Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells impair relaxation of rat thoracic aortic rings
Shao-xun WANG, Qi ZHANG, Man SHANG, Su WEI, Miao LIU, Yi-lu WANG, Meng-xiao ZHANG, Yan-na WU, Ming-lin LIU, Jun-qiu SONG, Yan-xia LIU
Introduction
Microvesicles (MVs) are small vesicles of 0.1~1 μm diameters released from activated or apoptotic cells.MVs are not merely passively shed by-products circulating in the bloodstream, their release is regulated tightly. MVs shed by diあerent cells contain a subset of cell surface proteins derived from the plasma membrane of the original cells, which allow them to function as messengers that mediate many biological processes[1-3]. In addition, MVs also carry various bioactive molecules, such as cytokines,mRNA and DNA derived from their metrocyte,which can be transferred into target cells and mediate a cascade of biological eあects.
Vascular endothelial dysfunction is a major abnormality in many diseases such as acutemyocardial ischemia, diabetes, and atherosclerosis[4, 5]. Recent studies indicated that patients with diabetes, atherosclerosis, ischemic heart disease and many other cardiovascular diseases have increased circulating levels of MVs [6], suggesting that under pathological states, MVs released from activated or apoptotic cells may in return play a critical role in the pathogenesis of vascular endothelial dysfunction.Our previous studies demonstrated that circulating levels of MVs increased in myocardial I/R injury rats, and MVs released from HUVECs exerted a proapoptotic effect on cultured H9c2 cardiomyocytes[7]. Myocardial ischemia/reperfusion (I/R) causes endothelial cells to generate reactive oxygen species(ROS). The accumulation of ROS can trigger myocardial oxidative stress. As a result, it could induce the impairment of endothelial function [8].MVs released by injured endothelial cells under the condition of I/R may carry ROS, inflammatory cytokines and other relative bioactive molecules[9]. One study reported that increased circulating levels of MVs from hypoxic rats could induce endothelial dysfunction in aorta of rats by decreasing NO production [10]. Because the MVs used in this study are a mixture with different cellular origins including platelets, endothelial cells, erythrocytes and lymphocytes, it is not clear whether the MVs from endothelial cells (EMVs) play an important role on endothelial function. Whether these EMVs can induce impairment of endothelium relaxation and the underlying mechanisms have not been elucidated.Our hypothesis is that H/R-EMVs released by HUVECs treated with H/R have eあects on relaxation of thoracic aortic ring of rats. To test this hypothesis,the hypoxia/reoxygenation (H/R) injury model of HUVECs was established in vitro to mimic I/R in vivo. The H/R-EMVs derived from this model was used to induce eあects on the endothelium relaxation of thoracic aortic rings of rats.
Materials and Methods
Materials
HUVECs EA.hy926 were purchased from the Cell Bank of Chinese Academy of Sciences. High glucose Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Hyclone. acetylcholine (ACh), phenylephrine (PE)and sodium nitroprusside (SNP) were purchased from Sigma. NO, SOD, and MDA kits were purchased from Beyotime Institute of Biotechnology.Anti-eNOS and anti-p-eNOS antibody were obtained from Cell Signaling Technology. The Hypoxic Modular Incubator was purchased from Billups-Rothenberg.
Preparation of hypoxia/reoxygenation injury model in HUVECs
HUVECs 2×105were seeded and cultured in 6 wells for 24 h. Normal medium was changed with 2 ml hypoxic buffer of following composition (mmol/L):NaH2PO40.9; NaHCO36.0; CaCl21.0; MgSO41.2;NaCl 98.5; KCl 10.0; HEPES 20.0; sodium lactate 40.0. Plates were instated into a hypoxic chamber.そe gas mixture containing 95% N2and 5% CO2were filled into chamber at a rate of 20 L/min. そe hypoxic chamber was closed after 15 min and was placed into incubator in 95%N2and 5% CO2condition in 37°C for 12 h. HUVECs were reoxygenated in incubator in 95%O2and 5% CO2condition in 37°C for an additional 4 h.
