Integration of QTL detection and marker assisted selection for improving resistance to Fusarium head blight and important agronomic traits in wheat
*
aJiangsu Provincial Key Laboratory of Crop Genetics and Physiology/Key Laboratory of Plant Functional Genomics of Ministry of Education, College of Agriculture,Yangzhou University,Yangzhou 225009,China
bKey Laboratory of Crop Germplasm and Biotechnology,Ministry of Agriculture/National Key Facility for Crop Gene Resources and Genetic Improvement,Institute of Crop Science,Chinese Academy of Agricultural Sciences,Beijing 100081,China
Integration of QTL detection and marker assisted selection for improving resistance to Fusarium head blight and important agronomic traits in wheat
Chao Lva,b,Yanxia Songb,Lifeng Gaob,Qin Yaob,Ronghua Zhoub,Rugen Xua,Jizeng Jiab,*
aJiangsu Provincial Key Laboratory of Crop Genetics and Physiology/Key Laboratory of Plant Functional Genomics of Ministry of Education, College of Agriculture,Yangzhou University,Yangzhou 225009,China
bKey Laboratory of Crop Germplasm and Biotechnology,Ministry of Agriculture/National Key Facility for Crop Gene Resources and Genetic Improvement,Institute of Crop Science,Chinese Academy of Agricultural Sciences,Beijing 100081,China
A R T I C L E I N F O
Article history:
Received 10 May 2013
Received in revised form
12 September 2013
Accepted 15 October 2013
Available online 5 November 2013
Triticum aestivum
Fusarium head blight(FHB),caused by Fusarium graminearum,is one of the most destructive wheat(Triticum aestivum L.)diseases worldwide.Identification of quantitative trait loci(QTL) conferring FHB resistance followed by markerassisted selection(MAS)is an efficientapproach to breed FHB-resistant varieties.In this study,38 additive QTL and 18 pairs ofepistatic QTL for FHB resistance were detected in four environments using a population of recombinant inbred lines(RILs)derived from varieties Neixiang 188 and Yanzhan 1.Six QTL clusters were located on chromosomes 2D,4B,4D,5A,5Dand 7B,suggesting possible polytrophic functions.Six elite lines with good FHB resistance and agronomic traits were selected from the same population using the associated markers.Our results suggest that MAS of multiple QTL will be effective and efficient in wheat breeding.
©2013 Production and hosting by Elsevier B.V.on behalf of Crop Science Society of China and Institute of Crop Science,CAAS.
1.Introduction
Fusarium head blight(FHB),caused by Fusarium graminearum Schwabe,is a common disease in wheat(Triticum aestivum) and barley(Hordeum vulgare),that causes yield losses and threatens human health[1–3].Due to global warming and agronomic practices,such as irrigation and retained stubble that may carry the pathogen,FHB has become more frequent and more severe in recent years.The disease has gradually extended to the northern major wheat production areas of China.[4]In the Yangtze River valley and Northeast Spring Wheat Zone,FHB regularly causes 10%–15%of yield losses, and nearly 50%in epidemic years[5].
Resistant varieties play an important role in controlling FHB. However,there are relatively few resistance genes used in wheat breeding in China.FHB resistance is a quantitative trait controlled by major and minor genes[3,6–10]located on all wheat chromosomes,except 7D[9].Chinese variety Sumai 3,which carries the major resistance QTL Fhb1,is widely recognized as the best resistance source and is extensively used in wheat breeding programs worldwide[6,11–14].
Marker assisted selection(MAS)is a promising method for breeding in the genomics era.Thousands of QTL and genes conferring traits of agronomic importance have been identified in major crops,and these can be used to accelerate MAS.At present,QTL detection and functionalanalysis are separate from MAS.Many molecular markers for targeting genes/loci are not usefulduring the selectionprocess because oflow polymorphism across different genetic backgrounds and incomplete association with target traits.In this study,we attempted to select promising breeding lines with FHB resistance and good agronomic traits by combining QTL analysis and MAS.In a recombinant inbred line(RIL)population derived from cultivars Yanzhan 1(YZ1)and Neixiang 188(NX188)FHB resistance and other important agronomic traits were simultaneously selected using molecular markers,and severalelite lines were produced.
2.Materials and methods
2.1. Plant materials
One hundred and ninety nine F7:8RILs were developed by single-seed descent from the cross YZ1×NX188.YZ1 is an early maturing cultivar released in Henan Province of China, in 2000;NX188,a high yielding cultivar with wide adaptation and released in 2000,was the fourth most widely planted cultivar in China(470,000 ha)in 2004.
2.2. Evaluation of agronomic traits
The RILs and their parents were planted in Beijing and in Luoyang,Henan province,in the 2003–2004 and 2004–2005 wheat seasons.All lines were phenotyped as single relicates in four environments.Thirty seeds of each line were sown in a two-row plot of 2 m in length.Plant height(PH)was measured in the field at maturity.Spike length(SL),spikelet number per spike(SPI),spike compactness(SC,SC=SPI/SL),grain number per spike(GNS),and thousand-grain weight(TGW)were measured after harvest.
2.3. FHB evaluation
FHB responses were assayed under natural conditions in the 2005–2006 and 2006–2007 cropping seasons in Jianyang, Fujian province.Although no wheat is commercially produced in the area extremely severe FHB infections are common.Field management was the same as that for agronomic evaluations. Sumai3,Mianyang 26,and Yangmai5 were used as the resistant, susceptible,and moderately susceptible controls,respectively.
About 15 and 20 days after flowering,30 spikes of each line were randomly selected.FHB severity in each spike was classified into five grades of symptoms on spikelets and spike rachi:0 for no incidence on spikelets and spike rachis,1 for ratio of incidence on spikelets less than 1/4 and no incidence on the rachis,2 for ratio of incidence on spikelets between 1/4 and 1/2 and no incidence on the rachis,3 for ratio of incidence on spikelets between 1/2 and 3/4 and incidence on spike rachis,4 for ratio ofincidence on spikelets of more than 3/4 or dead spikelets. [15],FHB disease index(DI)ofeach line was calculated as follows: DI=(Σseverity score of an individualspike×number ofspikes)/ (the highest severity score×totalnumber of spikes).
