Scale-up synthesis and characterization of 2,6-diamino-3,5-dinitropyrazine-1-oxide
Hai-bin WANG,Yan-hong WANG,Yong-xiang LI,Yu-cun LIU*,Ying-xin TAN
School of Chemical and Environmental Engineering,North University of China,Taiyuan 030051,China
Scale-up synthesis and characterization of 2,6-diamino-3,5-dinitropyrazine-1-oxide
Hai-bin WANG,Yan-hong WANG,Yong-xiang LI,Yu-cun LIU*,Ying-xin TAN
School of Chemical and Environmental Engineering,North University of China,Taiyuan 030051,China
2,6-diamino-3,5-dinitropyrazine-1-oxide(ANPZO),as an insensitive high explosive,with a high yield and excellent purity has been prepared at pilot plant scale by an improved method.The synthesized ANPZO is characterized by IR,laser granularity measurement,SEM and HPLC. The particle analysis revealed that the improved method could offer desired product with average particle size of 40 μm and high purity (>98.45%).The experimental parameters exhibited that the detonation velocity of the formulation based on ANPZO was higher than that of the corresponding TATB formulation.The DSC curve showed that the exothermic decomposition of the product occurred at the temperature between 300.5°C and 360.4°C.Furthermore,the sensitivity test suggests its safe nature towards mechanical stimulus.
ANPZO;Synthesis;Characterization;Detonation velocity;Sensitivity
1.Introduction
The quest for new high energetic materials(HEMs)with highest possible performance and low vulnerability led to the development of insensitive high explosive(IHE)[1].ANPZO has attracted substantial attention from researchers in energetic material feld.ANPZO is an energetic heterocycle compound with a density of 1.913 g/cm3.Its structure with monoclinic space group is shown in Fig.1[2].ANPZO has about 20%and 81%more energy than TATB and HMX, respectively.Besides,ANPZO is thermally stable and insensitive to shock,spark as well as friction.It has impact sensitivity level approaching that of TATB.Moreover,ANPZO can be used in military and civil applications,such as insensitive booster,main charge explosive and deep oil well explorations, based on its excellent properties[3-6].Hence,ANPZO is emerging as a realistic high performance IHE material for several applications that require moderate performance and insensitivity.
In view of the above,it is worth working on the synthesis of ANPZONPZO was frst reported by Talawar et al.[7],since then different synthetic methods of ANPZO have been developed.Pagoria et al.[8]prepared ANPZO in low yield (about 36%).In fact,this method is limited by collection methoxylation intermediate and drastic nitration reaction condition.Tran et al.[9]obtained ANPZO in laboratory scale by an alternative approach.This method modifed the nitration reaction condition.However,this method has some limitations in terms of cost and product purity.Li[10]also synthesized ANPZO based on the alternative approach.It seems that those reported methods can not be used to prepare desirable ANPZO with convenient methods.Moreover,the preparation of ANPZO by the reported methods suffers from three of the main drawbacks:(a)diffculty of collecting alkoxylation intermediate due to its sublime at room temperature;(b)low yield of product;and(c)expensive cost of synthesis.Therefore,these disadvantages limit its engineering production and further application.
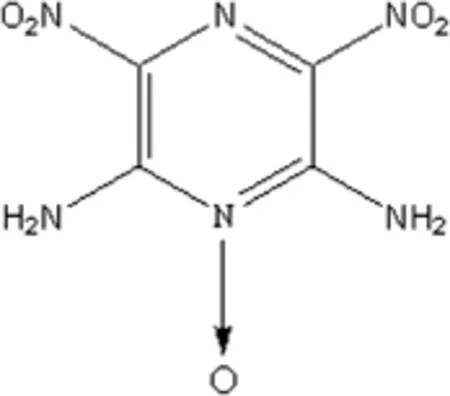
Fig.1.Structure of ANPZO.
In order to solve these problems mentioned above,an intensive study on the synthesis of ANPZO hasbeen taken.However, no detailed information about actual process parameters for the synthesis of ANPZO is readily available in open literature.
The paper discusses the characterization of ANPZO.In short,the highlights of this study are as follows:(a)detailing the synthetic parameters;(b)improving the overall yield of product;(c)reducing the cost of production;and(d)recovering and recycling expensive trifuoroacetic acid(TFA).