Preparation of H/R-EMVs
Hypoxic buffer was collected in 15 ml centrifuge tubes and centrifuged at 2 700 g, 4°C for 20 min to remove cell debris. The 90 μl supernatant without cell debris was collected and fixed in 4 %paraformaldehyde (PFA, 30 μl) for 1h and then stored at -80°C for flow cytometric analysis. Remaining supernatant was collected in 13.2 ml ultracentrifuge tubes and subjected to a centrifugation at 33 000 rpm for 150 min to pellet H/R-EMVs. そe pellet was re-suspended in 200 μl PBS and kept at -20°C for subsequent experiments.
Flow cytometric analysis and protein quantification of H/R-EMVs
The procedure was carried out as previously described [8]. In brief, fixed cell-free supernatant was blocked with mouse serum then incubated with anti-PE-CD144 antibody or its anti-PE Mouse IgG1 isotype respectively. After being diluted in 400 μl PBS, 50 000 calibration beads in diameter of 1 μm were added immediately prior to analysis by FACS Calibur flow cytometer. Dot plots of forward scatter (FSC) vs. side scatter (SSC) were established.Gain settings were adjusted place 1 μm beads in the upper log for scatter. Events < 1 μm in diameter and CD144 positive were defined as H/R-EMVs. Protein quantification of H/R-EMVs was performed by using a BCA protein assay.
Preparation of thoracic aortic rings of rats treated with H/R-EMVs
All animal procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23,revised 1996) and approved by the local animal care and use committee. Rat thoracic aorta obtained from male Wistar rats (body wt 250±10 g) were carefully removed and placed in Krebs solutions of following composition (mmol/L): NaCl 118; KCl 4.7; KH2PO41.2; MgSO4·7H2O 1.2; CaCl2·2H2O 2.5;NaHCO325 and glucose 10. Aorta were cleared from periadventitial tissue and cut into 3-4 mm rings.Control aortic rings were incubated in DMEM with 10% FBS, while H/R-EMVs treated aortic rings were incubated with 2, 5, 10, 20 μg/ml H/R-EMVs in the same medium at 37°C in a 95% O2and 5%CO2atmosphere cell culture incubator for 4 h. そen,aortic rings were placed in 10 ml tissue chambers filled with Krebs solution. Tissue baths were gassed with 95% O2-5% CO2and maintained at 37°C , pH 7.4. Rings were suspended by two parallel stainless steel wires, and tension was recorded isometrically with a force transducer connected to a computerbased data acquisition system.
Relaxations of thoracic aortic rings of rats induced by ACh or SNP
Aortic rings were allowed to equilibrate for 60 min under a basal resting tension of 2.0 g. After the equilibration, aortic rings were treated with phenylephrine (PE, 10-6mol/L) to test their contractility, and then washed for several times with Krebs solution until the tension was restored to a stable level around baseline. The rings were treated with PE again. When they arrived at the stable maximal contractile response which usually took 15 min [11], the relaxations of aortic rings response to ACh or SNP in concentrations of 10-9-10-6mol/L were examined to detect the endothelium-dependent or independent relaxation.
Measurement of NO, SOD, MDA in thoracic aortic rings of rats
Rat aortic rings stored at -80°C were put into 4°C refrigerator to keep freeze in thawing condition and washed by normal saline. Aortic rings in normal saline (W:V=1 g: 9 ml) were homogenated on ice and then centrifuged at 3 000 rpm for 5 min. Supernatant was collected into eppendorf tubes for subsequent experiments. Concentrations of NO and MDA were measured by method of Griess Reagent and thiobarbituric acid, respectively. Activity of SOD was detected by method of xanthine oxidase. The final concentrations of NO, SOD and MDA were calculated by the equations supplied by the kits producer.
