Children’s exercise capacity at high altitude in Tibet
Bianba, Lars Bo Andersen, Hein Stigum, Ouzhuluobu, Espen Bjertness
1. Research Center for High Altitude Medicine, Tibet University Medical College, Lhasa 850002, China;
2. Department of Community Medicine, Institute of Health and Society, University of Oslo, Oslo 0316, Norway;
3. Department of Sports Medicine, Norwegian School of Sport Sciences, Oslo 4014, Norway; 4. Department of Exercise Epidemiology, Institute of Sport Science and Clinical Biomechanics, University of Southern Denmark, Odense 5230,
Denmark; 5. Division of Epidemiology, Norwegian Institute of Public Health, Oslo 4404, Norway
Introduction
そere are several areas in the world with mountains higher than 3 500 m above sea level, but the permanently populated major high-altitude regions are the Himalayas of Asia, the Andes of South America and the Rocky Mountains of North America. そe Himalayan (Qinghai-Tibetan) Plateau is the largest of them all and people have lived there for a long time, approximately 22 000 years [1, 2]. そe Andian Altiplano has been selected for an intermediate duration of time, approximately 9 000~12 000 years,while the Rocky Mountain region was not inhabited permanently until approximately 150 years ago [3]. A lifelong high-altitude residence is a health challenge for both natives and immigrants living in Tibet due to a unique environment, including a tough climate and exposure to hypobaric hypoxia, with a low ambient partial pressure of oxygen. Approximately 17 million of the world’s population live at altitudes higher than 3 500 m above sea level [4]. In the Tibet Autonomous Region (TAR) of China, more than three million people [5] live at similar altitudes, of whom 90.5% are native Tibetans and 8.2% are Han immigrants from lowland China (Han Chinese) who have lived in Tibet for only one-three generations,as well as some smaller ethnic groups. The effect of high-altitude residence on exercise capacity are not fully understood, but this effect is often more pronounced at the highest altitudes and in immigrant lowlanders, as compared with native highlanders in Tibet [6]. A comparison of exercise capacity and the associated factors between native Tibetans and Han Chinese may contribute to the knowledge of how native highlanders adapt to high altitude.
Maximal oxygen uptake (exercise capacity) is a vital parameter in the evaluation of adaptation to high altitude, providing an index of the integrated function of the oxygen transport system. It is therefore widely recognized as the best single indicator of cardiopulmonary fitness. It is well known that a capacity for exercise performance is progressively limited at an increasing altitude measured from approximately 1 500 m above sea level [7]. Previous studies of maximal oxygen uptake in population at high altitude have mainly focused onadults and adolescents, studies of the physiological aspects of children’s capacity to work and live at diあerent altitudes and by ancestry in Tibet are scarce,although differences similar to those seen in adults may be expected to occur.
そis paper will focus on the comparison studies of children’s exercise capacity at high altitudes in Tibet.
Definition and measurement of exercise capacity
Both treadmill running and cycle ergometry are appropriate for assessment of maximal oxygen uptake. Treadmill is the apparatus of choice for determining maximal oxygen uptake in the laboratory setting, whereas cycle ergometry is more suitable in field experiments because it is easily movable. Five to eight percent lower maximal oxygen uptake has been reported during maximal work on a cycle ergometer compared to treadmill testing in children [10]. The underestimation in cycle tests compared with treadmill is systematic and can therefore be corrected, and a maximal multistage cycle ergometer exercise test in children has successfully been used in large populations [11].
Exercise capacity (maximal oxygen uptake) can be measured directly, using an oxygen analyser, or indirectly through the development of equations for estimation from the maximal power output(Wmax). Such estimations have been done in selected populations [12-16], but not in children living in Tibet, previously. A direct measurement of maximal oxygen uptake, using an oxygen analyzer, is often not feasible for screening purposes in many places in the world, because it is impractical for testing a large number of examinees as it requires special conditions and relatively expensive equipment [8]. An indirect estimation of maximal oxygen uptake from maximal power output may, however, be more appropriate,and it has been used in sea level populations [12-16]. Wmaxcan provide a valid indicator of the direct measurement of exercise capacity [12-16], and has been used in the evaluation of the adaptation to live and work at high altitude [17-19].