The FHB resistance score of a genotype was graded by DI from 1 to 7[15,16],representing R,R–MR,MR,MR–MS,MS, MS–S and S,respectively.Where R=resistant,S=susceptible, and M=moderate disease.
2.4. Construction of a linkage map and QTL analyses
A total of 328 publicly available SSR and DArT markers were mapped on 25 linkage groups(http://wheat.pw.usda.gov/GG2/ index.shtml)[17]covering a total genetic distance of 3848.2 cM and providing partial linkage groups for all chromosomes.QTL for agronomic traits and FHB resistance were analyzed separately.Composite interval mapping(CIM)was performed using QTLNetwork 2.0 software[18]onthe individual line means in order to detect additive QTL,epistatic QTL, and QTL×environment interaction(QE).QTL nomenclature followed the protocols of McIntosh et al.[19],in which the research institution is abbreviated as“caas”(Chinese Academy of Agricultural Sciences).
3.Results
3.1. FHB response and agronomic traits
Consistent FHB responses of both parents and RILs were observed during the 2005–2006 and 2006–2007 cropping seasons, and the correlation coefficient was 0.56(P<0.01).NX188 had a significantly lower DIand resistance score than YZ1.FHB DIand resistance scores for the RIL population showed a continuous distribution with transgressive segregation,particularly,some lines exhibiting higher resistance than the resistant parent (Table 1).
The frequency distributions for six agronomic traits were continuous with broad variation and transgressive segregation in allenvironments(Table 1).
3.2. QTL for FHB resistance and agronomic traits
A total of 38 additive and 18 epistatic QTL for FHB and agronomic traits were detected across all environments (Table 2 and Fig.1).Variation at single loci explained 0.40%–34.96%of the phenotypic variation.These QTL were distributed on 17 wheat chromosomes except for 1A,1D,7A and 7D. Twenty QTL had negative additive values,indicating that alleles from YZ1 reduced the phenotypic effect,whereas the alleles from NX188 increased the phenotypic values.At the remaining 18 loci,alleles from NX188 had positive additive values.
Additive QTL for FHB resistance were detected on chromosomes 2D,4B,4D,5B and 5D.The contribution of single QTL ranged from 1.01%to 12.86%(Table 2 and Fig.1).QFHB.caas-5D and QFHB.caas-4D showed larger effects than others.Favorable alleles at these five additive loci were from both parents, such as QFHB.caas-4D,QFHB.caas-5B,and QFHB.caas-5D from NX188 and QFHB.caas-2D and QFHB.caas-4B from YZ1(Table 2).
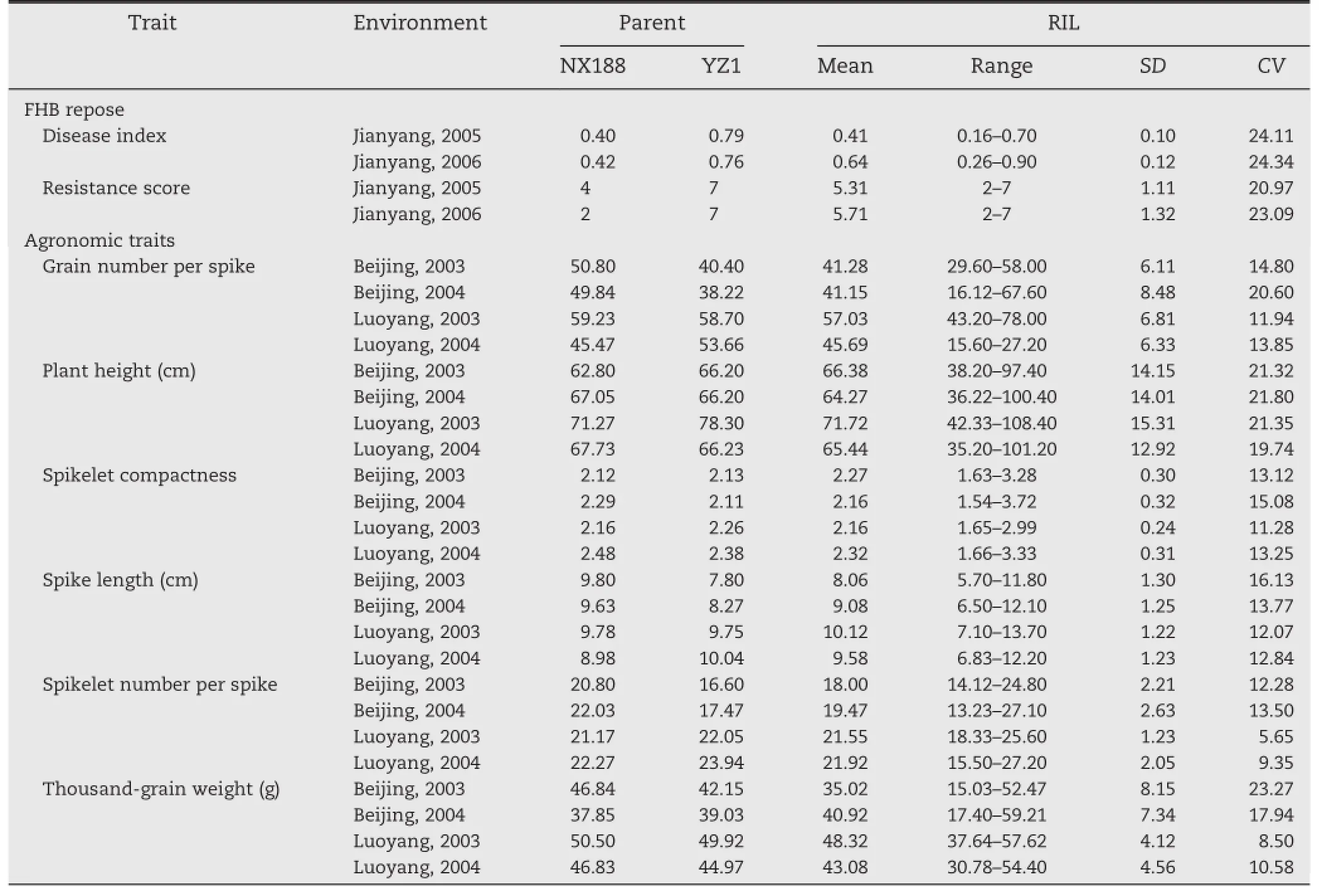
Table 1–Means,ranges,standard deviations(SD)and coefficients of variation(CV)of agronomic traits and FHB response of parents and RILs in two seasons.