Western blot for detecting the expression of t-eNOS and p-eNOS
Proteins from aortic rings homogenate were extracted with RIPA lysis buあer. Protein lysates were then centrifuged at 12 000 g at 4°C for 10 min, and the supernatant was collected. そe harvested proteins were used to perform Western blot analysis. In brief, the samples were electrophoresed on 8% SDSPAGE gel and transferred onto PVDF membranes.The membranes were blocked with 5% skim milk powder solution for 2 h at room temperature and incubated with primary rabbit anti-eNOS (1:400) or anti-phospho-eNOS (Ser-1177) (1: 500)antibodies at 4°C overnight. After washing for 3 times, membranes were incubated with horseradishperoxidase (HRP) conjugated IgG (1: 1 000) for 2 h at room temperature. Blottings were then developed with enhanced chemiluminescence reagents, and quantification of protein bands was performed using the densitometry with sofware Quantity One.
Statistical analysis
Data were expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Dunnett or Bonferroni post hoc test was used. P value less than 0.05 was regarded as statistically significant.Statistical evaluation was performed by using SPSS 17.0 sofware.
Results
Isolation and characterization of H/R-EMVs
Events were mainly located under the region of 1-μm beads (Fig.1). In contrast to the sample of purified water that showed scarce events in the defined area R (Fig.1A), we found that the large amount of H/R-EMVs (Fig.1B) located in the area are smaller than 1 μm.
To distinguish the true events from background noise, the H/R-EMVs were gated in R1 area, which was confirmed by 1-μm standard beads. Staining of anti-PE-CD144 further identified events in the R1 gate. The histogram showed the CD144 positive H/R-EMVs counted for 4.09 % of all events (Fig.1C).
Eあects of H/R-EMVs on ACh or SNP-induced relaxation
Compared with control, the relaxation of aortic rings induced by a cumulative increments of ACh was impaired by H/R-EMVs at the concentrations of 5, 10, 20 μg/ml (Emax: 81.1%±6.0%, 67.7%±4.0%,43.5%±9.0% and 93.6%±8.0% for 5, 10, 20 μg/ml and control, respectively, P<0.05, P<0.01) significantly.The relaxation of 2.5 μg/ml H/R-EMVs-treated aortic rings (Emax: 99.0%±2.4%) was slightly higher than control. The relaxation of aortic rings induced by ACh was impaired by H/R-EMVs in a dosedependent manner at the concentration range of 5-20 μg/ml (Fig.2A). Endothelium-independent relaxation induced by SNP was similar between control and H/R-EMVs treated groups (Fig.2B).
Eあects of H/R-EMVs on NO production
Compared with control, H/R-EMVs at the concentrations of 5, 10, 20 μg/ml reduced NO production in aortic rings in a dose-dependent manner significantly (P<0.01). There was no significant difference between 2.5 μg/ml group and control (Fig. 3).
Eあects of H/R-EMVs on t-eNOS and p-eNOS expression Compared with control, H/R-EMVs in the concentration of 2.5, 5, 10, 20 μg/ml did not aあect the expression of t-eNOS of aortic rings. Furthermore,there were no significant differences of t-eNOS expression between H/R-EMVs treated groups.However, compared with control, H/R-EMVs at the concentrations of 5, 10, 20 μg/ml decreased the expression of p—eNOS-Ser-1177 in a dose-dependent manner significantly (P<0.01, Fig. 4).
Eあects of H/R-EMVs on SOD, MDA production
Compared with control, H/R-EMVs in the concentration of 5, 10, 20 μg/ml reduced the activity of SOD (Fig.5 A), meanwhile increased level of MDA(Fig.5 B) in aortic rings in a dose-dependent manner significantly (P<0.01). There were no significant differences between 2.5 μg/ml group and control in both assays.
Discussion
Microvesicles derived from blood and vascular cells can be detected in the circulating blood and have emerged as a new concept of biomarkers in cardiovascular diseases. Vascular endothelium lines the entire cardiovascular system and performs a series of vital functions including control of microvascular permeability, coagulation, inflammation and vasorelaxation. Vascular endothelial dysfunction is the main reason to induce various and highly prevalent cardiovascular diseases [12, 13]. There was a report that the increased level of EMVs in pathologic conditions played roles in pathogenesis of endothelial dysfunction [14]. However, if EMVs generated in H/R conditions associated with vascular dysfunction has not been elucidated.