We applied similar methods previously used in studies on children [20, 21]. Wmaxwas measured during the progressive cycle ergometer test conducted on an electronically braked cycle ergometer, according to a previously validated protocol [15]. Heart rate was measured throughout the test, and recorded at the end of each work load during the cycling and at the maximal exercise level(Polar Electro OY, Kempele, Finland). The bicycle was electronically calibrated once every test day and mechanically calibrated after being moved, and the height of the seat was regulated so that the heel was flat when the leg was fully extended. The children started at a power output of 20 W if their weight was less than 30 kg, with a rise in the power output of 20 W for every three minutes. If their weight was greater than 30 kg, the children started at a power output of 25 W, with a rise in the power output of 25 W for every three minutes. The children cycled with a pedalling rate of 70-80 revolutions per minute(rpm), and the power output was increased until the child was no longer able to maintain a pedalling frequency of at least 30 rpm. Two criteria were used for determining whether the maximal effort was achieved. (1) A subjective judgment (hyperpnea,facial flushing, unsteady gait, sweating) by the test leader that the child could no longer continue, even after vocal encouragement [13, 20, 22]. (2) A heart rate equal to or above 185 beats per minute [20, 22].
Wmaxwas determined as watts in the last fully completed power output (Wh), plus the increment in watts (Wd) of the last step, multiplied by the number of seconds completed of the last step (t) and then divided by 180 seconds [15]:
Wmax= Wh+Wd· t ·180—1
Exercise capacity (endurance exercise) among children in Tibet
A quite large number of studies on exercise capacity have been performed among children at sea level,using both direct and indirect measurements of maximal oxygen uptake [14, 16, 20, 23-29]. However,only a few studies [27, 30-32] on exercise capacity among children at high altitude have been conducted and the studies are hampered by low sample size and lack of representatives. We, therefore, conducted comparison studies on exercise capacity (Wmax)across ancestry and by residential altitude [33].
Wmaxwas assessed using a cycle ergometer(Monark Ergomedic 839, Varberg, Sweden) in Lhasa Tibetan children (201 boys and 182 girls), Lhasa Han children (216 boys and 142 girls) and in Tingri Tibetan children (87 boys and 64 girls).
Exercise capacity across ancestry
In relation to both a decrease in arterial oxygen content and a limitation in maximal cardiac output,moving lowlanders to higher altitudes is associated with a decreased aerobic exercise capacity [34], while a higher pre-exposure residential altitude modulates the negative effect [34]. However, if high altitude natives move to a lower altitude, they will exhibit a better exercise capacity than natives living at the same, lower altitude, as demonstrated by Curran et al. [35]. The relatively better maximal exercise capacity in high altitude natives may primarily be due to their exposure to a high altitude environment during childhood [36].
We measured Wmaxin Tibetan and Han Chinese children living at the same altitude in Lhasa in order to investigate the variation of exercise capacity across ancestry. Initially, 812 children aged 9 to 10 years old from 20 primary schools in Lhasa with an altitude of 3 700 m above sea level were invited. Eventually, the data includes 383 Lhasa Tibetan and 358 Lhasa Han Chinese, rest of the children were lack of the cycle ergometer test due to having diきculties on touching the pedal or missing.
Lhasa Tibetan children achieved 10% and 4%(5.8% in boys and 2.1% in girls) higher levels of absolute Wmax(W) and relative Wmax(W/kg) than Lhasa Han Chinese children (Tab.1), respectively. In the regression analyses (Tab.2), the Wmaxfor Lhasa Tibetans was 86 W (95% confidence interval: 83.2,88.2), and Lhasa Han Chinese had a 5 (-6.4, -2.9) W lower Wmax. All the selected physiological covariates(Hb, SaO2, HR and FVC) were associated with Wmax.For example, for every increase in maximal heart rate of one beat per minute, the Wmaxincreased by 0.4 W; for a one litre increase in lung volume (FVC)the Wmaxincreased by 13.2 W, whereas a one kg body mass increase was associated with a 1 W increase in Wmax. Girls had an 8.8 W significantly lower Wmaxthan boys. そe ancestry was significantly associated with Wmax after an adjustment for the explanatory physiological variables, which indicated that the eあects of ancestry on Wmaxwere not due to the eあects of these intermediate variables. Compared to Lhasa Tibetan, Lhasa Han Chinese had a 4.1 W significantly lower Wmaxby adjustment. An additional analysis was conducted in which the Lhasa Han Chinese was divided into highland-born- and Lhasa Han Chinese lowland-born children. Compared to Lhasa Tibetan,highland-born- and lowland-born Han Chinese had a 5.3 W and 3.7 W significantly lower Wmax,respectively. Maximal heart rate, arterial oxygen saturation (SaO2) at rest and at maximal exercise, and lung volume did not aあect the associations between ancestries, with Wmax.
