory andanticholinesterase effects, as well as activities against ageing and neurodegenerative disease[23]. Recently, the species of this plant have attracted more attention of consumers due to the antimicrobial, antifungal, insecticidal and antioxidative effects of this herb on human health[24].
RegardingO. majoranawhich is an herb and perennial native to southern Europe and the Mediterranean, it is used in food for flavoring sausages, meats, salads and soups. Traditionally, it is used as a folk remedy against asthma, the headache, and rheumatism[25].
In this research, authors aimed to study the chemical composition and larvicidal activity of essential oil ofO. majoranacultivated in central Morocco againstCx. pipiensfor the first time.
2. Materials and methods
2.1. Plant harvest and extraction of essential oil
Plant material of theO. majoranaplant was harvested in March 2010 at the city of Taounate. A total of 200 g of the plant material ofO. majoranawas subjected to a water distillation for 3 h, using a modified Clevenger type apparatus. The essential oil collected by decantation at the end of the distillation has been dried over anhydrous sodium sulfate to remove traces of residual water. The essence thus obtained was opaque in small vials and stored at 4 °C before use.
2.2. Chemical study and identification composed
Chemical analysis of the essential oil was performed with the aid of a gas chromatograph coupled to mass spectrometry (GC-MS).
Then gas phase chromatographic analyses were carried out with the aid of a Trace GC Ultra apparatus equipped with one injector in Split Play, a VB-5 column (30 m×0.25 mm, film thickness 0.25 µm). The operating conditions are as follows: carrier gas: helium; solvent: ethyl acetate; injection temperature: 220 °C; injection volume: 1 µL; flow rate: 1.4 mL/min; temperature program: from 40 °C to 180 °C to 4 °C/ min, with a level of 20 min to 300 °C.
The coupling with the mass spectrometer Polaris QMS was done with a temperature of 300 °C interface. The operating conditions are as follows: type electron impact ionization (70 eV); injector temperature was 200 °C. The database used was NIST M Search.
The identification of the constituents was assigned on the basis of comparison of their retention indices and mass spectra with those given in the literature[26,27].
2.3. Characteristic breeding site
The collection of larvae ofCx. pipienswas performed in a breeding site located in the urban area of the city of Fez, appointed Grand Canal (402 m altitude, 30°03’37’’ N and 5°08’35’’ E). This gite is characterized by a very high density of larval belonging to Culicidae. The warm water from a thermal spring named Ain Lah promotes the proliferation of larvae ofCx. pipiens.
2.4. Collection of larvae of Cx. pipiens
Larvae were collected using rectangular plastic tray that inclined 45° to the water surface. Larvae harvested were maintained breeding in rectangular trays with an average temperature of (22.6±2.0) °C in the Entomology Unit at the Regional Diagnostic Laboratory Epidemiological and Environmental Hygiene falling within Regional Health Directorate of Fez.
2.5. Identification of larvae
The identification of morphological characters of larvae has been performed using the identification key of Moroccan Culicidae and the identification software dealing with mosquitoes of Mediterranean Africa[28].
2.6. Larval susceptibility testing
A stock solution (10%) of essential oil in ethanol and a dilution series: 100, 200, 300, 400, 500 and 600 mg/L were prepared. Preliminary experiments enabled us to select a range of concentrations for test. About 1 mL of each solution prepared was placed in beakers containing 99 mL of distilled water in contact with 20 larvae of stage 3 and 4. The same number of larvae was placed in a beaker containing 99 mL indicator of distilled water plus 1 mL ethanol (control). Three replicates were carried out for each dilution and for the control. After 24 h of contact, living and dead larvae were counted.
The results of susceptibility testing were expressed in percentage of mortality versus concentrations of essential oils used. If the percentage of mortality in control is greater than 5%, the percentage of mortality in larvae exposed to the essential oil shall be corrected by using Abbott’s formula[29]. % Mortality corrected=(% Mortality observed-% Mortality Control)/(100-% Mortality Control)×100.
If the control mortality exceeds 20%, the test is invalid and must be repeated.
2.7. Processing of data
For the entry and processing of data, the log-probit analysis (Windl version 2.0) software developed by CIRADCA/MABIS was used[30].
3. Results
3.1. Yield of essential oil
The yield of essential oil ofO. majoranais 0.8%. Thisessential oil yield was calculated on the basis of the dry matter.