In this study, aortic rings were incubated with H/R-EMVs for 4 h, the effects of H/R-EMVs on endothelium relaxation of aortic rings were detected. Relaxation of aortic rings induced by ACh is dependent on NO produced by endothelial cells,the result showed that endothelium-dependent relaxation of aortic rings was impaired by H/R-EMVs in response to ACh directly. Relaxation of aortic rings induced by SNP is not dependent on NO produced by endothelial cells since SNP is a kind of NO-donor. The result showed that relaxation of aortic rings induced by SNP was not aあected by H/R-EMVs. そese results indicate that H/R-EMVs may impair endothelial function through NO dependent pathway.
NO is a short-lived bioactive free radical and is known as vasorelaxation factors. NO derived from endothelial cells plays an essential role in maintaining endothelial function, such as vasorelaxation,proliferation and apoptosis. It diffuses into the vascular smooth muscles and results in the relaxation of vascular smooth muscles [15, 16]. During coronary ischemia/reperfusion condition, there was an impairment of endothelial function with a decrease in NO production [17]. In our present study, the concentration of NO2-which is the metabolic product of NO was measured by Griess Reagent, and the production of NO was calculated according to the concentration of NO2-in aortic rings. そis study demonstrated that the production of NO in aortic rings was decreased by H/R-EMVs significantly,which may be the reason for the impairment of relaxation of aortic rings in response to ACh. The balance of NO production is vital for endothelial function, and the decrease of NO production is able to result in endothelial dysfunction.

Fig. 1 Characterization of H/R-EMVs by flow cytometry. A:Representative dot plot of forward scatter (FSC) vs side scatter (SSC) for evaluation of background noise in the sample of purified water. B: Representative dot plots for H/R-EMVs. H/R-EMVs were identified as events with a size of< 1 μm within the gate R1. C: Representative histogram for CD144 positive H/R-EMVs. H/R-EMVs labeled with PE IgG isotype control----black line area; H/R-EMVs labeled with anti-PE-CD144 antibody---- right shifted gray area.
T-eNOS is the main enzyme of NO synthesis in vascular system. It is activated by calcium and calmodulin in endothelial cells. It not only regulates blood pressure and vascular tone, but also plays roles in many pathological processes including cardiac ischemia/reperfusion. Under the condition of ischemia/reperfusion, there was a decrease activity of t-eNOS and p-eNOS [18, 19]. In endothelial cells,NO is synthesized from the substrate L-argentine via t-eNOS, and the phosphorylation of a specific serine residue (Ser-1177) in t-eNOS is important for its enzymatic activity, p-eNOS is the main source which NO derived from [20]. It means that the decreased expression of p-eNOS will result in the decreased production of NO. Our results showed that the expression of t-eNOS in aortic rings was not aあected by H/R-EMVs treatment; however, the expression of p-eNOS was decreased by H/R-EMVs in a dosedependent manner. The results indicated that the mechanism of the decrease of NO production is related to the decreased expression of p-eNOS.

Fig. 2 Effects of H/R-EMVs on endothelium-dependent or independent relaxation of thoracic aortic rings of rats. A:ACh-induced relaxation of thoracic aortic rings of rats (n =5) after incubated with H/R-EMVs for 4 h. B: SNP-induced relaxation of thoracic aortic rings of rats (n = 5) after incubated with H/R-EMVs for 4 h. Values are means ± SD,*P<0.05, ** P<0.01 vs control; ##P<0.01 vs 2.5 μg/ml; $P<0.05,$$P<0.01 vs 5 μg/ml; &&P<0.01 vs 10 μg/ml.
