柠檬酸甘薯淀粉对齐口裂腹鱼生长及肠道菌群的影响
陈菲菲,邬应龙*
(四川农业大学食品学院,四川 雅安 625014)
柠檬酸甘薯淀粉对齐口裂腹鱼生长及肠道菌群的影响
陈菲菲,邬应龙*
(四川农业大学食品学院,四川 雅安 625014)
目的:探讨饲料中添加柠檬酸甘薯淀粉(citrate sweet potato starch,CSPS)对齐口裂腹鱼生长及肠道微环境的影响。方法:选用平均体质量为(75.47±5.43) g的齐口裂腹鱼共180 尾,随机分为5 组,每组3 个重复,每个重复12 尾鱼。对照组饲喂基础日粮,实验组分别饲喂添加3.5%、7%、14%及28%的CSPS的日粮,连续饲喂60 d后解剖齐口裂腹鱼,称质量后计算饵料系数、摄食率、特定生长率,取出肠管测定肠道黏膜皱褶高度,并对肠道菌群进行聚合酶链式反应变性梯度凝胶电泳(polymerase chain reaction-denaturing gradient gel electrophoresis,PCR-DGGE)分析。结果:7%及14%的剂量组的末质量、摄食率、特定生长率显著高于对照组(P<0.05);14%、28%剂量组前肠黏膜皱褶高度显著低于对照组(P<0.05)。在中肠,对照组中肠道菌群与3.5%剂量组相似度最高。在前后肠,对照组与其他各剂量组肠道菌群相似度均较低,但50.0≤q<75.0(中度相似)。结论:低剂量(7%)的CSPS能够促进生长,而高剂量(28%)会抑制生长,CSPS能影响齐口裂腹鱼的肠道菌群。
柠檬酸甘薯淀粉酯;齐口裂腹鱼;摄食率;黏膜皱褶高度;肠道菌群
碳水化合物对蛋白质的节约效应是指在饲料中含有适量的碳水化合物能够减少饲料蛋白质的分解供能,从而提高鱼类对蛋白质的利用,起到节约饲料蛋白质的作用。然而饲料中碳水化合物含量过高,会导致鱼的生长受阻,鱼体脂肪含量也会增加[1-3]。1993年由欧洲的抗性淀粉研究行动委员会将抗性淀粉定义为不被健康人体的小肠所消化吸收的淀粉及淀粉降解物的总称[4]。有研究发现抗性淀粉可有效降低血脂和预防脂肪肝的形成,可降低高碳水化合物饲料对鱼生长造成的影响,且适当摄入抗性淀粉可以减少结肠炎的发生,预防结肠癌、便秘等[5-6]。抗性淀粉有益于肠道健康、有益于肠道有益菌的生长,在人类和动物体上均有报道[7-9],但抗性淀粉对鱼类肠道的影响尚未见报道。鱼类肠道的形态发育和肠道菌群既影响营养物质的吸收,又影响肠道的免疫功能。本实验拟通过考察不同剂量的柠檬酸甘薯淀粉(citric sweet potato starch,CSPS)对齐口裂腹鱼(Schizothorax prenanti Tchang)肠道形态发育和肠道菌群的影响,为CSPS应用于鱼饲料的开发提供参考。
1 材料与方法
1.1 材料与试剂
柠檬酸甘薯淀粉 实验室自制(抗性淀粉质量分数45.96%);E.Z.N.A®Stool DNA Kit 美国Omega公司;Tris 美国Sigma 公司;甲叉双丙烯酰胺、丙烯酰胺、去离子甲酰胺、尿素(分析纯) 美国Amresco公司。
1.2 仪器与设备
