Risk assessment of ischemic stroke associated pneumonia
Department of Neurology, Wuhan Central Hospital, Wuhan 430014, China
Corresponding Author:Jun-min Hu, Email: hjm-69@163.com
Risk assessment of ischemic stroke associated pneumonia
Lin Li, Lin-hong Zhang, Wu-ping Xu, Jun-min Hu
Department of Neurology, Wuhan Central Hospital, Wuhan 430014, China
Corresponding Author:Jun-min Hu, Email: hjm-69@163.com
BACKGROUND:Cerebral stroke is a disease with a high disability rate and a high fatality rate. This study was undertaken to assess the risk of stroke associated pneumonia (SAP) in patients with ischemic stroke using A2DS2 score.
METHODS:Altogether 1 279 patients with ischemic stroke who were treated in our department from 2009 to 2011 were retrospectively analyzed with A2DS2 score. A2DS2 score was calculated as follows: age ≥75 years=1, atrial fi brillation=1, dysphagia=2, male sex=1; stroke severity: NIHSS score 0–4=0, 5–15=3, ≥16=5. The patients were divided into three groups according to A2DS2 score: 620 in score 0 group, 383 in score 1–9 group, and 276 in score ≥10 group. The three groups were comparatively analyzed. The diagnostic criteria for SAP were as follows: newly emerging lesions or progressively infiltrating lesions on post-stroke chest images combined with more than two of the following clinical symptoms of infection: (1) fever ≥38 °C; (2) newly occurred cough, productive cough or exacerbation of preexisting respiratory tract symptoms with or without chest pain; (3) signs of pulmonary consolidation and/or wet rales; (4) peripheral white blood cell count ≥10X109/L or ≤4X109/L with or without nuclear shift to left, while excluding some diseases with clinical manifestations similar to pneumonia, such as tuberculosis, pulmonary tumors, non-infectious interstitial lung disease, pulmonary edema, pulmonary embolism and atelectasis. The incidence and mortality of SAP as well as the correlation with ischemic stroke site were analyzed in the three groups respectively. Mean± standard deviation was used to represent measurement data with normal distribution and Student's t test was used. The chi-square test was used to calculate the percentage for enumeration data.
RESULTS:The incidence of SAP was significantly higher in the A2DS2 score≥10 group than that in the score 1–9 and score 0 groups (71.7% vs. 22.7%, 71.7% vs. 3.7%, respectively), whereas the mortality in the score≥10 group was signi fi cantly higher than that in the score 1–9 and score 0 groups (16.7% vs. 4.96%, 16.7% vs. 0.3%, respectively). The incidences of cerebral infarction in posterior circulation and cross-MCA, ACA distribution areas were signi fi cantly higher than those in the SAP group and in the non-SAP group (35.1% vs.10.1%, 11.4% vs. 7.5%, respectively). The incidence of non-fermentative bacteria infection was signi fi cantly increased in the score≥10 group.
CONCLUSIONS:A2DS2 score provides a basis for risk strati fi cation of SAP. The prevention of SAP needs to be strengthened in acute ischemic stroke patients with a A2DS2 score≥10.
Ischemic stroke; A2DS2 scoring tool; Stroke associated pneumonia; Function of deglutition; NIHSS scoring; Location of ischemic stroke; Non-fermentative bacteria; Risk strati fi cation
INTRODUCTION
Cerebral stroke is a disease with a high disability rate and a high fatality rate. Many studies have demonstrated that complications during post-stroke hospitalization have an impact on the disability rate and fatality rate. Stroke associated pneumonia (SAP) refers to infective pulmonary parenchymal inflammation[1]in stroke patients without previous pulmonary infection, and is a principal cause for exacerbation of post-stroke condition. Therefore, early identification of patients at a high risk of SAP may help physicians to monitor the disease and prescribe proper treatment. A2DS2 scoring tool[2]isrecognized as an effective method for predicting SAP. In this study, the A2DS2 scoring tool was used to evaluate the risk and prognosis of pneumonia in cerebral stroke patients treated in our department from 2009 to 2011.
