秀丽莓茎中酚类成分研究
刁玉林,热增才旦,2,王如峰,刘 斌*
1北京中医药大学中药学院,北京 100102;2 青海大学医学院中藏药研究中心,西宁 810001
Introduction
Rubus,belonging to Rosaceae,is a large genus with more than 750 known plants distributed all over the world and mainly concentrated in North America and East Asia.More than 200 different plants of this genus grow in the mainland of China,especially in southwestern China[1].Modern pharmacological study showed its antibacterial[2],anti-inflammatory[3],anti-neoplastic[4],anti-allergic[5],anti-oxidative[6],anti-aging,free radical scavenging[7],hepatic protective[8],analgesic[9],and hypolipidemic activities[10].The shortage of further chemical basis research restricted the development of this plant.The present article described the isolation and structural elucidation of a new natural product named 4-acetoxy-3-methoxybenzoic acid (1),as well as 7 known phenol derivatives,viz,2-(β-D-glucopyranosyloxy)-6-hydroxybenzoic acid (2),vanillic acid (3),resveratrol (4),2,6-dimethoxy-4-hydroxyphenol-1-O-β-D-glucopyranoside (5),trans-4-hydroxy cinnamic acid (6),caffeic acid (7)firstly obtained from the stems of R.amabilis and gallic acid (8).(Fig.1)
Materials and Methods
General experimental procedures

Fig.1 Chemical structures of phenol constituents (1-8)isolated from the stems of R.amabilis
UV spectra were obtained on a UV 210A Shimadzu spectrometer.1H and13C NMR spectra were recorded in deuterated solvent with Bruker DRX-500 spectrometers operating at 500 MHz for1H NMR experiments,and 125 MHz for13C NMR experiment,respectively.Coupling constants were expressed in Hertz (Hz)and chemical shifts were given on a δ (ppm)scale with tetramethylsilane as internal standard.Negative ion ESI-MS and HR-ESI-MS were recorded on a VG-20-250 and VG-ZAB-HS spectrometer (VG,Manchester,UK).Column chromatography separations were performed using Diaion HP 2MGL (Mitsubishi Chem,Beijing,China),AB-8 (The Chemical Plant of NanKai University,Tianjin,China)and Silica gel (Qingdao Haiyang Chemical Co.,Qingdao,China)as stationary phase.TLC was carried on silica gel G precoated plates(Qingdao Haiyang Chemical Co.,Qingdao,China).The TLC plate was monitored by spraying with 10%H2SO4solution in ethanol followed by heating.
Fungal material
The dried stems of R.amabilis were collected from Huzhubeishan in Qinghai,China and identified by Prof.Chunsheng Liu (Beijing University of Chinese Medicines).An authentic sample was kept in School of Chinese Pharmacy,Beijing University of Chinese Medicines.
Extraction and isolation
The dried stems of R.amabilis (10.0 and 15.0 Kg)were powdered and extracted exhaustively with 70%EtOH and water,respectively,under reflux.The extract(extracted with 70% EtOH)was concentrated to the small volume (1 g crude herbal per mL),and applied on extraction with petroleum ether,chloroform,ethyl acetate,n-butanol and water.The ethyl acetate fraction was concentrated under reduced pressure,and the residue (200 g)was subjected to column chromatography(CC,20 ×100 cm)on macroporous adsorption resin gradient eluted with CHCl3-MeOH to obtain two fractions (Fraction 1 ∶CHCl3-MeOH=45 ∶55-30 ∶70 and Fraction 2∶CHCl3-MeOH=25∶75-10∶90).Fraction 1,was further fractionated on silica gel gradient eluted with CHCl3-MeOH-H2O (9∶1∶0.1,8.5∶1.5∶0.1,8∶2∶0.2,7.5∶2.5∶0.3,7∶3∶0.5,6.5∶3.5∶0.8,6∶4∶1 and 5∶5 ∶1),and ODS eluted with a step gradient of H2O-MeOH (1∶0-0∶1)to give 2(10 mg)and 5(10 mg).Fraction 2 was fractionated repeatedly on silica gel gradient eluted with CHCl3-CH3OH (10∶1-0∶1),to obtain 4(20 mg)from Fr 2.The residue (300 g)extracted with water and fractioned with ethyl acetate was subjected was subjected to CC (10 ×150cm)on silica gel (CHCl3-MeOH,1∶0-0∶1),and recrystallized to obtain 1(20 mg),3(50 mg),6 (40 mg),7 (15mg)and 8(37 mg).