WH-866涡旋混合器 江苏太仓华美生化仪器厂;5415D高速离心机 德国Eppendorf 公司;聚合酶链式反应(polymerase chain reaction,PCR)仪、变性梯度凝胶电泳(denaturing gradient gel electrophoresis,DGGE)系统 美国Bio-Rad公司;DYY-8C型电泳仪 北京六一仪器厂。
1.3 方法
1.3.1 CSPS的制备及定量测定
参考相关文献[10-14],以甘薯淀粉为原料,制备得到CSPS。
1.3.2 动物分组与管理
齐口裂腹鱼购于雅安芦山雅鱼场。选用平均体质量为(75.47±5.43)g的健康齐口裂腹鱼180尾随机分为5 组,每组设3 个重复,每个重复12 尾。实验组饲料分别在基础饲料中添加质量分数3.5%、7%、14%及28%的CSPS,日粮组成及营养成分见表1[15]。
实验前齐口裂腹鱼先集中驯养2 周,实验开始时,鱼体称质量分组,将鱼的体质量调整至各组间差异不显著(P>0.05),分别饲养在玻璃缸(规格为0.60 m×0.45 m×0.45 m)里。昼夜24 h用增氧机充气。每天早中晚分别饲喂1次,每次换水量约为50%,每天的饲料投喂量为齐口裂腹鱼总体质量的2.5%。实验期间水温保持在20~22 ℃。
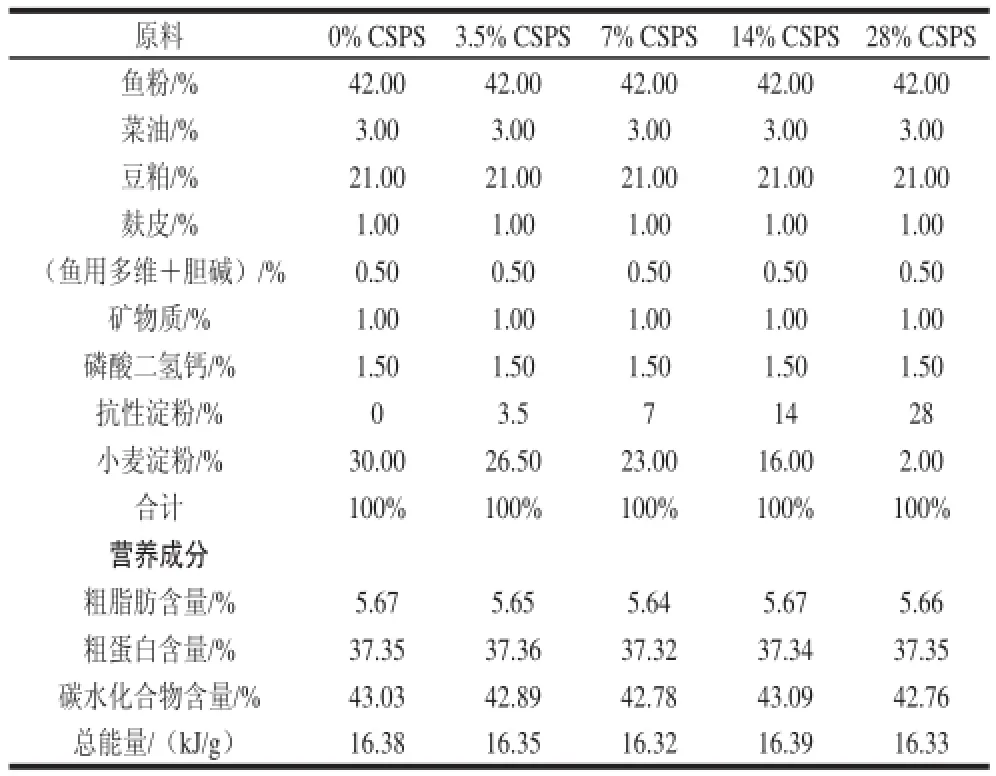
表1 实验饲料配方和化学成分Table 1 Formulation and chemical composition of experimental diets
1.3.3 肠道内容物及肠组织的采集
实验进行至60 d时,对各组齐口裂腹鱼饥饿24 h后,从每组中随机抽取5 尾鱼,按常规方法解剖,称其体长、体质量并记录,之后迅速取出齐口裂腹鱼的前肠、中肠和后肠,将内容物迅速转移至灭菌1.5 mL 离心管中,于-20 ℃保存备用。取各肠段长度约1 cm,用甲醛缓冲液固定、包埋并制作横断面切片,常规苏木精-伊红(hematoxylin and eosin,HE)染色。
1.3.4 肠绒毛的形态观察和测量
在40 倍的Nikon显微镜下观察着色较好的HE染色切片,并应用爱普图像处理分析软件4.0测量肠道黏膜皱褶高度。
1.3.5 肠道微生物DNA的提取
采用美国Omega公司的E.Z.N.A.®Stool DNA Kit试剂盒提取,提取的DNA于-20 ℃保存备用。
1.3.6 肠道微生物16S rDNA的V3区PCR扩增
选取16S rDNA通用引物[16]:上游引物为5’-CGCCCG GGGCGCGCCCCGGGGCGGGGCGGGGGCGCGGGGG GCCTACGGGAGGCAGCAG-3’,携带GC夹板;下游引物为5’-ATTACCGCGGCT GCTGG-3’。采用25 μL PCR反应体系:ddH2O 8.75 μL、2×mix 12.5 μL、上游引物和下游引物各1.25 μL、DNA 模板2.5 μL。PCR 扩增程序为:94 ℃预变性5 min;94 ℃变性30 s、54 ℃退火30 s、72 ℃延伸1 min,39 个循环;72 ℃延伸10 min。产物用2%琼脂糖凝胶电泳检测,用Bio-Rad凝胶成像系统拍照。
1.3.7 DGGE分析
采用Bio-Rad Dcode系统进行DGGE,DGGE条件:凝胶梯度为35%~65%,使用1×TAE 缓冲液,60 ℃、120 V固定电压电泳10 h。采用硝酸银染色并拍照。