3.2. Chemical composition of essential oil
The results of the analysis of essential oil by GC-MS showed that the major components ofO. majoranaare 4-terpinene (28.96%), γ-terpinene (18.57%) and α-terpinene (12.72%) (Table 1). The monoterpene hydrocarbons constitute the major fraction (51.7%), followed by oxygenated monoterpenes with 44.38%. The sesquiterpene hydrocarbons represented only 3.67% of all the constituents identified (99.75%).

Table 1 Chemical composition of essential oil of O. majorana analyzed by GC-MS.
3.3. Larvicidal activity of essential oil of O. majorana against Cx. pipiens
3.3.1. Variation in mortality rate
After exposure to different concentrations of essential oil ofO. majoranafor 24 h, the mortality rate of larvae ofCx.pipiensat stage 3 and 4 varied from 17.3% to 100% (Figure 1).
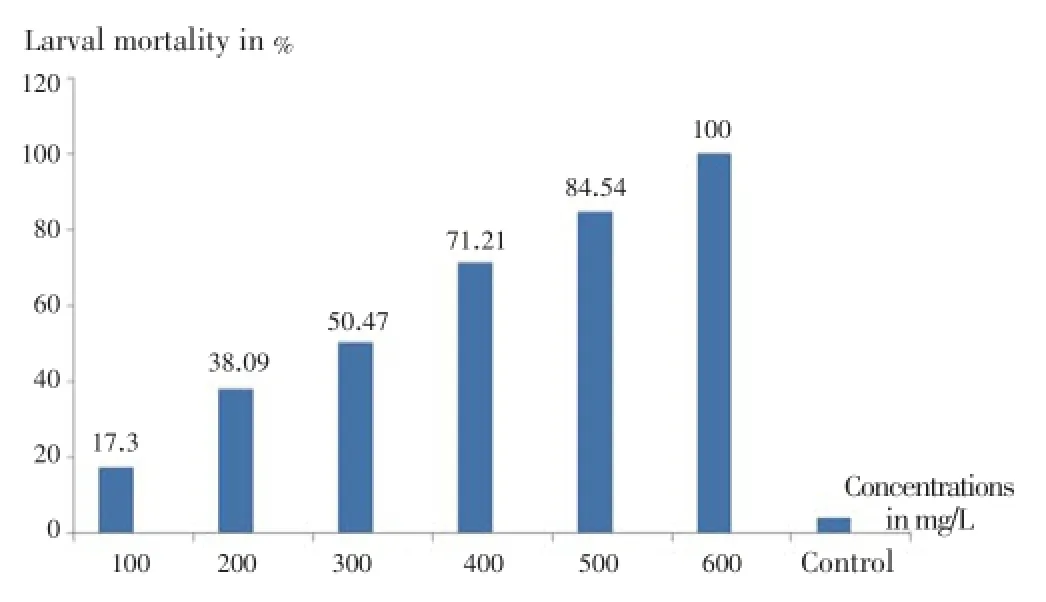
Figure 1. Mortality (%) of larvae of Cx. pipiens varied depending on the concentration of essential oil (mg/L) of O. majorana after 24 h exposure.
The lowest concentration necessary to achieve 100% mortality of larvae ofCx. pipienswas evaluated at 600 mg/L (Figure 1).
3.3.2. Lethal concentrations LC50and LC90
After 24 h, the essential oil from the leaves ofO. majoranaexhibited significant larvicidal activity; the LC50and LC90of the essential oil ofO. majoranais 258.71 mg/L (lower limitupper limit: 126.99-527.06 mg/L) and 580.49 mg/L (lower limitupper limit: 354.51-950.53 mg/L) respectively.Chi-square values (equation of the regression lineY=3.65193+8.81146Xand calculated χ2=16.3978) of the essential oil show significant larvicidal activity.
4. Discussion
The essential oil yield ofO. majorana(0.8%) obtained in this study, is relatively low compared to some plants that are exploited industrially as the source of essential oils[20]. The yield of the plantsO. majoranacultivated in a nursery located in Soliman in the North-East of Tunisia was found between 0.04% to 0.09%[31], but that obtained from a species of Indian country is around 1.7%[32]. The yield ofOriganum vulgare,which is an Eurasian species and belong to the same family (Lamiaceae) withO. majoranawas found in the order of 7.4%[33].
Plant essential oils, in general, have been recognized as an important natural resource of insecticides[34]. The leaves of theOriganumherb are rich in essential oil which confers its characteristic and fragrance. Several studies have shown that the essential oil ofOriganumis composed of majority constituents, giving it the biological activities. The extraction product can vary in quality, quantity and composition according to climate, soil composition, geographical location, seasonal variation, plant organ, age and vegetativecycle stage, and harvesting time[35,36].