Structural identification
4-acetoxy-3-methoxybenzoic acid (1)was obtained as a pale white crystal (MeOH).Bromocresol green reaction showed yellow points in TLC indicated carboxyl contained in the structure.UV (MeOH)λmax (log ε):246sh (2.83)nm.Its mass spectrum showed a molecular ion peak at m/z 211([M +H]+)consistent with the molecular formula C10H10O5elucidated from1H NMR,13C NMR,HSQC and HMBC results.The1H NMR spectrum of 1 displayed characteristic signals for a substituted aromatic ring in the form of ABX system at δ 7.44 (1H,d,J=1.5 Hz,H-2),7.34 (1H,dd,J=8.0,1.5 Hz,H-6)and 6.72 (1H,d,J=7.8 Hz,H-5).Two three-proton signals as a singlet at δ 1.76 (3H,s)and 3.50 (3H,s)were attributed to the protons of methyl and methoxyl,respectively.The13C NMR spectrum of 1 exhibited signals for a aromatic ring at δC113.3 (C-2),114.2 (C-5),122.5(C-6),129.5 (C-1),146.4 (C-3)and 148.3 (C-4).The carbonyl signals appeared at δC170.0 and 173.8,respectively,and one of them might be assigned to an ester carbonyl group.In addition,the signals at δC23.4 and 55.4 were assigned to -CH3and -OCH3carbons,respectively.Based on above evidence,two possible similar structures could be deduced as shown in Fig.2.

Fig.2 The possible structures of compound 1:(a)4-acetoxy-3-methoxybenzoic acid;(b)3-acetoxy-4-methoxybenzoic acid.
The HMBC spectrum of 1 showed correlations of C-4 with δH7.44,7.34 and 6.72,and -OCH3with δC146.4 and 148.3.This demonstrated that the structure of this compound should be structure a because there would be no correlation of δH7.34 (H-6)with δC148.3 observed in structure b.This structure was further confirmed by the stronger correlation signal of δH3.75 (-OCH3)with δC146.4 (Fig.3).Additionally,the isolation of compound 3 could further prove the given structure.Finally,the spectra data were given in Table 1.

Table 1 1H NMR(500 MHz,DMSO-d6),13 C NMR (125 MHz,DMSO-d6)* and HMBC data of compound 1
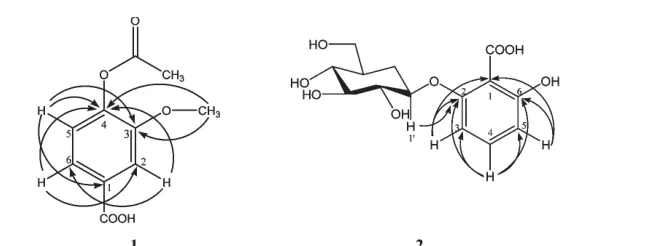
Fig.3 Selective HMBC correlations for 1 and 2 (H→C)
2-(β-D-glucopyranosyloxy)-6-hydroxybenzoic acid(2)was obtained as yellowish powder (MeOH)with positive FeCl3and Molish reaction results,which indicated this compound may be a phenolic glycoside.The ESI-MS (m/z 315[M-H]-,653[2M+Na]-)in combination with HR-ESI-MS (m/z):315.0714[M-H]-,calcd 315.0716 revealed a molecular formula C14H18O8,suggesting the presence of six degrees of unsaturation.In the1H NMR spectrum of 2,three downfield peaks at δH6.64 (1H,d,J=7.8 Hz),7.07 (1H,t,J=7.8 Hz)and 6.43 (1H,d,J=7.8 Hz)were attributed to H-3,H-4 and H-5 in an ABC spin coupling system,respectively.One single-proton signal at δ 4.43 with a coupling constant of 7.2 Hz showed the existence of a β oriented glucosyl group.Five multiplets at δH3.25-3.74 were assignable to the glucosyl group.