1.4 数据处理
根据体长、体质量的数据应用下列公式计算饵料系数及摄食率。

式中:mf为饲料投喂总量/g;mt为实验结束时鱼体总质量/g;m0为实验初始时鱼体总质量/g;m为饲料摄入量/g;t为养殖时间/d。
应用SPSS 16.0统计软件的单因素方差分析进行生物学统计,结果用±s表示,显著性水平确定为0.05。DGGE指纹图谱通过Quantity One 4.6.2软件,采用非加权组平均法(unweighted pair-group method with arithmetic means,UPGMA)进行相似性聚类分析,多样性指数用Shannon-Weiner指数(H’)表示,按照如下公式计算[17]。
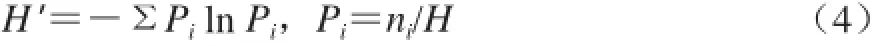
式中:ni为某一条带的峰高;H为泳道密度曲线所有的峰高的总和。
2 结果与分析
2.1 CSPS对齐口裂腹鱼生长指标的影响
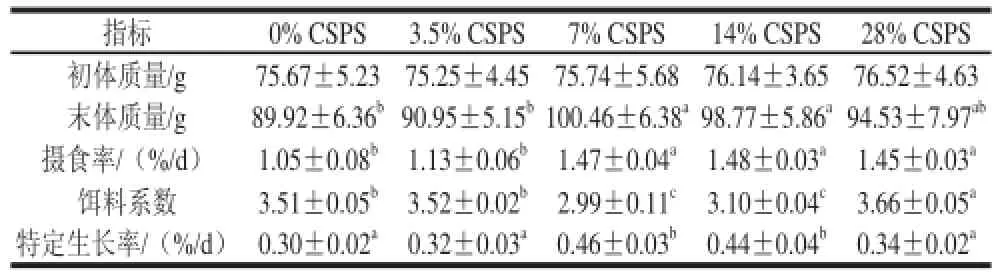
表2 齐口裂腹鱼的生长指标变化Table 2 Effect of dietary supplementation of CSPS on growth indexes of Schizothorax prenanti Tchang
由表2可知,与对照组相比,其他各组的体质量与摄食率均有所增加。末体质量:7%及14% CSPS处理组显著高于对照组(P<0.05),分别增加11.72%及9.84%;摄食率:7%、14%及28% CSPS处理组显著高于对照组(P<0.05),增加了40.00%、40.95%及38.10%;饵料系数:7%及14% CSPS处理组显著低于对照组(P<0.05),减少了17.39%及13.23%;特定生长率:7%及14% CSPS处理组显著高于对照组(P<0.05),增加了53.33%及46.67%。
2.2 CSPS对齐口裂腹鱼肠道黏膜皱褶高度的影响
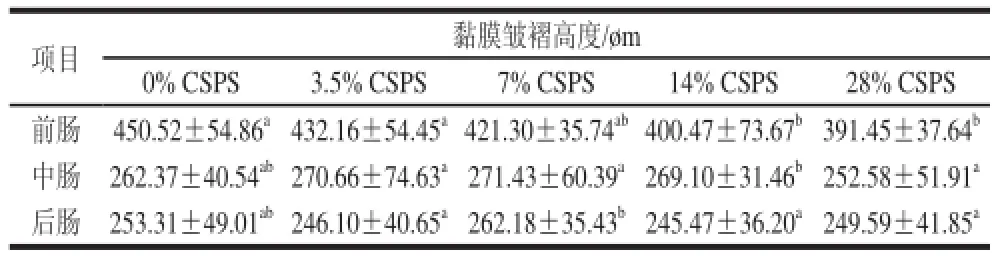
表3 齐口裂腹鱼肠道黏膜皱褶高度变化Table 3 Effect of dietary supplementation of CSPS on plica mucosa height of Schizothorax prenanti Tchang
由表3可知,14%及28%的剂量组前肠黏膜皱褶高度显著低于对照组(P<0.05);添加CSPS的各实验组的齐口裂腹鱼中后肠道黏膜皱褶高度与对照组相比,差异均不显著(P>0.05)。
2.3 CSPS对齐口裂腹鱼肠道菌群的影响
2.3.1 齐口裂腹鱼肠道菌群16S rDNA的V3区PCR扩增结果
以提取的微生物DNA为模板,进行PCR扩增,产物通过2%琼脂糖凝胶检测后发现,均能得到约200 bp的目的产物,且条带清晰明亮,满足DGGE分析的要求,如图1所示。

图1 齐口裂腹鱼肠道菌群16S rDNA V3区扩增结果Fig.1 16S rDNA V3 region amplification of intestinal flora in Schizothorax prenanti Tchang
2.3.2 齐口裂腹鱼肠道菌群PCR-DGGE指纹图谱的建立与分析