METHODS
General data
A total of 1 279 patients with SAP were treated in our department from 2009 to 2011. In these patients, ischemic stroke was confirmed in accordance with the diagnostic criteria[3]set up at the Fourth National Conference (1996) on the Diagnosis of Cerebrovascular Diseases by the Chinese Medical Association. The mean age of the patients (765 males and 514 females) was 68±12.3 years old.
Methods
The clinical data collected included prior medical history, results of routine blood test, biochemistry panel, ECG or dynamic ECG, head CT/MRI, chest X-ray photographs, pulmonary CT, and National Institute of Health Stroke Scale (NIHSS) scores at admission, swallowing function assessment, and sputum culture results. Patients' swallowing function was assessed using water swallowing test,[4]taking ≥grade 3 as existence of swallowing dysfunction. Ischemic stroke was divided into anterior circulation stroke (ACS) and posterior circulation stroke (PCS) by the imaging lesion site. ACS includes strokes in the middle cerebral artery (MCA) distribution area, anterior cerebral artery (ACA) distribution area, and cross-MCA and ACS distribution area. PCS includes stroke in the blood supply areas of the vertebrobasilar artery, such as the occipital lobe, temporal lobe, thalamus, cerebellum, and brain stem.
Diagnostic criteria for SAP
The criteria[1]2010 formulated by the Chinese Expert Consensus on Diagnosis and Treatment of Stroke-Associated Pneumonia were used, and the detailed clinical criteria for judgment included newly emerging lesions or progressively infiltrating lesions in post-stroke chest images combined with more than two of the following clinical symptoms of infection: (1) fever≥38 °C; (2) newly occurred cough, productive cough, or exacerbation of preexisting respiratory disease symptoms with or without chest pain; (3) signs of pulmonary consolidation, and/or moist rales; (4) peripheral WBC≥10X109/L or ≤4X109/L with or without nuclear shift to the left. Moreover, some diseases with similar clinical manifestations of pneumonia were excluded such as tuberculosis, pulmonary tumor, non-infective interstitial lung disease, pulmonary edema, pulmonary embolism, and pulmonary atelectasis.
Patient grouping by the A2DS2 scoring tool
A2DS2 scoring tool: age≥75 years=1, atrial fi brillation=1, dysphagia=2, male sex=1; stroke severity: NIHSS score 0–4=0, 5–15=3, and ≥16=5. Patients' conditions at admission were scored routinely by the A2DS2 scoring tool. The incidence and mortality of SAP were analyzed in the three groups of patients with scores 0, 1–9, or ≥10. Analysis was made of the location of ischemic stroke and the incidence of SAP.
Statistical analysis
Statistical analysis was performed with the SPSS 12.0 statistical software. Mean±SD was used to represent measurement data with normal distribution and Student's t test was also used. The Chi-square test was used to calculate the percentage for enumeration data.
RESULTS
There were 620 patients in A2DS2 scores 0 group, 383 patients in scores 1–9 group, and 276 patients in scores ≥10 group (Table 1). The incidence of SAP, average length of hospital stay, and mortality were compared in the three groups (Table 2).