Five additive QTL were detected for GNS on chromosomes 2B,4B,5A,5B and 5D,with phenotypic contributions ranging from 3.63%to 10.13%(Table 2 and Fig.1).Alleles increasing GNS from NX188 were at QGNS.caas-4B,QGNS.caas-5B and QGNS.caas-5D,and the positive alleles at other loci were from YZ1.QE interactions were detected for all five QTL and accounted for 3.57%of the phenotypic variation.One pair of additive QTL showed interaction,accounting for 6.02%of the phenotypic variation(Table 3).
Five additive QTL for PH detected in Beijing and Luoyang were located on chromosomes 2D,4B,4D,5A and 5D explained phenotypic variation of 1.51%and 34.96%,respectively(Table 2 and Fig.1).Alleles at the QPH.caas-4D and QPH.caas-5D loci reducing PH were from YZ1,and the other alleles reducing height came from NX188.QPH.caas-4B and QPH.caas-4D were located in marker intervals co-inciding with dwarfing genes Rht-B1 and Rht-D1,respectively,and QPH.caas-2D.1 was identified at the position of Rht8.The effects of QPH.caas-4B and QPH.caas-4D were much greater than that of QPH.caas-2D.This result confirmed an earlier finding that the effects of Rht-B1 and Rht-D1 were much larger than thatof Rht-8[20].QPH.caas-5A and QPH.caas-5D had minor effects on reducing PH.Four pairs of QTL showed interactions(Table 3)that explained phenotypic variation of 4.44%.
Eight additive QTL for SL were detected on chromosomes 1B, 2D,4A,5A,5D,6A and 7B,and explained 4.12%–11.97%of the phenotypic variation(Table 2 and Fig.1).Of these QSL.caas-1B and QSL.caas-2D gave the largest effects.The map position of QSL.caas-2D was similar to that of QPH.caas-2D in the Rht8 region,suggesting that Rht8 affected SL.Alleles increasing SL were from NX188,viz.QSL.caas-1B,QSL.caas-4A.1,QSL.caas-5D and QSL.caas-6A,whereas the other four were from YZ1. Interactions between three pairs of QTL accounted for 3.54%of the total phenotypic variation(Table 3).
Additive QTL for SPIwere detected on chromosomes 1B,5A, 5B and 5D,and each explained 0.40%–23.99%of the phenotypic variation(Table 2 and Fig.1).All three favorable alleles with larger effects on increasing SPIwere from NX188 and explained 53.6%the variation.QE interactions were detected for all QTL, accounting for 9.78%of the phenotypic variation.These data indicated that spikelet numbers were affected by environmentalvariation.Interaction was detected between two pairs of QTL on four chromosomes(Table 3),and together accounted for 3.43%of the phenotypic variation.
Six additive QTL for SC were detected on chromosomes 2D,4A,5A,6B and 7B,and each explained between 2.83% and 17.34%of the phenotypic variation(Table 2 and Fig.1). All except QSC.caas-4A.1 increased SC and all were derived from NX188 and contributed for 39.31%of the phenotypic variation.QE interactions were detected for four of the QTL.The latter had a very small effect(0.22%)on phenotypic variation.Interactions between four pairs of QTL were detected(Table 3),and together accounted for 6.45%of the phenotypic variation.These results showed that spikecompactness was controlled by genes with additive and epistatic effects.
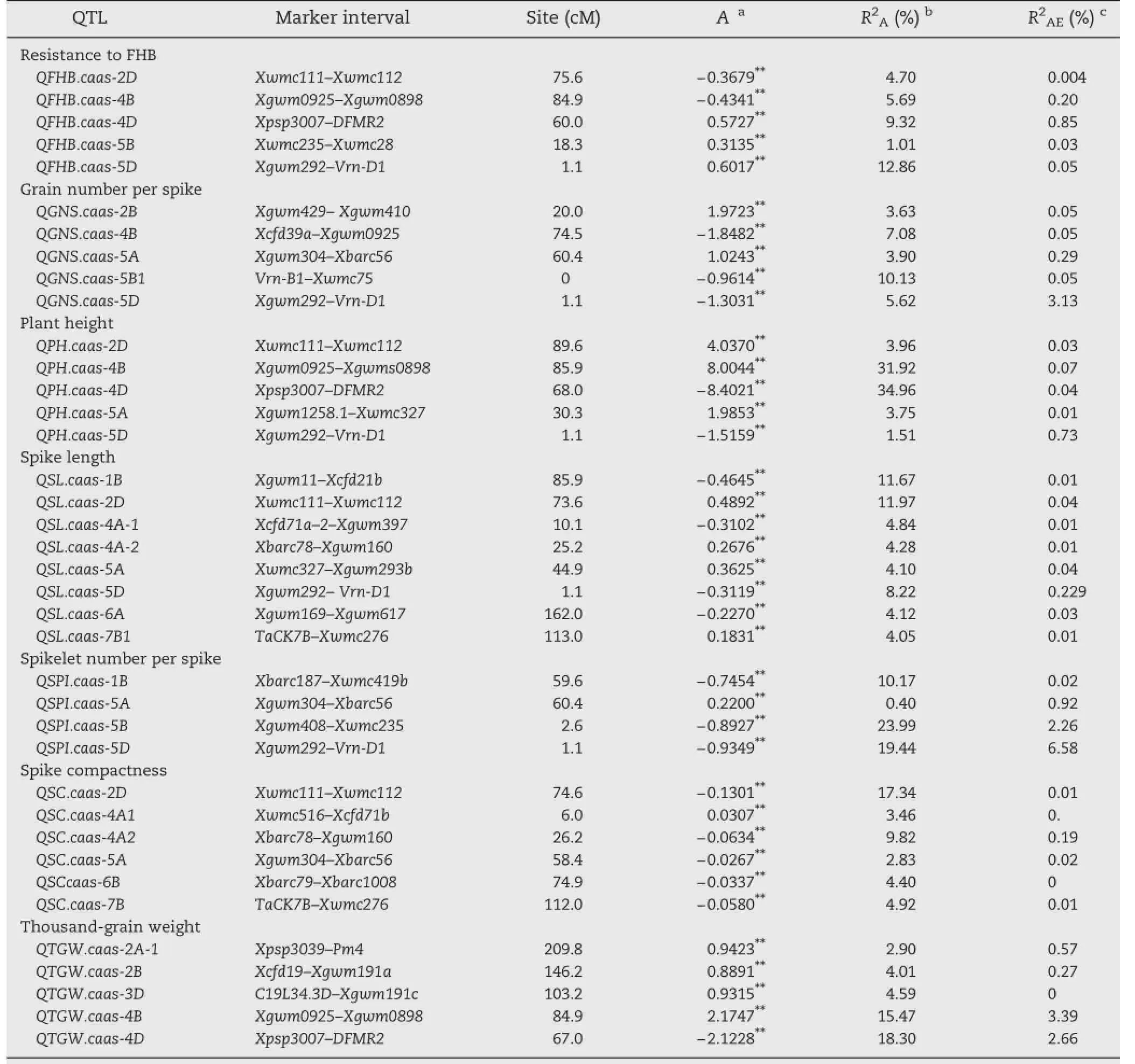
Table 2–QTL with additive effects and QE for agronomic traits and FHB resistance in two years.