2.Experiment
2.1.Materials
TATB was supplied by Liaoning Huafeng Chemical Industry Co.,Ltd.The other chemicals and reagents without further purifcation used in the present study were purchased from the market.
2.2.Synthesis
2.2.1.Synthesis of ANPZO
In this work,desired ANPZO was synthesized in a yield of 45%in large-scale production by the improved method with a four-step reaction process(see Fig.2).The frst step of this process is to use alkoxylation of 2,6-dichloropyrazine to obtain 2,6-dimethoxypyrazine(DMP).Then,the next two steps are to nitrify DMP to generate 2,6-dimethoxy-3,5-dinitropyrazine(DMDP),which undergoes a subsequent amination to offer 2,6-diamino-3,5-dinitropyrazine (ANPZ).The fnal step is to oxidize ANPZ to yield product ANPZO.
2.2.1.1.Synthesis of DMP.17.1 kg 2,6-dichloropyrazine was added to 125 L methanol.Then the mixture was stirred,and about 24.7 kg of sodium methoxide was added into the reaction solution within 40 min.The mixture was heated up to 60°C under refux condition for 5 h,then cooled and poured into 125 L water.The product was extracted with 50 L ether.The extracts were dried with magnesium sulfate and concentrated up to dryness at low temperature to give 14.7 kg white solid of DMP,with a yield of about 92%.
2.2.1.2.Synthesis of DMDP.14.7 kg DMP was added to 60 L of 98%sulfuric acid in 30 min.Then the suspension was stirred,and 22 kg sodium nitrate wasadded slowly. Throughoutthe addition,the temperature waskeptat 10-15°C by use of water-ethylene glycol cooling.After completion of addition,the sodium nitrate and the mixture was allowed to warm to 25°C and kept at 25°C for 4 h.The reaction mixture was gradually added into ice-cold water.The precipitate was fltered off,and the precipitated solid was washed with water and dried in air to offer 13.1 kg DMDP with a yield of about 54%.
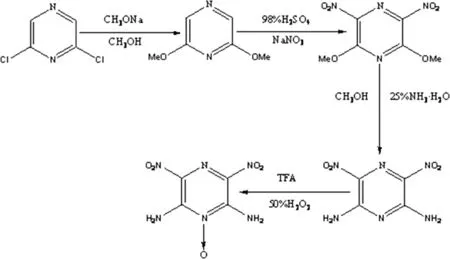
Fig.2.Reaction sequence of ANPZO.
2.2.1.3.Synthesis of ANPZ.13.1 kg DMDP was added to 74 L methanol.Then 66 L of 25%aqueous ammonia solution was added rapidly to the stirred suspension of DMDP.The mixturewas refuxed at 60°C for 6 h.After cooling the reaction mixture,the precipitate was fltered off,the precipitated solid was washed with water and dried in air to give 10.8 kg yellow ANPZ powder with yield of 95%.
2.2.1.4.Synthesis of ANPZO.10.8 kg ANPZ was added to 220 L TFA.22 L of 50%hydrogen peroxide was added to the stirred suspension of ANPZ through a constant fow pump within 35 min.The reaction mixture was heated to 30°C and stirred for 24 h.After 12 h,8 L of 50%hydrogen peroxide was added,and after 24 h,the reaction mixture was warmed to 50°C for a further 1 h.The reaction mixture was cooled to room temperature.The precipitate was fltered,the precipitated solid was washed with water and dried in air to yield 11.0 kg ANPZO as brilliant yellow powder with a yield of 95%and an overall yield of 45%.
The waste fltrate of TFA was neutralized by sodium hydroxide to obtain sodium trifuoroacetate followed by its acidifcation with concentrated sulfuric acid.The acidizing mixture was distilled at a temperature between 70°C and 75°C under atmospheric pressure.The results show that the recovery rate and purity of TFA are above 80%and 96%(see Fig.3),respectively.Moreover,the recovered TFA can be used in the synthesis of ANPZO.
2.3.Preparation of ANPZO and TATB formulations
95 g synthesized ANPZO was added to 500 mL water.The suspension of ANPZO was stirred and heated to 60°C.50 g of 10%Viton A binder solution was added dropwise to the ANPZO-water slurry through constant fow pump over a period of 20 min.After addition of Viton A binder solution, agitation was continued for 5 min at 60°C.The precipitate was fltered off,the precipitated solid was washed with water and dried in air to obtain coated ANPZO particles with 5% Viton A binder in a good free fowing form.