These results indicate that native Tibetan achieved higher exercise capacity compared to Han Chinese children at the same altitude. This may be due to a better oxygen transport system. Tissue oxygen extraction can be inferred from Hb and the diあerence between the oxygen saturation in arterialand mixed-venous blood (SaO2- SvO2) [37]. In comparison with Han Chinese, a lower Hb in adult native Tibetans has previously been reported [10, 38-41]. Additionally, the lower Hb in Tibetan subjects has been suggested to be a result of adaptation over many generations [41, 42], which has further been suggested to be more favorable than Andeans, who are characterized by a raised Hb [43]. Recent genetic studies identified genetic variants in native Tibetans living between 3 200 and 4 300 m compared with Han Chinese lowlanders [11, 44-48]. Three genes,EPAS1, EGLN1 and PPARA, have been shown to be associated with the relatively lower hemoglobin level in Tibetans [42], while no associations were found with EPAS1 and EGLN1 in Andeans [49]. Hence,genetic factors may explain the lower Hb in Lhasa Tibetans compared with Han Chinese in the present study.
The present Lhasa Tibetan children had a lower Hb than Han Chinese, but they sustained a higher SaO2during maximal exercise than Lhasa Han Chinese children. So, we suppose that the higher SaO2across the entire range of exercise intensities in native Tibetan children may indirectly reflect their smaller alveolar-arterial O2gradients ((A-a)DiffO2), thereby they may better maintain oxygen saturation during exercise compared to Han Chinese children [19, 50]. It has been suggested that diあusion capacity could be a decisive factor for oxygen transport during maximal exercise at high altitude [51]. As shown in several studies [52-57],an increased lung volume is also accompanied by an increased pulmonary diffusion capacity. Moreover,large lung volumes and chest circumferences have been reported among schoolchildren and adolescents who are native to high altitude [58, 59], though not in immigrants acclimatized to high altitude [60].Native Lhasa Tibetan children had a significantly larger FVC (boys: 2.22 ±0.29 L; girls: 2.06 ±0.33 L)and chest circumference (boys: 64.7 ±4.74 cm; girls:63.7 ±4.61 cm) than Lhasa Han Chinese children(FVC for boys: 2.04 ±0.30 L and for girls: 1.89 ±0.26 L; chest circumference for boys: 61.8 ±4.26 cm; girls:61.3 ±4.47 cm), even after adjusting for body mass and stature.
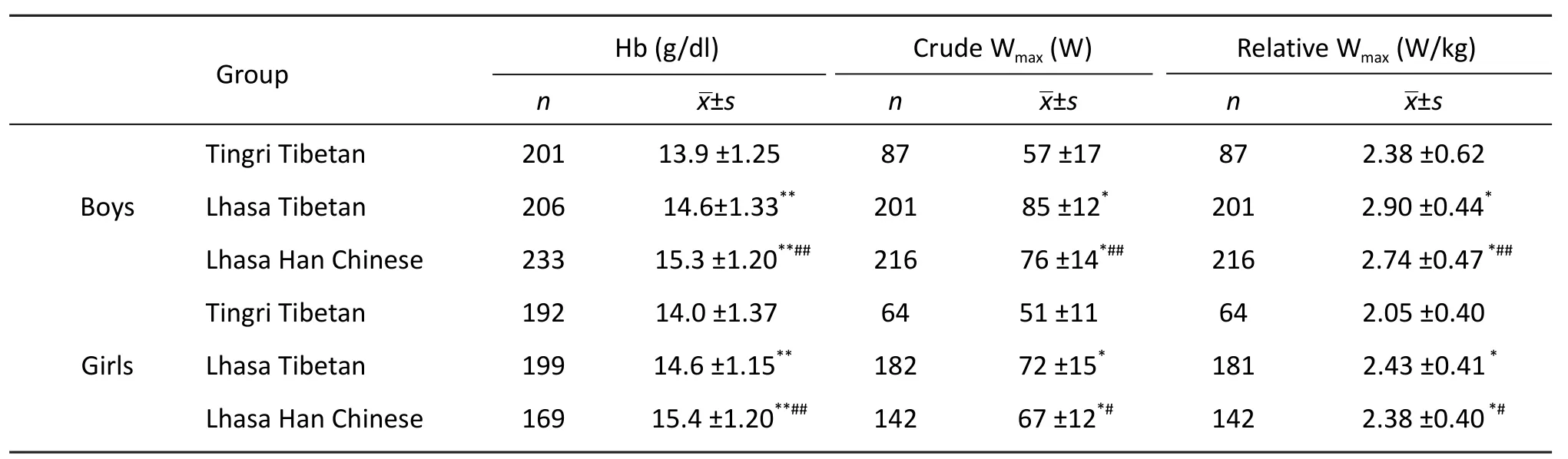
Tab. 1 Hemoglobin concentration and maximal power output (Wmax) of 9- to 10-year-old Lhasa Tibetans and Lhasa Han Chinese, and 9- to 10-year-old Tingri Tibetan children.

Tab. 2 Coefficients of maximal power output from three regression models, based on data from 9-10-year-old native Tibetan and Han Chinese children living in Lhasa at 3 700 m and native Tibetan children living in Tingri at 4 300 m above sea level.