Additive QTL for TGWwere detected on chromosomes 2A,2B, 3D,4Band 4D,and each one explained between 2.90%and 18.30% ofthe phenotypic variation(Table 2 and Fig.1).QTGW.caas-4B and QTGW.caas-4D,with the largest effects explained 15.47%and 18.30%of the phenotype variation,respectively.One favorable allele came from each parent.QE interactions were detected and explained 6.89%ofthe phenotypic variation in total.Interactions between three pairs of the QTL were detected(Table 3),accounting for 6.76%of the phenotypic variation.
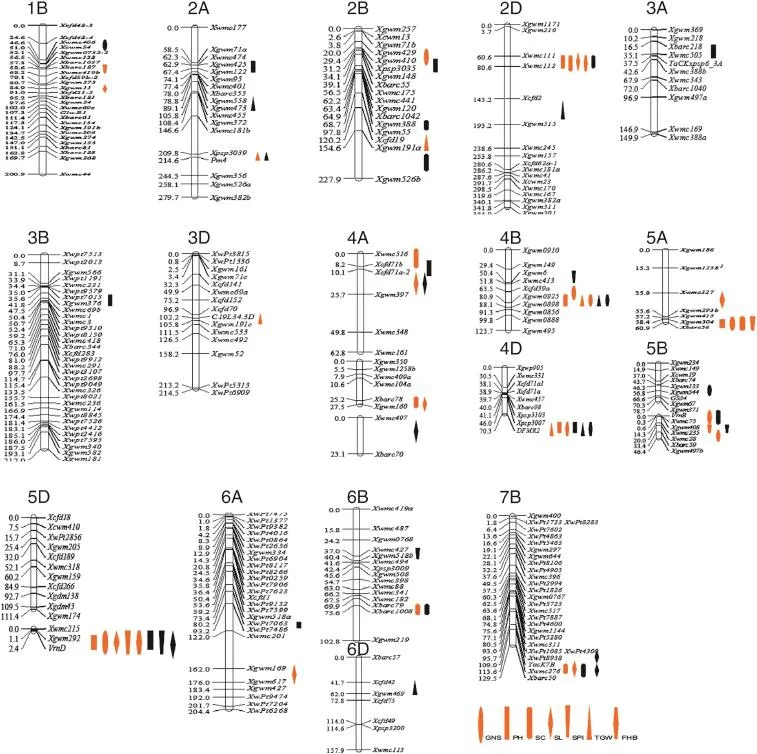
Fig.1–Positions of QTL associated with PH,SL,SPI,SC,GNS,TGWand FHB resistance in wheat.Red color indicates additive QTL and black indicates epistatic QTL.
Six gene clusters were detected for the 56 additive and epistatic QTL identified in this study,and were located on chromosomes 2D,4B,4D,5A,5B,5Dand 7B(Table 4 and Fig.1). These QTL clusters suggested polytrophic effects conferred by some loci.Four QTL(QPH.caas-2D,QSC.caas-2D,QSL.caas-2D and QFHB.caas-2D)were located in the region Xwmc111–Xwmc112 on chromosome 2D where Rht8 was located.The positive values for PHand SL and negative values for SCand FHB suggested that the allelic effects from YZ1 in this QTL cluster were for increasing PH,and SL,but decreasing SC and FHB (increasing FHB resistance)or alternately that the allele from NX188 decreased PHand SL but increased SCand FHB.Four QTL (QGNS.caas-4B,QPH.caas-4B,QTGW.caas-4B and QFHB.caas-4B) were located in the region Xgwm0925–Xgwm0898 on chromosome 4B,co-locating with dwarfing gene Rht-B1.The positive values for PH and TGW,and negative values for FHB and GNS suggested that alleles from YZ1 increased PH and TGW but reduced FHBresistance and GNS,or alternatively,the allele from NX188 with the effect of reducing PH and TGW but increasing FHB resistance and GNS.Three QTL(QPH.caas-4D,QTGW.caas-4D and QFHB.caas-4D)were mapped in the region between markers Xpsp3007 and DFMR2 on chromosome 4D,the position of dwarfing gene,Rht-D1.The allele from YZ1 for the QTL cluster reduced PH,TGWand FHB resistance or alternatively the allele from NX188 increased PH,TGWand FHB resistance.Three QTL (QGNS.caas-5A,QSC.caas-5A and QSPI.caas-5A)were in the region Xgwm304–Xbarc56 on chromosome 5A.The YZ1 allele in this QTL cluster had the effect ofincreasing GNS and SPIand reducing SC. Five QTL(QGNS.caas-5D,QPH.caas-5D,QSPI.caas-5D,QSL.caas-5D and QFHB.caas-5D)were mapped between Xgwm292 and Xgwm269 on chromosome 5D,the location of vernalization gene Vrn-D1.The NX188 allele at this locus had a large effect on simultaneously increasing FHB resistance,GNS,SL,and SPN,and with low interaction with PH.Finally,four QTL(two with additive and two epistatic effects)were mapped in the TaCK7B–Xwmc276region on chromosome 7B.TaCK7B is a cytokinin-oxidase/ dehydrogenase gene controlling cytokinin levels in plant tissues [21].