Table 2. The incidence of SAP, average length of hospital stay and mortality in the three groups
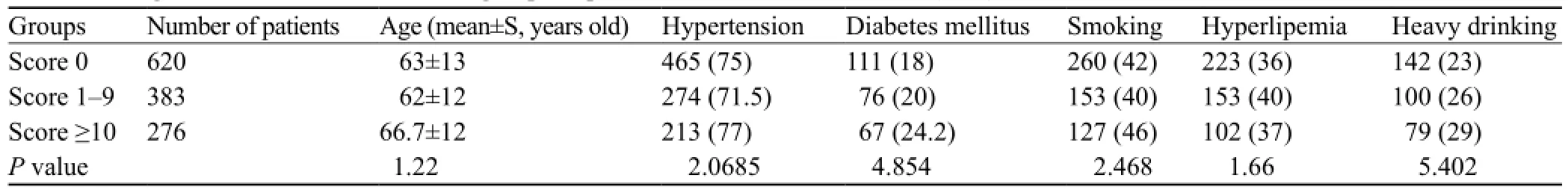
Table 1. The general conditions of the three groups of patients with cerebral infarction (n, %)

Table 3. The distribution area of cerebral infarction in the SAP and non-SAP groups (n,%)
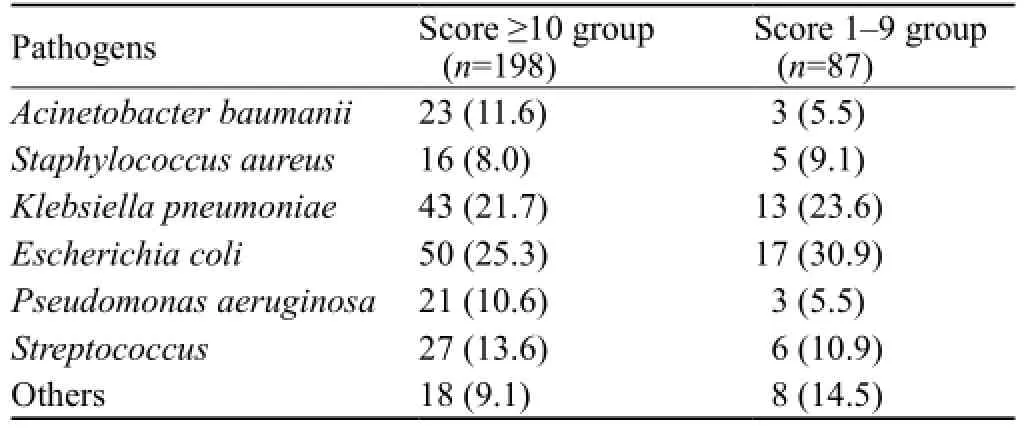
Table 4. The distribution of pathogens in the score≥10 group and score 1–9 group (n,%)
The infarction location of 276 patients in the A2DS2 score ≥10 group was as follows: MCA distribution area: 86 patients (31.2%); ACA distribution area: 49 patients (17.8%); PCA distribution area: 96 patients (34.8%), including 32 patients (11.6%) with large-area cerebral infarction in the cross-MCA and ACS distribution areas. Thirteen patients (4.7%) had both anterior and posterior circulation cerebral infarction. The infarction location of 383 patients in the score 1–9 group are as follows: MCA distribution area: 183 patients (47.8%); ACA distribution area: 141 patients (36.8%); PCA distribution area: 44 patients (11.5%), including 11 patients (2.9%) with large-area cerebral infarction in the cross-MCA and ACS distribution areas. Four patients (1%) had both anterior and posterior circulation cerebral infarction (Table 3).
198/198 patients in the A2DS2 score ≥10 group had a positive sputum culture, and 55/87 SAP patients in the score 1–9 group had a positive sputum culture (Table 4).
DISCUSSION
SAP is a potential, preventable stroke complication, and is associated with poor prognosis. It occurs mostly at acute and critical stage of stroke. Cerebral stroke and concurrent pneumonia may increase the fatality rate of patients in 30 days by 4 folds, extend the length of hospital stay by 3 folds, and result in a sharp increase of medical expenses.[5–7]The clinical features of SAP are: (1) diverse clinical manifestations; (2) diverse pathogens; (3) atypical clinical manifestations; (4) easy relapse of conditions; and (5) rapid change of conditions, readily complicated with pulmonary edema. The pathogenesis of SAP comprise immune dysfunction, dysphagia, or neurogenic pulmonary edema.[8]
Although there have been many studies investigating the risk factors of SAP at home and abroad, there are few speci fi c and effective scoring tools. Therefore, there is lacking risk stratification and precise therapeutic regimen of SAP. Sarah et al[2]carried out an assessment and analysis on multiple risk factors of SAP in 15 335 patients with acute ischemic stroke, and established the A2DS2 scoring tool. They found that the tool had excellent resolving power for prediction of SAP, and may be used in identification of patients at high risk and to guide preventive and management measures for pneumonia. In this study, 1 279 patients with acute ischemic stroke were investigated. SAP occurred in 308 patients with an incidence of 24.1%. They were evaluated using the A2DS2 tool. The incidence of SAP was 3.7% in patients with score 0, 22.7% in patients with scores 1–9, and up to 71.7% in patients with scores≥10. There was significant difference in incidences of SAP between the three groups (P<0.005). As the patients in the score ≥10 group often had disturbance of consciousness, bulbar paralysis and dysphagia because of older age and poor cardiac function, they were easy to experience silent aspiration, difficulty in drainage of endotracheal secretions, long bed rest time, requiring indwelling of nasal feeding tube, high frequency of proton pump inhibitor use, traumatic endotracheal intubation, and high ratio of mechanical ventilation. Moreover, stroke-induced immune mechanism abnormality was an important factor of SAP.