TATB was coated with 5%Viton A binder by the same method.
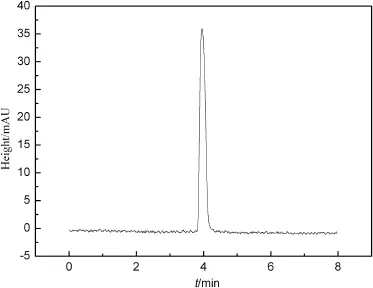
Fig.3.HPLC of ANPZO.
2.4.General methods
The crystal morphologies were examined by LE0438VP scanning electron microscope(SEM)(Britain)at 12 kV and 10 μA.A laser particle sizer(LS230,USA)was used for the study of particle size distribution.The structural feature of ANPZO was confrmed by using 8400S infra-red spectrophotometer(Japan),using KBr pellets.The purity of the synthesized ANPZO and recovered TFA was determined by HPLC system[11].The detonation velocity of formulations was tested by probes according to GJB-772A-97 standard method 702.1.The cylinder of coated ANPZO particles was molded at 120°C under 180 MPa pressure with a dwell time of 15-20 s,having a density of 1.80 g/cm3(94%TMD). Likewise,the coated TATB particles were pressed as a reference.The differential scanning calorimeter(DSC)analysis results were recorded on a Netzsch DSC 204 differential scanning calorimeter(Germany)by heating 1.155 mg of ANPZO sample in nitrogen(50 ml/min)atmosphere at a rate of 5°C/min.CGY-1 impact instrument was used to test the impact sensitivity of ANPZO,and each sample(50 mg)was tested 35 times to obtain a H50(height)for 50%probability of explosion with 2.5 kg of drop weight[12].
3.Results and discussion
3.1.Synthesis
ANPZO was synthesized on a multi-kilogram scale by the improved method including methoxylation,nitration,amination and N-oxidation which are all typical reactions in the preparation of explosives except for methoxylation.
2,6-Dichloropyrazine is methoxylated to yield DMP by replacing of both chlorine atoms in 2,6-dichloropyrazine by methoxy groups.As a result,the intermediate DMP is easier to collect and save in comparison to the convenient synthesis. Moreover,the next nitration can proceed more readily, because the presence of the methoxy groups on the pyrazine ring activates the system towards electrophilic attack.
The synthesis of DMDP involves the reaction of DMP with 98%sulfuric acid and sodium nitrate at 25°C.This nitration method not only improves the yield of DMDP and safety but also favors the scale-up synthesis of ANPZO.It can be explained that nitration may proceed completely and smoothly by the addition of electron donating substituents to the pyrazines[13].
During the amination,ANPZ is obtained by treating DMDP with aqueous ammonia in methanol instead of acetonitrile. Further experiments show that there is no obvious difference in the yields of ANPZ for different kinds of solvents such as methanol,acetonitrile and acetone.Hence,methanol is a better choice in terms of cost of synthesis of ANPZ.
The reported methods were used to prepare ANPZO containing 5-10%ANPZ without oxidation in the solid-liquid reaction[14].But the improved method is to use the reoxidation technology to prepare desired product by adding extra hydrogen peroxide into mother liquid at certain reaction time.
In a word,the advantages of the method proposed in this study,compared with the reported methods,are easy collection and preservation of intermediate DMP,milder nitration condition and higher safety in favor of scale-up synthesis, higher yield and purity of ANPZO,and remarkable reduction in the cost of synthesis of product due to the recovery and recycle of expensive TFA.
3.2.Infrared spectrum f ANPZO
As seen from Fig.4,the IR spectrum of ANPZO reveals the absorption bands and characteristics frequencies of orther groups.ANPZO exhibits the characteristic IR stretching frequencies at 3432,3404,3283 and 3229 cm-1due to NH2groups[15,16].Further,the other vibrations observed in IR spectrum at 924,815 and 533 cm-1may be attributed to the frequencies of pyrazine ring.The spectral pattern obtained from ANPZO is in good agreement with the structure,and therefore the structural features of ANPZO are confrmed by IR spectroscopy.