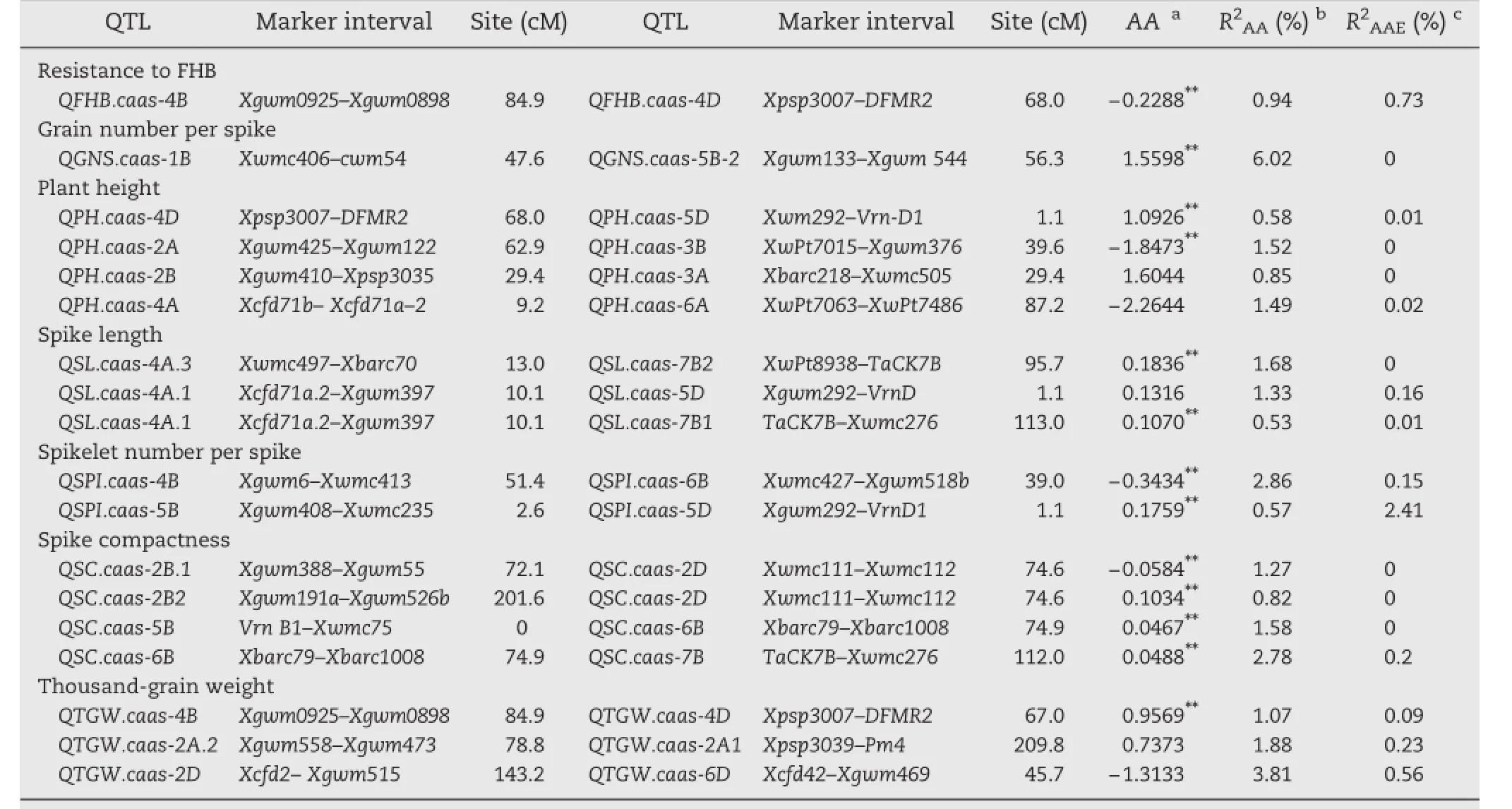
Table 3–Identified QTL with epistatic effects and QE for agronomic traits over two years.
3.3. MAS for developing elite lines with improved FHB resistance and agronomic traits RIL-68,RIL-130 and RIL-169 possessed four of the five resistance alleles).For other agronomic traits,these lines carried more favorable alleles than others.The lines should be useful as parents for conventional breeding and MAS because germplasm with both good FHB resistance and other agronomic traits is rare.
MAS was carried out to select elite lines with high FHB resistance and good agronomic traits.Among them,FHB was treated as first priority.Six elite lines were selected based on this criterion (Table 5).Allhad better agronomic traits(Table 6)than the others. No significant differences were detected between the observed and predicted values for allseven traits with SPIin the 2004–2005 cropping season(P=0.05)as the only exception.These results indicated a high efficiency of MAS in this study(Table 5).For example,for FHB resistance,RIL-151 and RIL-164 carried all five resistance alleles,and showed the best FHB resistance.RIL-31,
4.Discussion
4.1. Analysis of QTL for FHB resistance
Numerous sources of FHB resistance that have been genetically mapped to chromosomes are from many countries in Asia,North America,South America,and Europe[9].In this study,we identified five additive QTL associated with FHB resistance on chromosomes 2D,4B,4D,5B and 5D.Among them,QFHB.caas-4D and QFHB.caas-5D showed larger effectsTthan other QTL,explaining 7.01%and 12.87%of the phenotypic variation,respectively.Korean cultivar,Chokwang,was reported to carry Qfhs.ksu-5DL.1 for type IIFHBresistance[22].Aminor QTL (R2=4%)on chromosome 5DL was reported in a RIL population derived from a cross between European winter wheat cultivars Renan and Recital[23].While SSR marker Xgwm292 was closely linked to QFHB.caas-5D in this study,the same QTL for type II resistance was detected in a Wangshuibai/Wheaton RIL population[24].This indicated that QFHB.caas-5D conferred type II FHB resistance.In a similar region to QFHB-caas-4D,another QTL conferred Type I resistance using a different population [25,26].Thus,QFHB-caas-4D identified in this study was probably associated with Type Iresistance.In addition,QFHB-caas-4B was in the same region to that reported by Buerstmayr et al.[10].It therefore should be a reliable locus for FHB resistance.