[9]After stroke, IL-1β, TNF-α, and IL-6 secreted in cells, induced by cerebral injury, stimulated paraventricular nucleus of hypothalamus to release corticotrophin releasing factor (CRF). CRF bound to G-protein coupled receptors in prepituitary gland, to promote prepituitary gland to generate and secrete ACTH, leading to increased secretion of glucocorticoid. Glucocorticoid could inhibit production of proinflammatory mediators, and also promote release of antiinflammatory mediators.[10]Moreover, these patients had more signi fi cant neurogenicpulmonary edema.[11]Persistent and intense excitation of the sympathetic nerve resulted in high-pressure and highpermeability pulmonary edema, leading to refractory hypoxemia, and decreased local immunity and clearing ability of the respiratory tract. So, A2DS2 score provides a basis for risk stratification and treatment regimen of post-stroke pneumonia.
The infection rate of non-fermenter bacteria (such as Pseudomonas aeruginosa and Acinetobacter baumanii) was significantly increased in the A2DS2 score ≥10 group. Gram-negative non-fermentative bacilli are of low sensitivity to clinical common antibiotics, and are often resistant to many antibiotics. They have become the main pathogens in the intensive care unit of neurology, and patients infected by such strains are mostly of poor prognosis.[12]In the treatment of gram-negative nonfermentative bacilli, antimicrobial drugs should be used rationally according to the drug susceptibility results. The targeted gene transfer vector system can deliver a gene to specific target cells. Now, it is also a clinical research hotspot[13]that, in treatment of severe infection, an appropriate targeted gene transfer vector may be selected against invasive bacteria or viruses, and a correct gene is used to act on them to achieve respective therapeutic effects.
Meanwhile, through the correlation research on A2DS2 score and patient's infarction locations, we found that compared with patients with score <10, the incidences of cerebral infarction in posterior circulation and cross-MCA and ACA distribution areas, and cross-anterior and posterior circulation strokes were significantly higher in the SAP group compared with those in the score <10 group (P<0.005). Since neurogenic pulmonary edema may occur readily during infarction in those locations, the results were similar to those in the study by Walter.[14]
In conclusion, as stroke patients had factors of conscious disturbance, low responsiveness, difficulty in expectoration, and difficulty in finding focuses through bedside chest X-ray radiographs, etc, it is difficult to diagnose SAP in the early stage, and therefore it requires risk stratification for early prediction. A2DS2 score provides a basis for risk strati fi cation. However, we found that, some other risk factors (such as infarction locations) have non-ignorable effects on occurrence of SAP, and therefore the scoring tool may be further improved in future. Meanwhile, early clues may also be provided by early blood-related indexes of infection, such as CRP, procalcitonin, soluble triggering receptor-1 expressed on myeloid cells, and vasoactive propeptide, etc.[15]The prevention of SAP needs to be strengthened in acute ischemic stroke patients having a A2DS2 score ≥10. It may also effectively prevent and reduce occurrence and severity of SAP by the following measures: early intensive care, such as nasogastric feeding, and management of swallowing function; respective diet regimen selected according to the severity of dysphagia; enteral nutrition implemented rationally;[16]strengthened management of respiratory tract; bacterial culture carried out frequently for targeted use of antibiotics; and strengthened nutritional support symptomatic treatment, etc.
Funding:None.
Ethical approval:This study was approved by the Ethical Committee of Wuhan Central Hospital, Wuhan, China.