The13C NMR spectrum of 2 exhibited the presence of aromatic carbons at δC112.0 (C-1),160.0 (C-2),108.9 (C-3),131.3 (C-4),112.1 (C-5)and 164.0(C-6),carbonyl carbon at δC170.9 (C=O),anomeric carbon of the glucosyl group at δC106.5 (C-1')and the remaining five carbons of glucosyl group at between δC61.5 and 78.0.The HMBC spectrum of 2 showed correlations of H-1' with δC160.0,78.0 and 76.3,which indicated that the glucosyl group linked the carbon at δC160.0 of the aromatic ring to form glycoside.The HMBC correlations showed the linkages as illustrated in Fig.3.
The1H-1H COSY spectrum of 2 showed two sets of optional spin coupling proton correlation signals (δH4-H/3-H and 4-H/5-H)in the aromatic ring,which confirmed the structure given above.Complete1H and13C NMR assignments were accomplished by a combination of 2D NMR techniques,including HMQC,HMBC,and1H-1H COSY.
The spectra data were given as follows:1H NMR (in DMSO-d6,500 MHz)δ:6.64 (1H,d,J=7.8 Hz,H-3),6.43 (1H,d,J=7.8 Hz,H-5),7.07 (1H,t,J=7.8 Hz,H-4),4.43 (1H,d,J=7.2 Hz,H-1'),3.25(1H,m,H-2'),3.51 (1H,m,H-3'),3.39 (1H,m,H-4'),3.49 (1H,m,H-5'),3.74,3.65 (2H,m,H-6').13C NMR (in DMSO-d6,125 MHz)δ:112.0 (C-1),160.0 (C-2),108.9 (C-3),131.3 (C-4),112.1(C-5),164.0 (C-6),170.9 (C=O),106.5 (C-1'),73.9 (C-2'),76.4 (C-3'),70.4 (C-4'),78.0 (C-5'),61.5 (C-6').ESI-MS (m/z)(negative mode):315[M-H]-,653 [2M+Na]-.HR-ESI-MS:m/z 315.0714 [M-H]-(calculated for C14H18O8,315.0716).
Vanillic acid (3)was obtained as colourless needle crystal (MeOH).1H NMR (CD3OD,500 MHz)δ:7.56 (1H,brs,H-2),7.54 (1H,d,J=8.8 Hz,H-6),6.82 (1H,d,J=8.8 Hz,H-5),3.88 (3H,s,-OCH3);13C NMR (CD3OD,125 MHz)δ:123.1 (C-1),113.9 (C-2),152.7 (C-3),148.7 (C-4),115.8(C-5),125.3 (C-6),170.0 (C=O),56.4 (-OCH3).
Resveratrol (4)was obtained as white crystal(MeOH).1H NMR (500 MHz,CD3OD)δ:7.34(2H,d,J=8.4 Hz,H-2',H-6'),6.96 (1H,d,J=16.2 Hz,H-α),6.80 (1H,d,J=16.2 Hz,H-β),6.76 (2H,d,J=8.4 Hz,H-3',H-5'),6.43 (2H,d,J=1.8 Hz,H-2,H-6),6.15 (1H,t,J=2.1 Hz,H-4);13C NMR (125 MHz,CD3OD)δ:138.6 (C-1),103.1 (C-2,6),157.0 (C-3,5),100.0 (C-4),127.7 (C-1'),126.1 (C-2',6'),113.9 (C-3',5'),155.8 (C-4'),124.3 (C-α),126.7 (C-β).ESI-MS m/z 227[M-H]-.
2,6-dimethoxy-4-hydroxyphenol-1-O-β-D-glucopyranoside (5)was obtained as white powder (MeOH)with positive FeCl3and Molish reaction.1H NMR (500 MHz,CD3OD)δ:3.69 (6H,s,2 ×-OCH3),4.43(1H,d,J=9.0 Hz,H-1'),6.13 (2H,s,H-3,5);13C NMR(125 MHz,CD3OD)δ:129.3 (C-1),154.7 (C-2,6),94.6 (C-3,5),156.3 (C-4),106.2 (C-1'),75.7 (C-2'),77.8 (C-3'),71.4 (C-4'),78.2(C-5'),62.7(C-6'),56.8 (2 ×-OCH3).ESI-MS m/z 331[M-H]-.