图2 齐口裂腹鱼肠道菌群16S rDNA V3区段扩增产物的DGGE图谱Fig.2 DGGE profile of 16S rDNA amplified products of intestinal flora in Schizothorax prenanti Tchang

图3 齐口裂腹鱼肠道菌群的PCR-DGGE图谱的聚类分析Fig.3 Cluster analysis of PCR-DGGE profiles of intestinal flora in Schizothorax prenanti Tchang

表4 齐口裂腹鱼前肠肠道菌群的PCR-DGGE图谱的相似系数Table 4 Similarity coefficients of PCR-DGGE profiles of foregut flora iinn Schizothorax prenanti Tchang

表5 齐口裂腹鱼中肠肠道菌群的PCR-DGGE图谱的相似系数Table 5 Similarity coefficients of PCR-DGGE profiles of midgut flora iinn Schizothorax prenanti Tchang

表6 齐口裂腹鱼后肠肠道菌群的PCR-DGGE图谱的相似系数Table 6 Similarity coefficients of PCR-DGGE profiles of hindgut flora in Schizothorax prenanti Tchang
齐口裂腹鱼肠道菌群16S rDNA V3区片段的DGGE图谱的建立及其聚类分析和相似系数见图2、3。由参考文献[18]可知,当0<q(相似系数,下同)<25.0时,极不相似;当25.0≤q<50.0时,中等不相似;当50.0≤q<75.0时,中等相似;当75.0≤q<100.0时,极相似。对前、中、后肠菌群聚类分析结果如图3和表4~6所示。前肠中,14%与28%剂量组相似度最高,为75.5,被聚为一类,而对照组与其他组相似度最低,为50.3。在中肠,3.5%剂量组与对照组相似度最高,为76.5,被聚为一类,7%剂量组与其他组相似度最低,为55.5。后肠中,3.5%与7%的剂量组相似度最高,而28%剂量组与其他组相似度最低,为55.0。这说明高剂量的CSPS会显著改变齐口裂腹鱼的肠道菌群结构。
3 讨论与结论
肠道是机体消化、吸收营养物质的重要场所,肠绒毛作为肠道的重要组成部分,在吸收营养物质上至关重要。Caspary[19]报道指出肠绒毛高度增加,会使肠道接触营养物质的面积增大,从而有利于肠道对营养物质的吸收,所以肠绒毛的形态直接与生长发育有关。本实验结果表明:与对照组相比,添加了CSPS的实验组,摄食率上升;CSPS的添加对齐口裂腹鱼肠道结构有局部损伤。实验组中齐口裂腹鱼的前肠黏膜皱褶高度有明显降低。已有研究表明,鱼类可以分辨不同饲料中常规营养素和微营养素的组成差异[20]。鱼类需要摄食足够的必需营养物质,以满足其能量需求[21],本实验中,CSPS剂量组可能因为肠黏膜对营养物质的吸收差而必须增加摄食量来满足对营养物质的需要,以致摄食率上升,体质量增加。所以一定剂量的CSPS能有效提高鱼的摄食率,反而促进了生长。而过高剂量的抗性淀粉,会影响肠道消化酶的活性[22],同时会增加肠道的排空速度,所以肠道绒毛有局部损伤。