3.3.SEM and laser granularity measurement of ANPZO
The SEM image of ANPZO is shown in Fig.5.The surface of ANPZO synthesized by the improved method has cubic crystal morphology with smooth particle surfaces.The average particle size of ANPZO is 40 μm,which is measured using a laser particle sizer(see Fig.6).This average particle size is slightly bigger than the reported data[14].It was found that the particle size of ANPZO becomes bigger as reaction temperature increases.This is because the crystal growth rate increases with the reaction temperature.Further,the experiments show that the morphology of ANPZO crystals changes from cubic shape to cubic rod shape with the increase in stirring speed.For instance,ANPZO with cubic rod-shaped crystal morphology was obtained under a higher stirring speed(500 rpm/min).It may be attributed to the fact that a higher stirring speed can decrease the thickness of retention layer,which contributes to the rapid growth of ANPZO crystal along its radial direction[17,18].
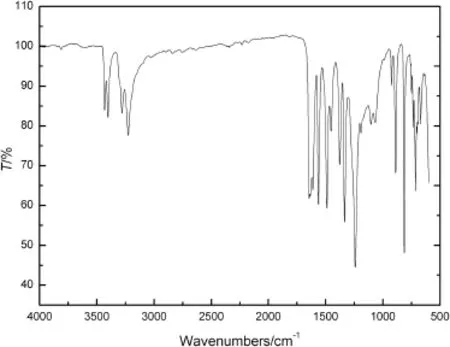
Fig.4.IR spectrum of ANPZO.

Fig.5.SEM image of ANPZO.
3.4.Properties of formulations
The coated ANPZO and TATB samples were pressed into 25.10 mm diameter and 25.20 mm long cylindrical samples in the detonation velocity test.The properties of the formulation are summarized in Table 1.The detonation velocity of ANPZO formulation is obviously higher than that of TATB formulation.It indicated that ANPZO has greater power than TATB due to its favorable oxygen balance and unique pyrazine ring structure.In addition,the new ANPZO formulation consisting of a mixture of coarse ANPZO(70 mass%)and fne ANPZO (25 mass%)with 5%Viton A binder has a detonation velocity of 8250 m/s with a pressing density of 1.86 g/cm3.It can be explained that the mixing of coarse and fne ANPZOs can increase the pressing density of formulation,so the new ANPZO formulation gets a higher detonation velocity.
3.5.DSC curve
The DSC curve of ANPZO is shown in Fig.7.The DSC results obtained from the DSC plot,with a heating rate of 5°C/min,indicates that a clear exothermic decomposition occurs between 300.5°C and 360.4°C with peak maximum (Tmax)at 345.3°C.Further,the energy output during the decomposition reaction is 598.2 J/g.The DSC data show that ANPZO has higher thermal stability than the most known high explosive.
3.6.Sensitivity test
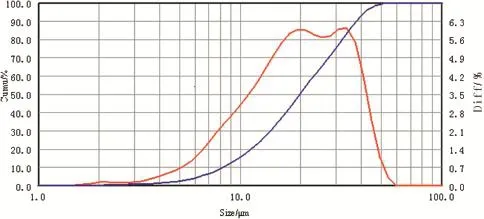
Fig.6.Laser granularity measurement of ANPZO.
ANPZO and TATB were subjected to impact sensitivity test.The test data of impact sensitivity are listed in Table 2. The results of drop hammer test show that both ANPZO and TATB are insensitive to mechanical impact.This is because of the presence of amino groups adjacent to nitro groups in both molecules[19].Therefore,they display an excellent insensitivity.However,it was found that ANPZO is sensitive to particle morphologies.For example,the impact sensitivities of ANPZO particle with needle-shape and ANPZO particle with cubic morphology are 85 cm and 115 cm,respectively.Needle-shaped particle has very coarse surface and exterior defects,which can lead to the increase in hot spots[20], consequently the former is more sensitive to impact than the latter.