Exercise capacity by residential altitude
High altitude populations are constantly challenged by an environment of hypobaric hypoxia with a low ambient partial pressure of oxygen, thus influencing the capacity for work and exercise. Moreover, the capacity is reduced with an increasing residential altitude [34]. It was well known that capacity for exercise performance is progressively limited with increasing altitude measured from about 1 500 m above sea level [7]. A reduction in the inspired partial pressure of oxygen (PiO2) at altitude will result in a decreased maximal exercise capacity [61].
We measured Wmaxin native Tibetan children living at two diあerent residential altitudes (3 700 mvs4 300 m) in order to investigate the alteration of exercise capacity by altitude. We collected data from entire five primary schools in Tingri in which a total of 151 native Tibetan children (boys: 87vsgirls: 64)participated in the bicycle test, due to whether did not go to school at the days of data collection, refused to participate, or participated but did not permit to use their data for publication.
Lhasa Tibetan children achieved 46% and 20%(21.8% in boys and 18.5% in girls) higher levels of absolute Wmax(W) and relative Wmax(W/kg) than Tingri Tibetan children, respectively. In the linear regression analyses, Wmaxwas 86 W (95% confidence interval: 83.2, 88.2) for Lhasa Tibetans, and a 16(-18.8, -14.0) W lower Wmaxwas found in Tingri Tibetans. All the included physiological variables(Hb, SaO2, HR and FVC) were associated with Wmax(Tab. 2). However, after an adjustment for the explanatory physiological variables, Tingri Tibetan had an 18.1 lower maximal power output, indicating residential altitude was still significantly associated with Wmax.
These results indicate that native Tibetan 9-10-year-old children living at lower resident altitude presented a higher exercise capacity than native Tibetans at higher residential altitude. It may be due to the reduction in the PiO2. Children’s growth and development are also closely correlated with their work, exercise performance and altitude adaptation[62, 63]. Our studies reveal that the Tingri Tibetan children were shorter (122.2 cm vs 134.8 cm; P<0.001 for boys; 122.8 cm vs 135.9 cm; P<0.001 for girls)and lighter (22.5 kg vs 29.7 kg; P<0.001 for boys; 22.4 kg vs 29.4 kg; P<0.001 for girls) than the Tibetan children from Lhasa of the same age. Such findings are consistent with a previous comparative study of Tibetan children and adolescents aged 7-18 years living at various altitudes from 2 261 m to 4 040 m in Qinghai-Tibet. そe children from the higher altitude(4 040 m) presented a slower growth (stature),lagging behind their counterparts at 2 261 m by approximately two years [64]. It was indicated that hypoxia may be the main agent responsible for this delayed growth [64]. Furthermore, Greksa et al. [65]suggested that socioeconomic factors (SES) slowed the growth of high-altitude children, as in the present study we only have information on SES in Lhasa Han Chinese and Lhasa native Tibetans. It is therefore a challenge to identify an indicator of SES that is valid for both Lhasa and Tingri. For example, in Tingri,parents generally have a low- or no education and no income, but they may have many Yak or sheep, hence indicating a high SES. In contrast, in Lhasa, parents seldom own animals, and most males have both an education and income. We speculate that a lower Hb among Tingri Tibetan children compared with Lhasa Tibetan children reported in the present study, which stands in contrast to previous reports of an increase in Hb with an increasing altitude [41], may be due to poor nutrition, including less iron-rich foods at high altitude.
Development equations for estimation of exercise capacity
Rationale for development equations
Several equations for estimation maximal oxygen uptake from Wmaxhave been developed based on data from children living at sea level [14-16,21, 26] but none at high altitude. We may use the equations derived from sea level population,however, considerations were taken in case of the disparities between constituencies. First of all is the differentiation in the SaO2during maximal work between sea level and high altitude. The SaO2at maximal exercise remains around 95% at sea level[66], but it may decrease to around 82%-84% at altitudes of 3 700-4 400 m above sea level [35, 51,57]. そen, it has been demonstrated that the exercise capacity in children at high altitude is influenced by developmental factors in concert with nutrition and environmental characteristics [67]. Children at high altitude were commonly found to have poor nutritional status and stunting [68-70]. It is therefore may cause the diあerent body composition of children at high altitude compared to those from sea level.Last but not least, different work efficiency due to variation in cycle ergometer skills may exist between children from high altitude compared to children living at sea level. A comparison study [20] reported an underestimation of aerobic fitness by a maximal bicycle test as compared to running in Tanzanian 9-10 years old children. そe study suggested that lack of bicycle skills may affect the results by reason of two thirds of the participants had poor cycle skills.