In this work, major components of essential oil ofO. majoranaare: 4-terpinene with an pourcentage of 28.96%, γ-terpinene with 18.57% and α-terpinene with 12.72%. The composition of the essential oil of that same species cultivated in Tunisia has levels of 64.01% to 71.4% in oxygenated monoterpenes, 21.73% to 29.92% in hydrocarbon monoterpenes and 1.47% to 4.05% in sesquiterpene hydrocarbons[31]. According to work by Banchioet al.[37], the major components ofO. majoranahave been terpinen-4-ol (55.09%), cis-sabinene hydrate (8.37%), α-terpineol (9.09%) and trans-sabinene hydrate (13.20%).
The very important larvicidal activity observed in the essential oil ofO. majoranacould be explained by the chemical composition of this oil and the action or effect of compound majority. Indeed, it was recently reported by some authors thatOrigunumspecies have an insecticidal activity against insects[24,33,38].
Thus, essential oils of theOriganum vulgare,an Eurasian plants species, have been also found to have a greater larvicidal activity against mosquitoCulexsp. The percentage mortality of speciesOriganumvulgarehas been found in the order of (88.6±7.2)%[33].
Similar studies performed by Traboulsiet al. have shown the insecticidal activity of four plants including genusOriganumagainst larvae ofCx. pipiens. LC50obtained were between 16 and 89 mg/L[39]. Phenolic compounds such as carvacrol (61%) and thymol (21.8%) were quantitatively the most important in the essence ofOriganum syriacum. The evaluation of larvicidal activity of these compounds, in the same conditions, demonstrated that thymol (LC50=36 mg/ L) and carvacrol (LC50=37.6 mg/L) were responsible for this activity[39].
Taking into account the absence of studies on the essential oils ofO. majoranaagainst specifically the speciesCx. pipiens, we tried to compare the action of a plant of the species ofOriganumagainstCulex. Thus, the LC50and LC90obtained from the plantOriganumvulgareEuro-Asian species against the mosquitoCulexsp. were respectively 256 and >500 mg/L[33]. These results are close to those found in our study.
This study has shown the larvicidal action of the essential oil ofO. majoranaagainstCx. pipiens. This essential oil can be an effective alternative in the fight against mosquito vectors of disease.
In our study, realized for the first time in Morocco, we evaluated the chemical composition of essential oil ofO. majoranaby GC-MS, which allowed us to identify 24 compounds. The major compounds are: 4-terpinene (28.96%), γ-terpinene (18.57%), α-terpinene (12.72%) and sabinene (8.02%). This oil showed larvicidal property against larvae ofCx. pipiens. The oil also showed an interesting larvicidal property against larvae ofCx. pipienswith LC50value of 258.71 mg/L LC90value of 580.49 mg/L.
We plan to continue this study to clarify the nature of the compounds responsible for the activity by fractionation carried out in parallel with biological tests and study evaluating the larvicidal activity against other mosquito larvae and other plants including harvested aqueous extracts.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We thank everyone who contributed to this work.
[1] Krida G, Diancourt L, Bouattour A, Rhim A, Chermiti B, Failloux AB. [Assessment of the risk of introduction to Tunisia of the Rift Valley fever virus by the mosquito Culex pipiens]. Bull Soc Pathol Exot 2011; 104: 250-259. French.
[2] Barzon L, Pacenti M, Franchin E, Squarzon L, Lavezzo E, Cattai M, et al. The complex epidemiological scenario of West Nile virus in Italy. Int J Environ Res Public Health 2013; 10: 4669-4689.
[3] Ziegler U, Skrypnyk A, Keller M, Staubach C, Bezymennyi M, Damiani AM, et al. West Nile virus antibody prevalence in horses of Ukraine. Viruses 2013; 5: 2469-2482.
[4] Figuerola J, Baouab RE, Soriguer R, Fassi-Fihri O, Llorente F, Jimenez-Clavero MA. West Nile virus antibodies in wild birds, Morocco, 2008. Emerg Infect Dis 2009; 15: 1651-1653.
[5] Fassil H, El Harrak M, Marie JL. Epidemiological aspects of West Nile virus infection in Morocco. Med Sante Trop 2012; 22: 123-125.
[6] Changbunjong T, Weluwanarak T, Taowan N, Suksai P, Chamsai T, Sedwisai P. Seasonal abundance and potential of Japanese encephalitis virus infection in mosquitoes at the nesting colony of ardeid birds, Thailand. Asian Pac J Trop Biomed 2013; 3(3): 207-210.