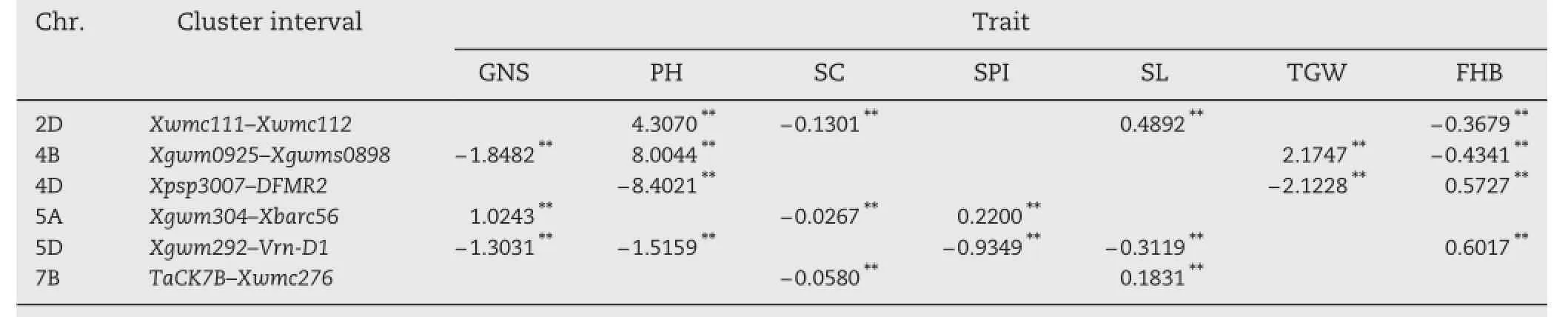
Table 4–Additive QTL clusters and their effects on FHB and agronomic traits.
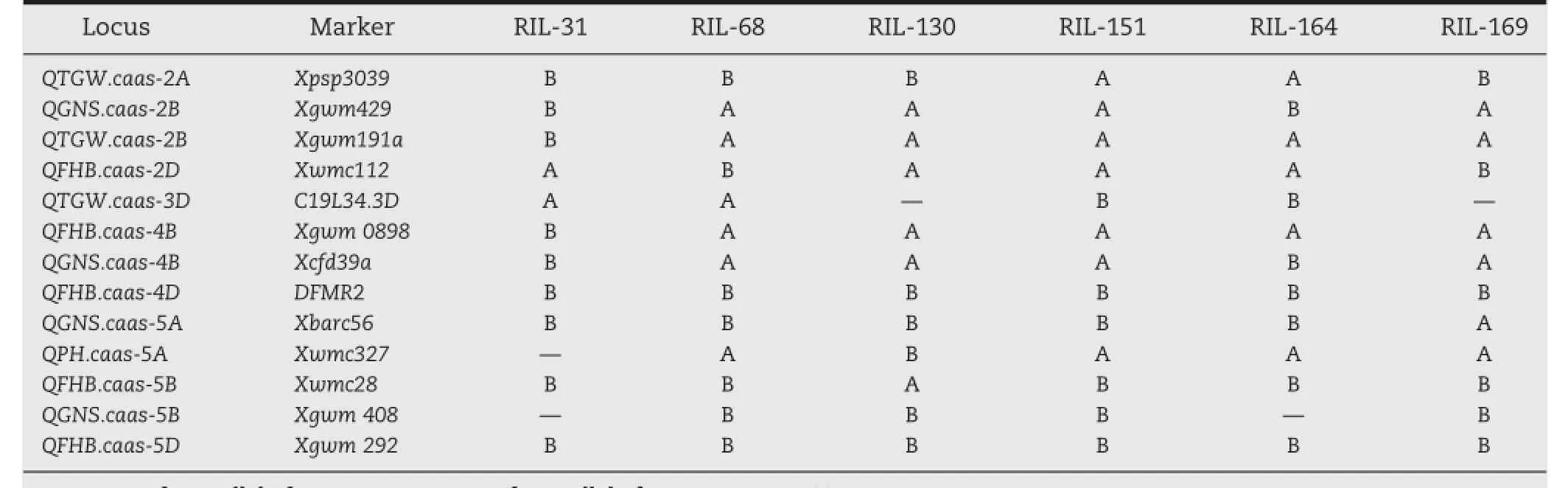
able 5–Genotypes of six selected RILs.
4.2. Correlation between FHB resistance and agronomic traits
Mechanisms of FHB resistance in wheat can be addressed from the viewpoint of morphology,physiology and biochemistry. Negative correlations between visual FHB symptoms and some agronomic traits such as plant height have been reported[2,9]. Co-localizations were also found between FHB resistance and QTL for plant height and spike architecture in barley[27].In this study,the locations of QPH.caas-2D,QPH.caas-4B and QPH.caas-4D were the same as QFHB.caas-2D,QFHB.caas-4B and QFHB.caas-4D, respectively.QFHB.caas-4D was located in the interval Xpsp3007–DFMR2,and QFHB.caas-2D was located between Xwmc11 and Xwmc112.Wheat dwarfing genes Rht-B1 and Rht8 are located on chromosomes 4D and 2D,respectively.DFMR2 was used for detecting Rht-B1 allelic variation[28].Compared with the high density wheat integration map[29],Xwmc112 was very close to Xgwm261 which is closely linked to Rht8.Since plant height was reduced,the probability of soil surface spore infection was increased,and the high humidity environment was conducive to FHB disease development.In the same or a similar interval between Xgwm292 and Vrn-D1,there were five additive QTL conferring different traits,including QFHB-caas-5D(Fig.1).These co-localizations showed that linkages may exist between genes for FHB resistance and agronomic traits that are independent of pleiotropic effects.