肠道微生物种类多,有一部分不能分离培养。因此,传统培养法不能反映出菌群多样性。变性梯度凝胶电泳技术是采用细菌通用引物扩增菌群总DNA的16S rDNA可变区,通过分析其核酸序列多样性而获知菌群多样性情况,比传统培养法更能获得丰富的菌群信息。本实验中,前肠菌群聚类分析表明:对照组与其他组相似度最低,这表明了在齐口裂腹鱼饲料中添加CSPS会对其前肠菌群结构和种类有所影响;14%与28%剂量组相似度最高,聚为一类。这可能是当剂量达到14%以上,出现一个明显的影响。CSPS做为一种抗性淀粉,虽然有报道指出其低剂量利于有益菌的生长[23-24],但过高的剂量可能会影响肠道健康进而对肠道菌群产生不良影响。而在中肠,3.5%剂量组与对照组相似度最高,被聚为一类,而7%剂量组与其他组相似度最低。这表明3.5%剂量组和对照组菌群更接近,7%的剂量组的中肠菌群可能与其他各组存在着很大不同。这可能是7%剂量组中CSPS促进乳酸菌、双歧杆菌等有益菌[25-26]的生长达到一个显著水平。后肠中,3.5%与7%的剂量组相似度最高,这表明低剂量的CSPS促进了后肠有益菌的生长,而3.5%剂量组也达到了显著水平。因此可以推测,在齐口裂腹鱼饲料中添加CSPS会对肠道菌群产生影响;低剂量(3.5%、7%)的CSPS可能会有利于有益菌的生长,但高剂量的反而产生不良影响。
综上所述,添加CSPS实验组的鱼由于吸收差而代偿性的增加了摄食量,致使鱼体质量增加,即促进了鱼的生长。也正是由于鱼体质量增加,其消化酶活性也增加,从而加快肠道的排空速度,所以肠道绒毛有局部损伤。CSPS具体如何改变齐口裂腹鱼的肠道菌群还有待进一步研究。
[1] DIAS J S J. Regulation of hepatic lipogenesis by dietary protein/ energy in juvenile European sea bass (Dicentrarchus labrax)[J]. Aquaculture, 1998, 161: 169-186.
[2] GRISDALE-HELLAND B, HELLAND S J. Macronutrient utilization by Atlantic halibut (Hippoglossus hippoglossus): diet digestibility and growth of 1 kg fish[J]. Aquaculture, 1998, 166: 57-65.
[3] 田丽霞, 刘永坚, 冯健, 等. 不同种类淀粉对草鱼生长、肠系膜脂肪沉积和鱼体组成的影响[J]. 水产学报, 2002, 26(3): 247-251.
[4] ESCARPA A, GONZALEZ M C, MORALES M D. An approach to the influence of nutrients and other food constituents on resistant starch formation[J]. Food Chemistry, 1997, 60(4): 527-532.