The production of NO not only can be affected by the expression of p-eNOS, but also by the level of oxidative stress (OS). Production of NO can be reduced by superoxide anion O2-produced in OS and generate deleterious metabolites such as peroxynitrite(ONOO-) [21]. SOD is an oxygen radical cleaner that could reduce the OS, while MDA is generated by oxidation reaction that has strong oxidizing property. SOD and MDA levels have been applied in evaluating cell ability against OS, in which SOD acts as an anti-OS factor and MDA represents cell OS damage [22]. そe decreased activity of SOD and increased level of MDA indicates the extent of the damage by OS. One of the possible mechanisms of the decreased production of NO in aortic rings may be the increased level of OS in aortic rings treated with H/R-EMVs. そis hypothesis has been proved by the measurement of SOD and MDA. そe decreased activity of SOD and increased level of MDA indicated that OS occurred. The decrease of NO production and impairment of endothelial function may be associated with OS.

Fig. 3 Nitric oxide (NO) production in thoracic aortic rings of rats (n = 5) after incubated with H/R-EMVs for 4 hours.Values are means ± SD, **P < 0.01 vs control.
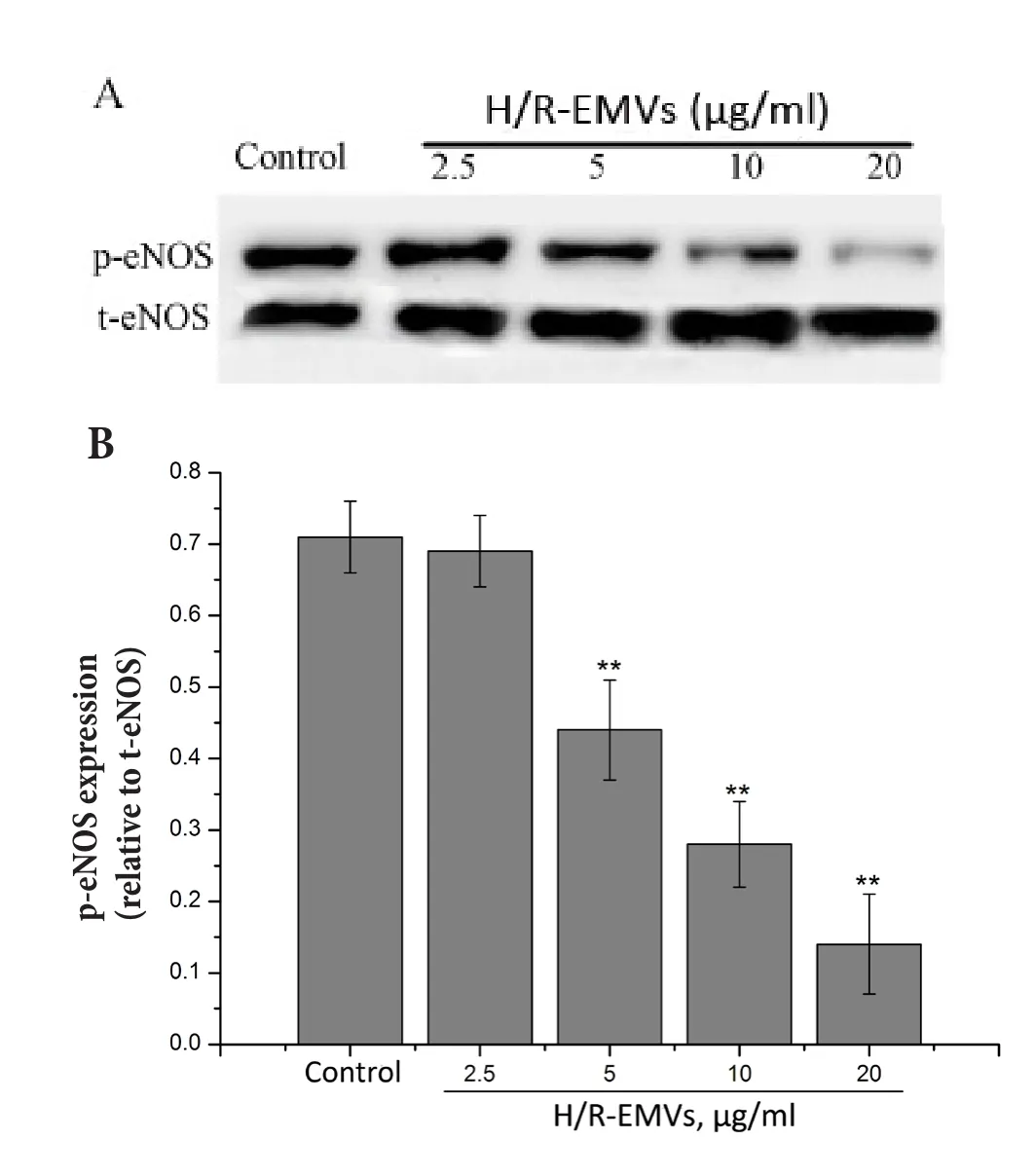
Fig. 4 t-eNOS and p-eNOS (Ser-1177) expressions in thoracic aortic rings of rats (n = 5) after incubated with H/R-EMVs for 4 hours. A: t-eNOS and p-eNOS (Ser-1177) expression in thoracic aortic rings of rats (n = 10) was assayed by Western blotting. B: Relative densitometry of the expression of p-eNOS (Ser-1177) to t-eNOS. Data are representative of three independent experiments, values are means ± SD. **P <0.01 vs control.

Fig. 5 Effects of H/R-EMVs on SOD and MDA production.A: Activity of SOD in thoracic aortic rings of rats (n = 5)after incubated with H/R-EMVs for 4 h. B: Level of MDA in thoracic aortic rings of rats (n = 5) after incubated with H/R-EMVs for 4 hours. Values are means ± SD, **P<0.01 vs control in SOD assay, ##P<0.01 vs control in MDA assay.