4.3. Simultaneous QTL detection and MAS
Gene/QTL detection and MAS are often carried out separately. Although hundreds ofgenes/QTL have been detected,progress inutilization MAS has been slow.The main reason for this was that markers detected in one population are often not applicable to other populations.In the present study,we combined gene/QTL detection and MAS using a RIL population.The advantages ofthis approach are as follows:(1)all the markers detected are efficient for MAS,and do not need to be validated again;(2)Gene/QTL detection and MAS are carried out simultaneously,shortening the time of MAS;(3)the genotypes of all selected new varieties/ elite lines are known,a feature that will be helpful in further genetic improvement.For example,of the five QTL for FHB resistance,there are four favorable alleles for FHB resistance in RIL-169,and only favorable allele QFHB.caas-2D was absent.To further improve its FHB resistance,RIL-169 and RIL-151 can be crossed in order to add QFHB.caas-2D in a genetic background that is largely shared with RIL-169(Table 5).New varieties with better FHB resistance and agronomic traits than RIL-169 will be easily bred.To carry out QTL detection and MAS simultaneously,the precondition is to construct a segregating population with both target traits and a better background of traits of agronomic importance.In the present study,six elite lines were selected from a cross of welladapted varieties.In conclusion,the results from this study suggest that QTL detection and MAS can be integrated using appropriate populations.This approach will significantly accelerate MAS in the future.
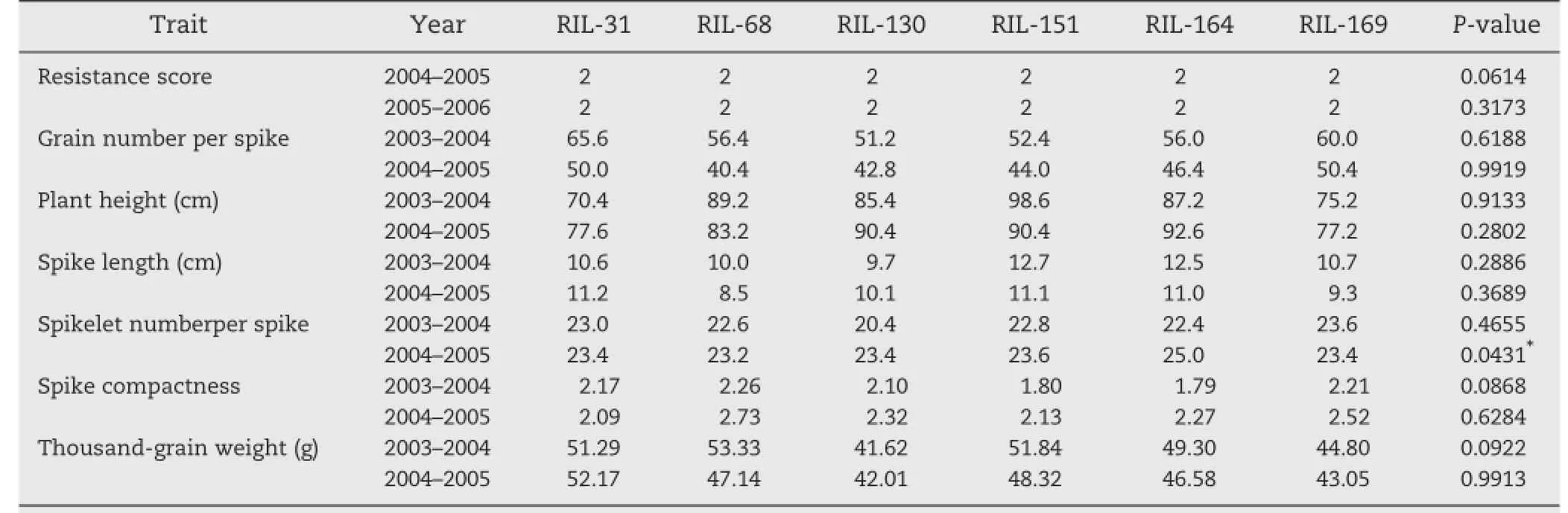
Table 6–Agronomic traits of six YZ1/NX188 RILs with superior resistance to FHB in RILs.
Acknowledgments
This study was supported by the National R&D Project of Transgenic Crops of the Ministry of Science and Technology of China and the Priority Academic Program Development of Jiangsu Higher Education Institutions.We thank Dr.Chunji Liu,CSIRO Plant Industry,Queensland,Australia,Dr.Yunbi Xu,Institute of Crop Science,Chinese Academy of Agricultural Sciences,for English improvement.
R E F E R E N C E S
[1]H.W.Schroeder,J.J.Christensen,Factors affecting resistance of wheat to scab caused by Gibberella zeae(Schw.), Phytopathology 53(1963)831–838.
[2]A.Mesterhazy,Types and components of resistance to Fusarium head blight of wheat,Plant Breed.114(1995) 377–386.
[3]G.H.Bai,G.Shaner,Managementand resistance in wheatand barley to Fusarium head blight,Annu.Rev.Phytopathol.42 (2004)135–161.
[4]P.Chen,D.Liu,W.Sun,New countermeasures of breeding wheat for scab resistance,in:H.J.Dubin,L.Gilchrist,J.Reeves, A.McNab(Eds.),Fusarium Head Scab:Global Status and Future Prospects,CIMMYT,Mexico,1997,pp.59–65.
[5]J.B.Yao,W.Z.Lu,Research advances in wheat breeding for scab resistance in China,J.Agric.Sci.16(2000) 242–248(in Chinese with English abstract).
[6]G.H.Bai,F.L.Kolb,G.E.Shaner,L.L.Domier,Amplified fragment length polymorphism markers linked to a major quantitative trait locus controlling FHB resistance in wheat, Phytopathology 89(1999)343–348.
[7]G.H.Bai,G.Shaner,H.Ohm,Inheritance of resistance to Fusarium graminearum in wheat,Theor.Appl.Genet.100 (2000)1–8.
[8]H.X.Ma,G.H.Bai,X.Zhang,W.Z.Lu,Main effects,epistasis, and environmental interactions of quantitative trait loci for Fusarium head blight resistance in a recombinant inbred population,Phytopathology 96(2006)534–541.
[9]H.Buerstmayr,T.Ban,J.A.Anderson,QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat:a review,Plant Breed.128(2009)1–26.