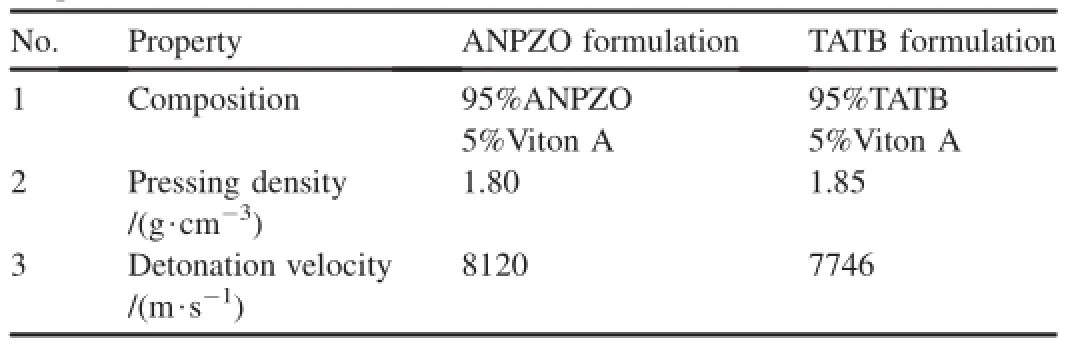
Table 1Properties of ANPZO and TATB formulations.
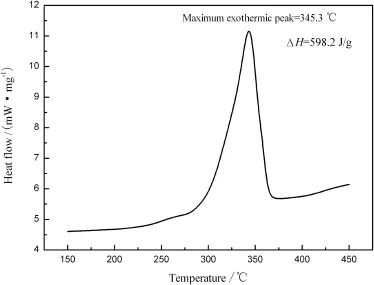
Fig.7.DSC curve of ANPZO.

Table 2Impact sensitivity data of ANPZO and TATB.
4.Conclusions
The improved method for the preparation of ANPZO with higher yield and excellent purity on industrial scale was established.The experimental results show that the improved method is effcient and economic for the preparation of ANPZO.It not only improves the yield and purity of ANPZO but also reduces the cost of production.The HPLC and SEM analysis indicated that ANPZO with higher purity(>98.45%)has an average particle size of 40 μm.The detonation velocity test revealed that ANPZO formulation has higher energy than TATB.The DSC plot and impact sensitivity test exhibited that ANPZO is thermally stable and insensitive to mechanical stimuli.Moreover, based on the excellent properties and scale-up synthesis of ANPZO,it is a very promising candidate for replacement of TATB as an insensitive high performance explosive.
Acknowledgments
We would like to express our gratitude to Wei-yan LI and Ping-li YU for their enthusiastic help for this work.
[1]Anniyappan M,Talawar MB,Gore GM,Venugopalan S,Gandhe BR. Synthesis,characterization and thermolysis of 1,1-diamino-2,2-dinitroethylene(FOX-7)and its salts.J Hazard Mater 2006;B137:812-9.
[2]Ma HX,Song JR,Zhao FQ,Gao HX,Hu RZ.Crystal structure,safety performance and density-functional theoretical investigation of 2,6-Diamino-3,5-dinitropyrazine-1-oxide (LLM-105). Chin J Chem 2008;26:1997-2002.
[3]P.F.Pagoria,G.S.Lee,A.R.Mitchell,R.D.Schmidt,The synthesis of amino-and Nitro-Substituted heterocycles as insensitive energetic materials,in Proceedings of 2001 Insensitive Munitions&Energetic Materials Technology Symposium,Bordeaux,France,https://e-reports-ext. llnl.gov/pdf/244204.pdf2001.
[4]Millar RW,Hamid J,Endsor R.Selection and synthesis of energetic heterocyclic compounds suitable for use in insensitive explosive and propellant compositions.Propell Explos Pyrot 2008;33:66-72.
[5]Badgujar DM,Talawar MB,Asthana SN,Mahulikar PP.Advances in science and technology of modern energetic materials:an overview.J Hazard Mater 2008;151:289-305.
[6]P.F.Pagoria,A.R.Mitchell,R.D.Schmidt,R.L.Simpson,F.Garcia,J.W. Forbes,Synthesis,Scale-up and Characterization of 2,6-Diamino-3,5-dinitropyrazine-1-oxide(LLM-105),JOWOG 9,Aldermaston,England, June 22-26,http://www.osti.gov/bridge/servlets/purl/672328-tIIGju/ webviewable/672328.pdf,1998.
[7]Talawar MB,Sivabalan R,Anniyappan M,Gore GM,Asthana SN, Gandhe BR.Emerging trends in advanced high energy materials. Combust Explo Shock 2007;43:62-72.