In conclusion, our findings suggest that H/R-EMVs impaire the relaxation of aortic rings in response to ACh directly, and decreased NO production in aortic rings. そe underlying mechanism involves decreased expression of p-eNOS and occurrence of oxidative stress. Although there are some evidences that H/R-EMVs may play a role in endothelial dysfunction,further studies should be carried out to clarify more underlying mechanisms.
Acknowledgements
そis work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (20101202110005), the Natural Science Foundation of Tianjin (11JCZDJC18300), the Research Foundation of Tianjin Municipal Education Commission (20110106) and the National Key Basic Research Program of China (973 Program,2011CB933100).
1. Laresche C, Pelletier F, Garnache-Ottou F, et al. Increased levels of circulating microparticles are associated with increased procoagulant activity in patients with cutaneous malignant melanoma [J]. J Invest Dermatol, 2014, 134(1):176-182.
2. Kwaan HC, Rego EM. Role of microparticles in the hemostatic dysfunction in acute promyelocytic leukemia[J]. Semin そromb Hemost, 2010, 36(8): 917-924.
3. Leroyer AS, Anfosso F, Lacroix R, et al. Endothelial-derived microparticles: Biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis [J]. Thromb Haemost, 2010, 104(3): 456-463.
4. Jansen F, Yang X, Hoelscher M, et al. Endothelial microparticle-mediated transfer of microrna-126 promotes vascular endothelial cell repair via spred1 and is abrogated in glucose-damaged endothelial microparticles[J].Circulation, 2013, 128(18): 2026-2038.
5. Chung BH, Kim JD, Kim CK, et al. Icariin stimulates angiogenesis by activating the MEK/ERK-PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells[J].Biochem Biophys Res Commun, 2008, 376(2): 404-408.
6. Hulsmans M , Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease[J]. Cardiovasc Res, 2013, 100(1):7-18.
7. Shang M, Zhang Q, Zhang MX, et al. Effects of endothelial microvesicles induced by A23187 on H9c2 cardiomyocytes[J]. Chin J Appli Physiol, 2013, 29(6): 559-564.