[7] Calistri P, Giovannini A, Hubalek Z, Ionescu A, Monaco F, Savini G, et al. Epidemiology of West Nile in Europe and in the Mediterranean Basin. Open Virol J 2010; 4: 29-37.
[8] Haas H, Tran A. Mosquito allergy. Arch Pediatr 2014; 21: 913-917.
[9] Berchi S, Aouati A, Louad K. Typology of favourable biotopes to the larval development of Culex pipiens L. 1758 (Diptera-Culicidae), source of nuisance at Constantine (Algeria). Ecologia Méditeranea 2012; 38: 5-16.
[10] Adeleke MA, Adebimpe WO, Hassan AO, Oladejo SO, Olaoye I, Olatunde GO, et al. Larval habitats of mosquito fauna in Osogbo metropolis, Southwestern Nigeria. Asian Pac J Trop Biomed 2013;3(9): 673-677.
[11] El Ouali Lalami A, Hindi T, Azzouzi A, Elghadraoui L, Maniar S, Faraj C, et al. [Inventory and seasonal distribution of Culicidae in the center of Morocco]. Faunistic Entomol 2010; 62: 131-138. French.
[12] El Ouali Lalami A, Cherigui M, Koraichi SI, Maniar S, El Maimouni N, Rhajaoui M. Imported malaria in northern central Morocco, 1997-2007. Sante 2009; 19: 43-47.
[13] Wang X, Li JL, Xing HJ, Xu SW. Review of toxicology of atrazine and chlorpyrifos on fish. J Northeast Agric Univ 2011; 18(4): 88-92.
[14] Chen W, Jing M, Bu J, Ellis Burnet J, Qi S, Song Q, et al. Organochlorine pesticides in the surface water and sediments from the Peacock River Drainage Basin in Xinjiang, China: a study of an arid zone in Central Asia. Environ Monit Assess 2011; 177: 1-21.
[15] Mohammed MP, Penmethsa KK. Assessment of pesticide residues in surface waters of Godavari delta, India. J Mater Environ Sci 2014; 5(1): 33-36.
[16] Brown AW. Insecticide resistance in mosquitoes: a pragmatic review. J Am Mosq Control Assoc 1986; 2: 123-140.
[17] Djogbénou L. Vector control methods against malaria and vector resistance to insecticides in Africa. Med Trop (Mars) 2009; 69: 160-164.
[18] Akiner MM, Simsek FM, Caglar SS. Insecticide resistance of Culex pipiens (Diptera: Culicidae) in Turkey. J Pestic Sci 2009; 34(4): 259-264.
[19] El Ouali Lalami A, El-Akhal F, El Amri N, Maniar S, Faraj C. State resistance of the mosquito Culex pipiens towards temephos central Morocco. Bull Soc Pathol Exot 2014; 107: 194-198.
[20] El Ouali Lalami A, El-akhal F, Oudrhiri W, Ouazzani CF, Guemmouh R, Grech H. [Thymus essential oils (Thymus vulagris and Thymus satureioidis) from center of Morocco: chemical composition and antimicrobial activity]. Les Technologies de Laboratoire 2013; 8: 31. French.
[21] Aouinty B, Oufara S, Mellouki F, Mahari S. Preliminary evaluation of larvicidal activity of aqueous extracts from leaves of Ricinus communis L. and from wood of Tetraclinis articulata (Vahl) Mast. on the larvae of four mosquito species: Culex pipiens (Linne), Aedes caspius (Pallas), Culiseta longiareolata (Aitken) and Anopheles maculipennis (Meigen). Biotechnol Agron Soc Environ 2006; 10: 67-71.
[22] El idrissi M, Elhourri M, Amechrouq A, Boughdad A. Study of the insecticidal activity of the essential oil of Dysphania ambrosioides L. (Chenopodiaceae) on Sitophilus oryzae (Coleoptera: Curculionidae). J Mater Environ Sci 2014; 5(4): 989-994.
[23] Loizzo MR, Menichini F, Conforti F, Tundis R, Bonesi M, Saab AM, et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem 2009; 117: 174-180.
[24] Azizi A, Yan F, Honermeier B. Herbage yield, essential oil content and composition of three oregano (Origanum vulgare L.) populations as affected by soil moisture regimes and nitrogen supply. Ind Crops Prod 2009; 29: 554-561.