[10]M.Buerstmayr,K.Huber,J.Heckmann,B.Steiner,J.C.Nelson, H.Buerstmayr,Mapping of QTL for Fusarium head blight resistance and morphological and developmental traits in three backcross populations derived from Triticum dicoccum×Triticum durum,Theor.Appl.Genet.125(2012) 1751–1765.
[11]B.L.Waldron,B.Moreno-Sevilla,J.A.Anderson,R.W.Stack, R.C.Frohberg,RFLP mapping of QTL for Fusarium head blight resistance in wheat,Crop Sci.39(1999)805–811.
[12]J.A.Anderson,R.W.Stack,S.Liu,B.L.Waldron,A.D.Fjeld,C. Coyne,B.Moreno-Sevilla,J.M.Fetch,Q.J.Song,P.B.Cregan, DNA markers for Fusarium head blight resistance QTLs in two wheat populations,Theor.Appl.Genet.102(2001) 1164–1168.
[13]J.A.Anderson,S.M.Chao,S.X.Liu,Molecular breeding using a major QTL for Fusarium head blight resistance in wheat,Crop Sci.47(2007)S112–S119.
[14]W.C.Zhou,F.L.Kolb,G.Bai,G.Shaner,L.L.Domier,Genetic analysis of scab resistance QTL in wheat with microsatellite and AFLP markers,Genome 45(2002)719–727.
[15]Z.Z.Liu,Z.Y.Wang,W.J.Zhao,A study on FHB resistance of wheat germplasm source,Acta Agric.Shanghai 1(2)(1985) 75–84(in Chinese with English abstract).
[16]L.Tamburic-Ilincic,D.Falk,A.Schaafsma,Fusarium ratings in the Ontario Winter Wheat Performance Trial(OWWPT) using an index that combines Fusarium head blight symptoms and deoxynivalenol levels,Czech J.Genet.Plant Breed.47(2011)S115–S122.
[17]Q.Yao,Y.X.Song,R.H.Zhou,T.H.Fu,J.Z.Jia,Quantitative trait loci for adult-plant resistance against yellow rust in a wheat-derived recombinant inbred line population,Sci. Agric.Sin.42(2009)4234–4241(in Chinese with English abstract).
[18]D.L.Wang,J.Zhu,Z.K.Li,A.H.Paterson,Mapping QTLs with epistatic effects and QTL×environment interactions by mixed linear model approaches,Theor.Appl.Genet.99 (1999)1255–1264.
[19]R.A.McIntosh,G.E.Hart,K.M.Devos,M.D.Gale,W.J.Rogers, Catalogue of gene symbols for wheat,http://grain.jouy.inra. fr/ggpages/wgc/98/Intro.htm#Intro61988.
[20]X.K.Zhang,S.J.Yang,Y.Zhou,Z.H.He,X.C.Xia,Distribution of the Rht-B1b,Rht-D1b and Rht8 reduced height genes in autumn-sown Chinese wheats detected by molecular markers,Euphytica 152(2006)109–116.
[21]L.Zhang,B.S.Zhang,R.H.Zhou,L.F.Gao,G.Y.Zhao,Y.X. Song,J.Z.Jia,Cloning and genetic mapping of cytokinin oxidase/dehydrogenase gene(TaCKX2)in wheat, Acta Agron.Sin.33(2007)1419–1425(in Chinese with English abstract).
[22]Z.P.Yang,J.Gilbert,G.Fedak,D.J.Somers,Genetic characterization of QTL associated with resistance to Fusarium head blight in a doubled-haploid spring wheat population,Genome 48(2005)187–196.
[23]L.Gervais,F.Dedryver,J.Y.Morlais,V.Bodusseau,S.Negre,M. Bilous,C.Groos,M.Trottet,Mapping of quantitative trait loci for field resistance to Fusarium head blight in an European winter wheat,Theor.Appl.Genet.106(2003)961–970.
[24]J.Yu,G.Bai,W.Zhou,Y.Dong,F.L.Kolb,Quantitative trait loci for Fusarium head blight resistance in a recombinant inbred population of Wangshuibai/Wheaton,Phytopathology 98 (2003)87–94.
[25]J.Yang,G.Bai,G.E.Shaner,Novel quantitative trait loci(QTL) for Fusarium head blight resistance in wheat cultivar Chokwang,Theor.Appl.Genet.111(2005)1571–1579.
[26]R.Draeger,N.Gosman,A.Steed,E.Chandler,M.Thomsett,J. Schondelmaier,H.Buerstmayr,M.Lemmens,M.Schmolke, A.Mesterhazy,Identification of QTLs for resistance to Fusarium head blight,DON accumulation and associated traits in the winter wheat variety Arina,Theor.Appl.Genet. 115(2007)617–625.
[27]Z.X.Ma,B.J.Steffenson,L.K.Prom,N.L.V.Lapitan,Mapping of quantitative trait loci for Fusarium head blight resistance in barley,Phytopathology 90(2000)1079–1088.
[28]H.Ellis,W.Spielmeyer,K.Gale,G.Rebetzke,R.Richards,“Perfect”markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat,Theor.Appl.Genet.105(2002)1038–1042.
[29]D.J.Somers,P.Isaac,K.Edwards,A high-density microsatellite consensus map for bread wheat(Triticum aestivum L.),Theor. Appl.Genet.109(2004)1105–1114.
*Corresponding author.
E-mail address:jiajizeng@caas.cn(J.Jia).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
2214-5141/$–see front matter©2013 Production and hosting by Elsevier B.V.on behalf of Crop Science Society of China and Institute of Crop Science,CAAS.
http://dx.doi.org/10.1016/j.cj.2013.10.004
Fusarium graminearum
FHB
Head scab
- The Crop Journal的其它文章
- Induction of avirulence by AVR-Pita1 in virulent U.S. field isolates of Magnaporthe oryzae,
- Molecular characterization ofα-gliadin genes from common wheatcultivar Zhengmai004 and their role in quality and celiac disease
- Three photosynthetic patterns characterized by cluster analysis of gas exchange data in two rice populations
- Near-infrared spectroscopy(NIRS)evaluation and regionalanalysis of Chinese faba bean(Vicia faba L.)
- Yield and tillering response of super hybrid rice Liangyoupeijiu to tillage and establishmentmethods
- BRIEF GUIDE FOR AUTHORS