8. Zhao M, He X, Bi XY, et al. Vagal stimulation triggers peripheral vascular protection through the cholinergic antiinflammatory pathway in rat model of myocardial ischemia/reperfusion[J]. Basic Res Cardiol, 2013, 108(3): 345.
9. Jansen F, Yang X, Franklin BS. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation [J].Cardiovasc Res, 2013, 98(1): 94-106.
10. Tual-chalot S, Guibert C, Muller B, et al. Circulating microparticles from pulmonary hypertensive rats induce endothelial dysfunction [J]. Am J Respir Crit Care Med,2010, 182(2): 261-268.
11. Yılmaz B, Usta C. Ellagic acid-induced endothelialiumdependent and endothelialium-independent vasorelaxation in rat thoracic aortic rings and the underlying mechanism[J]. Phytother Res, 2013, 27(2): 285—289.
12. Sharma S, Singh M, Sharma PL. Mechanism of hyperhomocysteinemia-induced vascular endothelium dysfunction -possible dysregulation of phosphatidylinositol-3-kinase and its downstream phosphoinositide dependent kinase and protein kinaseB[J]. Eur J Pharmacol, 2013,721(1-3): 365-372.
13. Hyseni A, Roest M, Braun SL, et al. Chronic dysfunction of the endothelium is associated with mortality in acute coronary syndrome patients [J]. そromb Res, 2013, 131(3):198-203.
14. Gao C, Li R, Liu Y, et al. Rho-kinase-dependent F-actin rearrangement is involved in the release of endothelial microparticles during IFN-Alpha-induced endothelial cell apoptosis [J]. J Trauma Acute Care Surg, 2012, 73(5): 1152-1160.
15. Gonzalez-Forero D, Moreno-Lopez B. Retrograde response in axotomized motoneurons: nitric oxide as a key player in triggering reversion toward a dedifferentiated phenotype[J]. Neuroscience, 2014, 283C: 138-165.
16. Joshua J, Kalyanaraman H, Marathe N, et al. Nitric oxide as a mediator of estrogen eあects in osteocytes[J]. Vitam Horm,2014, 96: 247-263.
17. Garcia-Villalon AL, Granado M, Monge L, et al. Purinergic component in the coronary vasodilatation to acetylcholine after ischemia-reperfusion in perfused rat hearts[J].J Vasc Res, 2014, 51(4): 283-289.
18. Kirtiman S, Ulvi BM, Bath W. Current therapeutic strategies to mitigate the eNOS dysfunction in ischaemic stroke [J].Cell Mol Neurobiol, 2012, 32: 319-336.
19. Ren-An Q, Juan L, Chuyuan L, et al. Study of the protective mechanisms of Compound Danshen Tablet (Fufang Danshen Pian) against myocardial ischemia/reperfusion injury via the Akt-eNOS signaling pathway in rats[J].J Ethnopharmacol, 2014, 156: 190-198.
20. Kim YH, Hwang JH, Noh JR, et al. Activation of NADPH:quinone oxidoreductase ameliorates spontaneous hypertension in an animal model via modulation of eNOS activity[J]. Cardiovasc Res, 2011, 91: 519-527.
21. Han J, Wang D, Yu B, et al. Cardioprotection against ischemia/reperfusion by licochalcone B in isolated rat hearts[J]. Oxid Med Cell Longev, 2014, 2014: 134862.
22. Xu Y, Wang S, Feng L, et al. Blockade of PKC-beta protects HUVEC from advanced glycation end products induced inflammation[J]. Int Immunopharmacol, 2010, 10(12):1552-1559.
- 中国应用生理学杂志的其它文章
- Children’s exercise capacity at high altitude in Tibet
- Hypoxic preconditioning: effect, mechanism and clinical implication (Part I)
- Role of HCN channels in the nervous system: membrane excitability and various modulations
- How to deal with cerebral palsy in 21st century
--A new epoch in clinic treatment - Hematological parameters in high altitude residents:Tibetan natives versus Han migrants
- A rat model of high altitude polycythemia rapidly established by hypobaric hypoxia exposure