[25] Perez Gutierrez RM. Inhibition of advanced glycation endproduct formation by Origanum majorana L. in vitro and in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med 2012; doi: 10.1155/2012/598638.
[26] Adams RP. Identification of essential oils components by gas chromatography quadrupole massspectroscopy. Carol stream: Allured Publishing Corporation; 2001, p. 455.
[27] Joulain D, König WA. The atlas of spectral data of spectral data of sesquiterpene hydrocabones. Hambourg: E .B. Verlag; 1998, p. 405.
[28] Brunhes J, Rhaim A, Geoffroy B, Angel G, Hervy JP. Les moustiques de l’Afrique Méditerranéenne: Logiciel d’identification et d’enseignement [CD-ROM]. Montpellier: IRD & IPT; 2000.
[29] Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol 1925; 18: 265-267.
[30] Giner M, Vassal M, Vassal C, Chiroleu F, Kouaik Z. WinDL Software version 2.0, CIRAD-CA. URBI/MABIS, Montpellier. 1999.
[31] Sellami IH, Maamouri E, Chahed T, Wannes WA, Kchouk ME, Marzouk B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind Crops Prod 2009; 30: 395-402.
[32] Pimple BP, Patel AN, Kadam PV, Patil MJ. Microscopic evaluation and physicochemical analysis of Origanum majorana Linn leaves. Asian Pac J of Trop Dis 2012; 2: S897-S903.
[33] Pavela R. Larvicidal effects of various Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol Res 2008; 102: 555-559.
[34] Ghosh A, Chowdhury N, Chandra G. Plant extracts as potential mosquito larvicides. Indian J Med Res 2012; 135(5): 581-598.
[35] Abu Lafi S, Odeh I, Dewik H, Qabajah M, Hanus LO, Dembitsky VM. Thymol and carvacrol production from leaves of wild Palestinian Majorana syriaca. Bioresour Technol 2008; 99: 3914-3918.
[36] Zein S, Awada S, Rachidi S, Hajj A, Krivoruschko E, Kanaan H. Chemical analysis of essential oil from Lebanese wild and cultivated Origanum syriacum l. (Lamiaceae) before and after flowering. J Med Plants Res 2011; 5: 379-387.
[37] Banchio E, Bogino PC, Zygadlo J, Giordano W. Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem Syst Ecol 2008; 36: 766-771.
[38] Pavela R. Insecticidal properties of several essential oils on the house fly (Musca domestica L.). Phytother Res 2008; 22: 274-278.
[39] Traboulsi AF, Taoubi K, El-Haj S, Bessiere JM, Rammal S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicideae). Pest Manage Sci 2002; 58: 491-495.
10.12980/APJTB.4.2014APJTB-2014-0392
*Corresponding author: El Ouali Lalami Abdelhakim, Regional Diagnostic Laboratory Epidemiological and Environmental Hygiene, Regional Health Directorate, EL Ghassani Hospital, Fez, Morocco.
Tel: +212 661937474
E-mail: eloualilalami@yahoo.fr
Methods:The analysis and the identification of the various constituents of essential oil were carried out by gas chromatography coupled with mass spectrometry. Biological test was performed according to a standard methodology inspired by the World Health Organization protocol with slight modification.
Results:This oil mainly consisted of monoterpene and sesquiterpenes. The majority compounds are 4-terpinene (28.96%), γ-terpinene (18.57%), α-terpinene (12.72%) and sabinene (8.02%). The lethal concentrations (LC50and LC90) measured for the essential oil Origanum majorana, were respectively of the order of 258.71 mg/L and 580.49 mg/L.
Conclusions:The results could be useful in search for newer, safer, and more effective natural larvicidal agents.
 Asian Pacific Journal of Tropical Biomedicine2014年9期
Asian Pacific Journal of Tropical Biomedicine2014年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A case report of cutaneous larva migrans in a Mexican population of high marginalization
- Acute brucellosis as unusual cause of immune thrombocytopenia: a case report and review of the literature
- Hematological and serum biochemical aspects associated with a camel (Camelus dromedarius) naturally infected by Trypanosoma evansi with severe parasitemia in Semnan, Iran
- Phytochemical screening and antioxidant activity of ethanol extract of Tithonia diversifolia (Hemsl) A. Gray dry flowers
- Rate of carcass and offal condemnation in animals slaughtered at Yazd Slaughterhouse, central Iran
- Formulation and evaluation of novel stomach specific floating microspheres bearing famotidine for treatment of gastric ulcer and their radiographic study