[8]Pagoria PF,Lee GS,Mitchell AR,Schmidt RD.A review of energetic materials synthesis.Thermochim Acta 2002;384:187-204.
[9]T.D.Tran,P.F.Pagoria,D.M.Hoffman,J.L.Cutting,R.S.Lee,R.L. Simpson,Characterization of 2,6-Diamino-3,5-Dinitropyrazine-1-Oxide (LLM-105)as an insensitive high explosive material,in Proceedings of 33rd International Annual Conference on ICT on energetic materialssynthesis,production and applications,Karlsruhe,Germany,June 25-28,https://e-reports-ext.llnl.gov/pdf/244397.pdf,2002.
[10]Li HB,Cheng BB,Li HZ,Nie FD,Li JS,Huang Z,Liu SJ.Synthesis of 2,6-Diamino-3,5-Dinitropyrazine-1-oxide, Chinese. J Org Chem 2007;27:112-5[Chinese].
[11]Sikder N,Bulakh NR,Sikder AK,Sarwade DB.Synthesis,characterization and thermal studies of 2-oxo-1,3,5-trinitro-1,3,5-triazacyclohexane (Keto-RDX or K-6). J Hazard Mater 2003;A96:109-19.
[12]Wang JY,Huang H,Xu WZ,Zhang YR,Lu B,Xie RZ,Wang PY,Yun N. Preflming twin-fuid nozzle assisted precipitation method for preparing nanocrystalline HNS and its characterization.J Hazard Mater 2009;162:842-7.
[13]Agrawal JP,Hodgson RD.Organic chemistry of explosive.1st ed.Chichester:John wiley&Sons Ltd;2007.
[14]D.M.Hoffman,K.T.Lorenz,B.Cunningham,F.Gagliardi,Formulation and Mechanical Properties Of LLM-105 PBXs,39th International Annual Conference of ICT,Karlsruhe,Germany,June 24-27,https://ereports-ext.llnl.gov/pdf/359699.pdf,2008.
[15]Bellamy AJ.A study of the synthesis and amination of 2,6-Dialkoxy-3,5-dinitropyrazines.Cent Eur J Energ Mater 2008;5:3-9.
[16]Jadhav HS,Talawar MB,Sivabalan R,Dhavale DD,Asthana SN, Krishnamurthy VN.Synthesis,characterization and thermolysis studies on new derivatives of 2,4,5-trinitroimidazoles:potential insensitive high energy material.J Hazard Mater 2007;143:192-7.
[17]Jiang RG,Liu ZT.Initiating explosive,1.Beijing:Ordnance Industry Press of China;2006[Chinese].
[18]Li WJ,Shi EW,Zhong WZ,Yin ZW.Growth mechanism and growth habit of oxide crystals.J Cryst Growth 1999;203:186-96.
[19]Zhou L.Base of explosion chemistry,1.Beijing:Beijing Institute of Technology Press;2005[Chinese].
[20]Armstrong RW,Ammon HL,Elban WL,Tsai DH.Investigation of hot spot characteristics in energetic crystals. Thermochim Acta 2002;384:303-13.
Received 1 April 2014;revised 25 June 2014;accepted 14 July 2014 Available online 23 August 2014
*Corresponding author.Tel.:+86 351 3922116;fax:+86 351 3922118. E-mail address:whaibin@nuc.edu.cn(Y.C.LIU).
Peer review under responsibility of China Ordnance Society.
http://dx.doi.org/10.1016/j.dt.2014.07.008
2214-9147/Copyright©2014,China Ordnance Society.Production and hosting by Elsevier B.V.All rights reserved.
Copyright©2014,China Ordnance Society.Production and hosting by Elsevier B.V.All rights reserved.
- Defence Technology的其它文章
- Analysis of parameter estimation using the sampling-type algorithm of discrete fractional Fourier transform
- Nitrogen analogs of TEX-A computational study
- Neural network modeling to evaluate the dynamic fow stress of high strength armor steels under high strain rate compression
- Experiment and simulation of launching process of a small-diameter steel cartridge case
- Reliability sensitivity analysis based on multi-hyperplane combination method
- Feasibility of surface-coated friction stir welding tools to join AISI 304 grade austenitic stainless steel