In total, prediction models based on measurements at sea level populations may not accurately predict maximal oxygen uptake at high altitude.Consequently, for the purpose of development formulas for estimating maximal oxygen uptake,we conducted a conventional study among 25 Tibetan children and 15 Han Chinese children aged 9-10 years in Lhasa at 3 700 m above sea level. The methods we applied were used in the European Youth Heart Study [21] that conducted in several European countries. そe methods were similar to a comparison study in Tanzanian and Norwegian schoolchildren as well [20].
Measurements
We directly measured maximal oxygen uptake(exercise capacity) using MetaMax II (CORTEX Biophysik MetaMax ® II portable CPX system,Germany) on a cycle ergometer [71]. Volume and gas calibration (against gas mixtures of known concentration of oxygen and carbon-dioxide) were conducted before each test period. Subjects breathed through a mouthpiece connected to a mass-flow sensor.
Wmaxwas estimated during the progressive cycle ergometer test, conducted on an electronically braked cycle ergometer (Monark Ergomedic 839, Varberg,Sweden) [71], according to a previous validated protocol [15]. Children were requested to have five minutes of practice on the ergometer cycle before the test to ensure that all inexperienced children had some cycling practice, and more details regarding the procedures and test criteria can be found elsewhere[71].
Suggested new equations for children living in Tibet
A standard multiple regression was used to develop a prediction model for maximal oxygen uptake.As independent variables, Wmax, sex, weight and peak heart rate were used to explain the variance of maximal oxygen uptake. In a pooled analysis ethnicity did not make any contribution to explain the variance in maximal oxygen uptake. そough, as mentioned earlier, the main ethnic groups in Tibet are native Tibetan and Han Chinese immigration.Likewise, the lack of significantly contribution of ethnicity could be caused by small sample size.Therefore, we decided to make the analyses on Tibetan and Han Chinese subjects separately.Eventually, two equation, BianbaeqTfor native Tibetan and BianbaeqHfor Han Chinese, were produced[71]. To further confirm the new equations are fit better for children living in Tibet, three sea level equations [14, 21, 26]were selected for comparison using mean squared error (MSE) and Bland-Altman plots according to several criteria based on the characteristics of our subjects. None of the three selected equations could accurately predict the direct measured maximal oxygen uptake, and predictions diあered in an unsystematic manner, including overor underestimation and no differentiation between genders [71]. Awkwardly, BianbaeqTand BianbaeqHshould be used with caution and they should preferably be tested in a large population before may be used as a general equation for all children in Tibet.Following are the new equations:

Conclusion
Native Tibetan children exhibit a better exercise capacity (Wmax) when they live at a lower altitude in Lhasa (3 700 m) compared with a higher altitude in Tingri (4 300 m). Native Tibetan children also have a better exercise capacity than Han Chinese children measured at their same residential altitude of 3 700 m in Lhasa. In addition to residential altitude and ancestry, selected physiological parameters,including higher SaO2and FVC, were associated with a higher Wmax, but did not impact significantly on the associations between residential altitude and ancestry with Wmax.
そe method of predicting maximal oxygen uptake from Wmaxin a progressive cycle ergometer test can be applied among children living at 3 700 m in Tibet, but need to be tested in other Tibetan and Han Chinese populations in order to verify whether it can be widely used as a standard equation for children living in Tibet. The estimates of maximal oxygen uptake based on equations derived from sea level data has its limitations, and needed to be verified in a larger population.
We recommend testing the new equations for the estimation of maximal oxygen uptake from Wmaxin studies of a larger sample of native Tibetan- and Han Chinese children. New studies will confirm if the equation can be widely used, and we also recommend conducting studies that using both a quantitative and qualitative methodology to identify the indicators of SES and diet in Tibet — a district of China with great inequalities in economic development between urban and rural areas. Lastly, we recommend studies on the factors associated with delayed growth across residential altitude and ancestry in Tibet, preferably a cohort study of children followed to young adulthood, and genetic factors.
Disclosure statement
Prefessor Bianba, Andersen, Stigum, Ouzhuluobu,and Bjertness have no conflicts of interest or financial ties to disclose.
Acknowledgements
We are very thankful to the Network for University Cooperation Tibet-Norway for supporting this study.そanks as well as to all the children who participated in this study and to our colleagues at the Tibet University Medical College for their great support in the data collection.
1. Aldenderfer MS. Moving up in the world: Archaeologists seek to understand how and when people came to occupy the Andean and Tibetan plateaus[J]. Am Sci, 2003, 91:542—549.
2. Niermeyer S, Zamudio S, Moore LG. The People. In:Hornbein TF, Schoene RB, et al. High altitude: an exploration of human adaptation[M]. New York: Marcel Dekker, 2001: 43-99.
3. Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives[J]. Am J Phys Anthropol, 1998 (Suppl 27): 25-64.
4. Huddleston B, Ataman E, Fe d’Ostiani L. Towards a GIS-based analysis of mountain environments and populations[M]. Rome: FAO, 2003.
5. Census. Census 2010 National Bureau of Statistics of China.6. Wu TY. Chronic mountain sickness on the Qinghai-Tibetan plateau[J]. Chin Med J (Engl), 2005, 118(2): 161-168.
7. Roach R, Kayser B. Exercise and hypoxia. In: Hornbein TF,Schoene RB, et al. High altitude: an exploration of human adaptation[M]. New York: Marcel Dekker, 2001: 663-705.
8. Rankovic G, Mutavdzic V, Toskic D, et al. Aerobic capacity as an indicator in diあerent kinds of sports[J]. Bosn J Basic Med Sci, 2010, 10(1): 44-48.
9. Astrand PO, Rodahl K. Textbook of work physiology:physiological bases of exercise[M]. Third ed. Now York McGraw-Hill, 1986.
10. Beall CM, Brittenham GM, Strohl KP, et al. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara[J]. Am J Phys Anthropol, 1998, 106(3): 385-400.
11. Beall CM, Cavalleri GL, Deng L, et al. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders[J]. Proc Natl Acad Sci, 2010, 107(25): 11459-11464.
12. Andersen LB. A maximal cycle exercise protocol to predict maximal oxygen uptake[J]. Scand J Med Sci Sports, 1995,5(3): 143-146.
13. Andersen LB, Henckel P, Saltin B. Maximal oxygen uptake in Danish adolescents 16-19 years of age[J]. Eur J Appl Physiol Occup Physiol, 1987, 56(1): 74-82.
14. Arngrimsson SA, Sveinsson T, Johannsson E. Peak oxygen uptake in children: evaluation of an older prediction method and development of a new one[J]. Pediatric Exerc Sci, 2008, 20(1): 62-73.
15. Hansen HS, Froberg K, Nielsen JR, et al. A new approach to assessing maximal aerobic power in children: the Odense School Child Study[J]. Eur J Appl Physiol Occup Physiol,1989, 58(6): 618-624.
16. Woynarowska B. The validity of indirect estimations of maximal oxygen uptake in children 11-12 years of age[J].Eur J Appl Physiol Occup Physiol, 1980, 43(1): 19-23.
17. Ge RL, Chen QH, Wang LH, et al. Higher exercise performance and lower VO2maxin Tibetan than Han residents at 4 700 m altitude[J]. J Appl Physiol, 1994, 77(2):684-691.
18. Grover RF, Reeves JT, Grover EB, et al. Muscular exercise in young men native to 3 100 m altitude[J]. J Appl Physiol,1967, 22(3): 555-564.
19. Wu T, Kayser B. High altitude adaptation in Tibetans[J].High Alt Med Biol, 2006, 7(3): 193-208.
20. Aandstad A, Berntsen S, Hageberg R, et al. A comparison of estimated maximal oxygen uptake in 9 and 10 year old school children in Tanzania and Norway[J]. Br J Sports Med, 2006, 40(4): 287-292.
21. Riddoch C, Edwards D, Page A, et al. そe European youth heart study-cardiovascular disease risk factors in children:rationale, aims, study design, and validation of methods[J].J Phys Act Health, 2005, 2: 115-129.
22. Anderssen SA, Cooper AR, Riddoch C, et al. Low cardiorespiratory fitness is a strong predictor for clustering of cardiovascular disease risk factors in children independent of country, age and sex[J]. Eur J Cardiovasc Prev Rehabil,2007, 14(4): 526-531.
23. Adegboye AR, Anderssen SA, Froberg K, et al.Recommended aerobic fitness level for metabolic health in children and adolescents: a study of diagnostic accuracy[J].Br J Sports Med, 2011, 45(9): 722-728.
24. Armstrong N, Williams J, Balding J, et al. そe peak oxygen uptake of British children with reference to age, sex and sexual maturity[J]. Eur J Appl Physiol Occup Physiol, 1991,62(5): 369-375.
25. Eiberg S, Hasselstrom H, Gronfeldt V, et al. Maximum oxygen uptake and objectively measured physical activity in Danish children 6-7 years of age: the Copenhagen school child intervention study[J]. Br J Sports Med, 2005, 39(10):725-730.
26. Kolle E, Steene-Johannessen J, Andersen LB, et al.Objectively assessed physical activity and aerobic fitness in a population-based sample of Norwegian 9- and 15-yearolds[J]. Scand J Med Sci Sports, 2010, 20(1): e41-47.