Con fl icts of interest:We have no con fl icts of interest to the report.
Contributors:Li L proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
1 Chinese Expert Consensus Group on Diagnosis and Treatment of Stroke-associated Pneumonia. Chinese expert consensus on diagnosis and treatment of stroke-associated pneumonia. Chin J Inter Med 2010; 49: 1075–1077.
2 Hoffmann S, Malzahn U, Harms H, Koennecke HC, Berger K, Kalic M, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke 2012; 43: 2617–2623.
3 Chinese Society of Neurology. Classification and diagnosis of cerebrovascular diseases. Chin J Neurosurgery 1997; 13: 2–5.
4 Tan C, Liu Y, Li W, Liu J, Chen L. Transcutaneous neuromuscular electrical stimulation can improve swallowing function in patients with dysphagia caused by non-stroke diseases: a meta-analysis. J Oral Rehabil 2013; 40: 472–480.
5 Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol 2008; 7: 341–353.
6 Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology 2003; 60: 620–625.
7 Katzan IL, Dawson NV, Thomas CL, Votruba ME, Cebul RD. The cost of pneumonia after acute stroke. Neurology 2007; 68: 1938–1943.
8 Guo W, Zhang J. Focusing on stroke-associated pneumonia. Chin J Inter Med 2011; 50: 191–192.
9 Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, et al. Stroke induced immunodepression: experimental evidence and clinical relevance. Stroke 2007; 38: 770–773.
10 Harms H, Hoffmann S, Malzahn U, Ohlraun S, Heuschmann P, Meisel A. Decision-making in the diagnosis and treatment of stroke-associated pneumonia. J Neurol Neurosurg Psychiatry2012; 83: 1225–1230.
11 Zhao H, Lin G, Shi M, Gao J, Wang Y, Wang H, et al. The mechanism of neurogenic pulmonary edema in epilepsy. J Physiol Sci 2014; 64: 65–72.
12 Liu KS, Wang YT, Lai YC, Yu SF, Huang SJ, Huang HJ, et al. Antimicrobial resistance of bacterial isolates from respiratory care wards in Taiwan: a horizontal surveillance study comparison of the characteristics of nosocomial infection and antimicrobialresistant bacteria in adult Intensive Care Units and two respiratory care facilities for mechanically ventilated patients at a tertiary care centre in Taiwan. Int J Antimicrob Agents 2011; 37: 10–15.
13 Gao J, Lu JC, Huang YF. Status quo of the researches on HSV vaccines. Zhonghua Nan Ke Xue 2009; 15: 60–64.
14 Walter U, Knoblich R, Steinhagen V, Donat M, Benecke R, Kloth A. Predictors of pneumonia in acute stroke patients admitted to a neurological intensive care unit. J Neurol 2007; 254: 1323–1329. Epub 2007 Mar 14.
15 Ye Z, Zhan H. Early diagnosis and prognosis assessment of infective diseases. Chin J Emerg Med 2013; 21: 667–669.
16 McCallum SL. National dysphagia diet: Implementation at a regional rehabilitation center and hospital system. J Am Diet Assoc 2003; 103: 381–384.
Received February 13, 2014
Accepted after revision June 16, 2014
World J Emerg Med 2014;5(3):209–213
10.5847/ wjem.j.issn.1920–8642.2014.03.009
 World journal of emergency medicine2014年3期
World journal of emergency medicine2014年3期
- World journal of emergency medicine的其它文章
- Domestic versus imported drug-eluting stents for the treatment of patients with acute coronary syndrome
- Comparison of plasma microRNA-1 and cardiac troponin T in early diagnosis of patients with acute myocardial infarction
- International normalized ratio as a predictor of mortality in trauma patients in India
- Can venous blood gas analysis be used for predicting seizure recurrence in emergency department?
- Knowledge and skills of neonatal resuscitation of health professionals at a university teaching hospital of Northwest Ethiopia
- Relationship between different surgical methods, hemorrhage position, hemorrhage volume, surgical timing, and treatment outcome of hypertensiveintracerebral hemorrhage