[5] 付蕾, 田纪春. 抗性淀粉制备、生理功能和应用研究进展[J]. 中国粮油学报, 2008, 23(2): 51-54.
[6] SUZUKI T, YOSHIDA S, HARA H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability[J]. British Journal of Nutrition, 2008, 100(2): 297-305.
[7] 李瑞, 廖振林, 方祥, 等. 抗性淀粉对HFA 小鼠肠道菌群的影响[J].中国微生态学杂志, 2013, 25(7): 762-765.
[8] HAENEN D, da SILVA C S, ZHANG Jing, et al. Resistant starch induces catabolic but suppresses immune and cell division pathways and changes the microbiome in proximal colon of male pigs[J]. Journal of Nutrition, 2013, 143(11): 123-129.
[9] MAKI K C, PELKMAN C L, FINOCCHIAROET, et al. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obesemen[J]. Nutrition and Disease, 2012, 142(4): 717-723.
[10] RENATA B, KAMILA J, KATARZYNA S. The effect of citric acidmodified enzyme-resistant dextrin on growth and metabolism of selected strains of probiotic and other intestinal bacteria[J]. Journal of Functional Foods, 2010(2): 126-133.
[11] SANG I S, CHANG J L, KIM D I, et al. Formation, characterization, and glucose response in mice to rice starch with low digestibility produced by citric acid treatment[J]. Journal of Cereal Science, 2007, 45: 24-33.
[12] CHANG J L, SANG I S. Structural characteristics of low-glycemic response rice starch produced by citric acid treatment[J]. Carbohydrate Ploymers, 2009, 78(3): 588-595.
[13] MA Xiaofei, CHANG P R, YU Jiugao, et al. Properties of biodegradable citric acid-modified granular starch/thermoplastic pea starch composites[J]. Carbohydrate Polymers, 2009, 75: 1-8.
[14] 于密军. 柠檬酸改性豌豆淀粉的研究[D]. 天津: 天津大学, 2008: 19-27.
[15] 段彪, 向袅, 周兴华, 等. 齐口裂腹鱼饲料中适宜脂肪需要量的研究[J].动物营养学报, 2007, 19(3): 232-236.
[16] LIU Huaide, WANG Lei, LIU Mei, et al. The intestinal microbial diversity in Chinese shrimp (Fenneropenaeus chinensis) as determined by PCR-DGGE and clone library[J]. Aquaculture, 2011, 317: 32-36.
[17] VANCE J M, JOYCE M S, RODERICK I M, et al. Molecular ecological analysis of dietary and antibiotic-induced alterations of the mouse intestinal microbiota[J]. Journal of Nutrition, 2001, 131(6): 1862-1870.
[18] PANGLOLI P, HUNG Y C, BEUCHAT L R, et al. Reduction of Escherichia coli O157:H7 on produce by use of electrolyzed water under simulated food service operation conditions[J]. Journal of Food Protection, 2009, 72(9): 1854-1861.
[19] CASPARY W F. Physiology and pathophysiology of intestinal absorption[J]. American Journal of Clinical Nutrition, 1992, 55: 299-308.
[20] ARANDA A, SANCHEZ-VAZQUEZ F J, MADRID J A. Effect of short-term fasting on macronutrient self-selection in sea bass[J]. Physiology and Behavior, 2001, 73: 105-109.
[21] SANCHEZ-VAZQUEZ F J, YAMAMOTO T, AKIYAMA T, et al. Macronutrient self-selection through demand-feeders in rainbow trout[J]. Physiology and Behavior, 1999, 66: 45-51.
[22] 黄瑾, 熊邦喜, 陈洁, 等. 鱼类消化酶活性与体长、体重和水质的相关性研究[J]. 水生态学杂志, 2012, 33(2): 121-126.
[23] 何梅, 洪洁, 杨月欣, 等. 抗性淀粉对大鼠肠道菌群的影响[J]. 卫生研究, 2005, 34(1): 85-87.
[24] 戴求仲, 刘绍伟, 李湘, 等. 饲粮直/支链淀粉比对黄羽肉鸡血液生化指标和后肠微生物菌群的影响[J]. 动物营养学报, 2010, 22(4): 904-910.