27. Mamen A, Resaland GK, DA MO, et al. Comparison of peak oxygen uptake in boys exercising on treadmill and cycle ergometers[J]. Gazz Med Ital Arch Sci Med, 2008, 167: 15-21.
28. Armstrong N. Aerobic fitness and physical activity in children[J]. Pediatr Exerc Sci , 2013, 25(4): 548-560.
29. Dencker M, Wollmer P, Karlsson MK, et al. Aerobic capacity related to cardiac size in young children[J]. J Sports Med Phys Fitness, 2013, 53(1): 42-47.
30. Fellmann N, Coudert J, Spielvogel H, et al. Physical fitness of children resident at high altitude in Bolivia[J]. Int J Sports Med , 1992, 13 (Suppl 1): S92-95.
31. Blonc S, Fellmann N, Bedu M, et al. Eあect of altitude and socioeconomic status on VO2maxand anaerobic power in prepubertal Bolivian girls[J]. J Appl Physiol, 1996, 80(6):2002-2008.
32. Obert P, Bedu M, Fellmann N, et al. Effect of chronic hypoxia and socioeconomic status on VO2maxand anaerobic power of Bolivian boys[J]. J Appl Physiol, 1993, 74(2): 888-896.
33. Bianba, Berntsen S, Andersen LB, et al. Exercise capacity and selected physiological factors by ancestry and residential altitude: cross-sectional studies of 9-10-year-old children in Tibet[J]. High Alt Med Biol, 2014, 15(2): 162-169.
34. Fulco CS, Rock PB, Cymerman A. Maximal and submaximal exercise performance at altitude[J]. Aviat Space Environ Med, 1998, 69(8): 793-801.
35. Curran LS, Zhuang J, Droma T, et al. Superior exercise performance in lifelong Tibetan residents of 4 400 m compared with Tibetan residents of 3 658 m[J]. Am J Phys Anthropol, 1998, 105(1): 21-31.
36. Frisancho AR, Martinez C, Velasquez T, et al. Influence of developmental adaptation on aerobic capacity at high altitude[J]. J Appl Physiol, 1973, 34(2): 176-180.
37. de Meer K, Heymans HS, Zijlstra WG. Physical adaptation of children to life at high altitude[J]. Eur J Pediatr, 1995,154(4): 263-272.
38. Curran LS, Zhuang J, Sun SF, et al. Ventilation and hypoxic ventilatory responsiveness in Chinese-Tibetan residents at 3,658 m[J]. J Appl Physiol, 1997, 83(6): 2098-2104.
39. Garruto RM, Chin CT, Weitz CA, et al. Hematological diあerences during growth among Tibetans and Han Chinese born and raised at high altitude in Qinghai, China[J]. Am J Phys Anthropol, 2003, 122(2): 171-183.
40. Moore LG. Comparative human ventilatory adaptation to high altitude[J]. Respir Physiol, 2000, 121(2-3): 257-276.
41. Wu T, Wang X, Wei C, et al. Hemoglobin levels in Qinghai-Tibet: different effects of gender for Tibetans vs Han[J]. J Appl Physiol, 2005, 98(2): 598-604.
42. Simonson TS, McClain DA, Jorde LB, et al. Genetic determinants of Tibetan high-altitude adaptation[J]. Human Genet, 2012, 131(4): 527-533.
43. Stuber T, Scherrer U. Circulatory adaptation to long-term high altitude exposure in Aymaras and Caucasians[J]. Prog Cardiovasc Dis, 2010, 52(6): 534-539.
44. Bigham A, Bauchet M, Pinto D, et al. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data[J]. PLoS Genet, 2010, 6(9):e1001116.
45. Peng Y, Yang Z, Zhang H, et al. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas[J]. Mol Biol Evol, 2011, 28(2): 1075-1081.
46. Simonson TS, Yang Y, Huff CD, et al. Genetic evidence for high-altitude adaptation in Tibet[J]. Science, 2010,329(5987): 72-75.
47. Xiang K, Ouzhuluobu, Peng Y, et al. Identification of a Tibetan-specific mutation in the hypoxic gene EGLN1 and its contribution to high-altitude adaptation[J]. Mol Biol Evol, 2013, 30: 1889-1898.
48. Yi X, Liang Y, Huerta-Sanchez E, et al. Sequencing of 50 human exomes reveals adaptation to high altitude[J].Science, 2010, 329(5987): 75-78.
49. Bigham AW, Wilson MJ, Julian CG, et al. Andean and Tibetan patterns of adaptation to high altitude[J]. Am J Hum Biol, 2013, 25(2): 190-197.
50. Zhuang J, Droma T, Sutton JR, et al. Smaller alveolar-arterial O2gradients in Tibetan than Han residents of Lhasa (3658 m)[J]. Respir Physiol, 1996, 103(1): 75-82.