Trans-4-hydroxy cinnamic acid (6)was obtained as white crystal (acetone)with positive FeCl3and bromophenol blue reaction results and mp 210~212℃.1H NMR(acetone-d6,500 MHz)δ:7.55 (2H,d,J=8.5 Hz,H-2,6),6.90 (2H,d,J=8.5 Hz,H-3,5),7.61 (1H,d,J=16.0 Hz,H-β),6.33 (1H,d,J=16.0 Hz,H-α);13C NMR (acetone-d6,125 MHz)δ:126.8 (C-1),130.6 (C-2,6),116.4 (C-3,5),160.2 (C-4),115.5 (C-α),145.3 (C-β),167.9 (C=O of COOH).
Caffeic acid (7)was obtained as yellow cubic crystal (MeOH)with mp 223-225 ℃.1H NMR (500 MHz,DMSO-d6)δ:7.40 (1H,d,J=16 Hz,H-7),7.01 (1H,d,J=1.5 Hz,H-2),6.95 (1H,dd,J=8.0,1.5 Hz,H-6),6.75 (1H,d,J=8.0 Hz,H-5),6.16 (1H,d,J=16 Hz,H-8);13C NMR (125 MHz,DMSO-d6)δ:125.7 (C-1),114.6 (C-2),144.6 (C-3),148.1 (C-4),115.1 (C-5),121.1 (C-6),145.6(C-7),115.8 (C-8),167.9 (C-9).
Gallic acid (8)was obtained as colourless needle crystal (CHCl3-MeOH)with mp 235-238 ℃.Positive FeCl3-K3Fe(CN)6and bromocresol green reaction results showed the existence of phenolic hydroxyl group and carboxyl group.The same Rfvalues were obtained with gallic acid reference substance in different solvent systems.1H NMR (500 MHz,CD3OD)δ:7.05 (2H,s,H-2,6);13C NMR (125 MHz,CD3OD)δ:121.9(C-1),110.3 (C-2,6),146.3 (C-3,5),139.5 (C-4),170.4 (C=O of COOH).
1 Chinese Herbal Editorial Committee.Flora of China.Beijing:Beijing Science and Technology Press,1985.Vol 37:210-218.
2 Puupponen PR,Nohynek L,Alakomi HL,et al.The action of berry phenolics against human intestinal pathogens.BioFactors,2005,23:243-251.
3 Choi J,Lee KT,Ha J,et al.Antinociceptive and antiinflammatory effects of Niga-ichigoside F1and 23-hydroxytormentic acid obtained from Rubus coreanus.Biol Pharm Bull,2003,26:1436-1441.
4 Lee JH,Ham YA,Choi SH,et al.Activity of crude extract of Rubus crataegifolius roots as a potent apoptosis inducer and DNA topoisomerase I inhibitor.Arch Pharmacol Res,2000,23:338-343.
5 Moon PD,Choi IY,Na HJ,et al.Rubus croceacanthus Leveille inhibits mast cell-mediated anaphylactic-like reaction and tumor necrosis factor-α secretion.Biol Pharm Bull,2004,27:1359-1363.
6 Beekwilder J,Jonker H,Meesters P,et al.Antioxidants in raspberry:on-line analysis links antioxidant activity to a diversity of individual metabolites.Agric Food Chem,2005,53:3313-3320.
7 Ju HK,Cho EJ,Jang MH,et al.Characterization of increased phenolic compounds from fermented Bokbunja (Rubus coreanus Miq.)and related antioxidant activity.J Pharm BiomedAnal,2009,49:820-827.
8 Badr AM,Ebtehal EI-D,Khalifa AE,et al.Rubus sanctus protects against carbon tetrachloride-induced toxicity in rat isolated hepatocytes:isolation and characterization of its galloylated flavonoids.Pharm Pharmacol,2009,61:1511-1520.
9 Márcia K,Danúbia S,Eliza SB,et al.Phytochemical and analgesic activity of extract,fractions and a 19-hydroxyursanetype triterpenoid obtained from Rubus rosaefolius (Rosaceae).Biol Pharm Bull,2007,30:999-1002.
10 Morimoto C,Satoh Y,Hara M,et al.Anti-obese action of raspberry ketone.Life Sci,2005,77:194-204.