[25] 马相杰, 汪立平, 赵勇, 等. 甘露寡糖对罗非鱼幼鱼肠道微生物的影响[J]. 微生物学通报, 2010, 37(5): 708-713.
[26] 李卫芬, 沈涛, 陈南南, 等. 饲料中添加枯草芽孢杆菌对草鱼消化酶活性和肠道菌群的影响[J]. 大连海洋大学学报, 2012, 27(3): 221-225.
Abstract: Xiaguan Tuocha is a functional drink and known to possess some health properties. In order to identify the chemical components and investigate preventive effects of Xiaguan raw (unfermented) Tuocha (XRT) on gastric injury in vivo, liquid chromatography-mass spectrometry (LC-MS) and cytokine analysis were used in this study, and the gastric secretion and pH of gastric juice were also tested. Twelve components were found in XRT, all of which contributed to the preventive effect on gastric injury. XRT at higher concentration reduced the levels of serum proinflammatory cytokines such as IL-6 and TNF-α compared with at lower concentration. The 1 000 mg/kg XRT showed the best inhibitory effect on gastric injury (78.7% inhibitory rate). The 1 000 mg/kg XRT treated rats also exhibited similarly low gastric secretion (2.0 mL) and higher pH of gastric juice (pH 3.1) compared with normal rats. Xiaguan Tuocha contained many functional components and exerted a strong preventive effect on gastric injury.
Key words: Tuocha; gastric injury; cytokine; rat
Effect of RS4-Type Sweet Potato Resistant Starch on Growth and Intestinal Microenvironment of Schizothorax prenanti Tchang
CHEN Fei-fei, WU Ying-long*
(College of Food Science, Sichuan Agricultural University, Ya’an 625014, China)
Objective: To investigate the effect of dietary supplementation of citrate sweet potato starch (CSPS), prepared using esterification with citric acid, on the growth and intestinal microenvironment of Schizothorax prenanti Tchang. Methods: Totally 180 fish with an average body weight of (75.47 ± 5.43) g were randomly divided into five groups of 12 fish in each group, with three replicates for each group. Control group was fed a basal diet, and three test groups were fed a diet supplemented with CSPS at doses of 3.5%, 7%, 14% and 28%, respectively. Sixty days later, all fish were weighed and dissected, and feed conversion rate, feeding rate and specific growth rate were calculated. After removing the intestine, the intestinal mucosa height was measured. The intestinal flora was analyzed by PCR-DGGE. Results: Compared with the control group, final body weight, feeding rate and specific growth rate were significantly increased in 7% and 14% CSPS groups (P < 0.05). Foregut plica mucosa height was significantly decreased in 14% and 28% CSPS groups (P < 0.05). As for the midgut, the intestinal flora in 3.5% CSPS group was more similar to in the control group. The foregut and hindgut intestinal flora revealed an extremely significant difference between the control group and three test groups, despite showing 50.0 ≤ q < 75.0 (moderate similar). Conclusion: The growth of Schizothorax prenanti Tchang is promoted by dietary supplementation of CSPS at a low dose (7%) but inhibited at a high dose (28%). At the same time, CSPS can affect the intestinal flora of Schizothorax prenanti Tchang.
citrate sweet potato starch; Schizothorax prenanti Tchang; food intake rate; plica mucosa height; intestinal flora
Chemical Composition of Xiaguan Raw Tuocha and Its Preventive Activity on HCl/Ethanol-Induced Gastric Injury in SD Rats
WANG Rui, ZHAO Xin*
(Department of Biological and Chemical Engineering, Chongqing University of Education, Chongqing 400067, China)
TS254
A
1002-6630(2014)13-0266-05
10.7506/spkx1002-6630-201413053
2013-10-10
四川农业大学“211”工程双支计划项目(2010)
陈菲菲(1988—),女,硕士研究生,研究方向为功能性食品。E-mail:672394220@qq.com
*通信作者:邬应龙(1963—),男,教授,博士,研究方向为功能性食品。E-mail:wuyinglong99@163.com