51. Chen QH, Ge RL, Wang XZ, et al. Exercise performance of Tibetan and Han adolescents at altitudes of 3 417 and 4 300 m[J]. J Appl Physiol, 1997, 83(2): 661-667.
52. Armstrong N, Welsman JR. Assessment and interpretation of aerobic fitness in children and adolescents[J]. Exerc Sport Sci Rev, 1994, 22: 435-476.
53. Cerny FC, Dempsey JA, Reddan WG. Pulmonary gas exchange in nonnative residents of high altitude[J]. J Clin Invest, 1973, 52(12): 2993-2999.
54. DeGraff AC Jr, Grover RF, Johnson RL Jr, et al. Diffusing capacity of the lung in Caucasians native to 3 100 m[J]. J Appl Physiol, 1970, 29(1): 71-76.
55. Guleria JS, Pande JN, Sethi PK, et al. Pulmonary diあusing capacity at high altitude[J]. J Appl Physiol, 1971, 31(4): 536-543.
56. Johnson RL Jr, Cassidy SS, Grover RF, et al. Functional capacities of lungs and thorax in beagles after prolonged residence at 3 100 m[J]. J Appl Physiol, 1985, 59(6): 1773-1782.
57. Sun SF, Droma TS, Zhang JG, et al. Greater maximal O2uptakes and vital capacities in Tibetan than Han residents of Lhasa[J]. Respir Physiol, 1990, 79(2): 151-161.
58. Beall CM, Baker PT, Baker TS, et al. The effects of high altitude on adolescent growth in southern Peruvian Amerindians[J]. Hum Biol, 1977, 49(2): 109-124.
59. Frisancho AR. Human growth and pulmonary function of a high altitude Peruvian Quechua population[J]. Hum Biol,1969, 41(3): 365-379.
60. Frisancho AR, Velasquez T, Sanchez J. Influence of developmental adaptation on lung function at high altitude[J]. Hum Biol, 1973, 45(4): 583-594.
61. Faulkner JA, Kollias J, Favour CB, et al. Maximum aerobic capacity and running performance at altitude[J]. J Appl Physiol, 1968, 24(5): 685-691.
62. Frisancho AR, Borkan GA, Klayman JE. Pattern of growth of lowland and highland Peruvian Quechua of similar genetic composition[J]. Hum Biol, 1975, 47(3): 233-243.
63. Frisancho AR, Matos J, Leonard WR, et al. Developmental and nutritional determinants of pregnancy outcome among teenagers[J]. Am J Phys Anthropol, 1985, 66(3): 247-261.
64. Zhang YB, Wang Y, Wu TY, et al. High altitude diseases[M].Xining: Qinghai people’s publishing house, 1985.
65. Greksa LP, Spielvogel H, Paredes-Fernandez L, et al. The physical growth of urban children at high altitude[J]. Am J Phys Anthropol, 1984, 65(3): 315-322.
66. Powers SK, Lawler J, Dempsey JA, et al. Effects of incomplete pulmonary gas exchange on VO2max[J]. J Appl Physiol, 1989, 66(6): 2491-2495.
67. Frisancho AR, Frisancho HG, Milotich M, et al.Developmental, genetic, and environmental components of aerobic capacity at high altitude[J]. Am J Phys Anthropol,1995, 96(4): 431-442.
68. Dang S, Yan H, Yamamoto S, et al. Poor nutritional status of younger Tibetan children living at high altitudes[J]. Eur J Clin Nutr, 2004, 58(6): 938-946.
69. Harris NS, Crawford PB, Yangzom Y, et al. Nutritional and health status of Tibetan children living at high altitudes[J].N Engl J Med, 2001, 344(5): 341-347.
70. Weitz CA, Garruto RM, Chin CT, et al. Growth of Qinghai Tibetans living at three different high altitudes[J]. Am J Phys Anthropolog, 2000, 111(1): 69-88.
71. Bianba B, Berntsen S, Andersen LB, et al. Estimation of peak oxygen uptake from maximal power output among 9-10 year-old children in Lhasa, Tibet[J]. J Sports Med Phys Fitness, 2010, 50(3): 274-280.
- 中国应用生理学杂志的其它文章
- Hypoxic preconditioning: effect, mechanism and clinical implication (Part I)
- Role of HCN channels in the nervous system: membrane excitability and various modulations
- How to deal with cerebral palsy in 21st century
--A new epoch in clinic treatment - Hematological parameters in high altitude residents:Tibetan natives versus Han migrants
- A rat model of high altitude polycythemia rapidly established by hypobaric hypoxia exposure
- Plasma endothelin-1 and nitric oxide correlate withligustrazine alleviation of pulmonary artery hypertension in patients of chronic cor pulmonale from high altitude plateau during acute exacerbation

