热分析-红外联用研究京尼平、京尼平苷的熔点、热分解机理及其动力学分析
肖卓炳 ,郭满满,郭瑞轲
吉首大学林产化工工程湖南省重点实验室,张家界427000
Introduction
Genipin and geniposide are two natural iridoids with a special structure of pyran combined with cyclopentane[1].Geniposide can be hydrolyzed to genipin,hence genipin plays the main form of efficacy with intestinal metabolism of geniposide[1,2]. Genipin,in the form of white crystalline powder,is easily dissolved in methanol,alcohol,ethyl acetate,diethyl ether and acetone but only slightly soluble in water. Its molecular formula is C11H14O5and relative molecular mass is 226.08.As a new drug intermediate,a variety of studies have shown that genipin has many important pharmacological activities such as cholagogue,hepatic protection,anti-inflammatory,anti-thrombosis,anti-tumor,antibiosis,treating gastritis and diabetes,inhibiting nerve toxicity,anti-depression[2-4].In addition,it has been also used as a biological agent crosslinked with other active ingredients,which promoted active ingredients controlled release and efficient transportation in vivo[5]. Because of good biocompatibility,anti-degradation and extensive application prospect,it is concerned with much interest.
The natural genipin combined with glucose occurs as glycoside,particularly soluble in water,of which the name is geniposide and the molecular formula is C17H24O10.As an important plant secondary metabolite,in addition to anti-anxiety,protection of nervous system and anti-tumor[6],there are also other extensive biological activities,such as protecting choleretic,hepatic and pancreatic tissue,regulating gastrointestinal functions,protecting endothelial cells from arteriosclerosis,improving cerebral ischemia,relieving pain and anti-inflammation,regulating blood sugar levels[6,7].
Considering the important pharmacological activities of these two natural products,they have highly clinical development prospects and are increasingly used.However,thermal stability and decomposition kinetics of these two compounds are less studied. Hence in this work,the melting points,thermal stability and decomposition kinetic mechanism of genipin and geniposide were studied by non-isothermal analysis kinetics methods and TG-IR combined technology,which provided important references for the technological parameters of extraction,processing and development of genipin and geniposide.
Experimental
Samples
Genipin and geniposide were both standards from Xi'an feida bio-tech Co.,Ltd.
Instruments
Differential scanning calorimetry analyzer (DSC 200 F3 Maia from Germany-NETZSCH);Thermo gravimetric analyzer (TGA/SDTA851e from Mettler-Toledo of Switzerland);TG-IR Analyzer (STA6000 and Spectrum100 from American PerkinElmer).
Thermal analysis
In this work the differential scanning calorimetry(DSC)analysis were used to study the melting points and purity of genipin and geniposide.The more impure substance in standards,the lower the melting point was and the wider DSC melting peak was.According to the manual of DSC analyzer,the sensitivity and temperature were calibrated with highly pure In,Sn,Zn,Bi and CsCl.On the thermal analysis for melting point and purity[8],firstly,3-5 mg samples were heated at 10 ℃/min from room temperature to the temperature below the melting point about -10-20 ℃while after one minute at the constant temperature they were heated to the temperature above the melting point about 20-30 ℃at 0.5 ℃/min.The analysis was under highly pure nitrogen atmosphere with the flow rate of 50 mL/min as the protective gas and 20 mL/min as sweep gas while the crucible made of Al was used as thermo reference material and container.
Thermal decomposition was carried out by the thermo gravimetric analyzer.8-10 mg samples were heated from room temperature to 700 ℃at 10 ℃/min under highly pure nitrogen atmosphere with the flow rate of 50 mL/min as the protective gas.The ceramic crucible made of Al2O3was used as the container.
TG-IR analysis
The evolved gas of thermal decomposition was tested by TG-IR combined technology.10 mg samples were heated from room temperature to 700 ℃at 10 ℃/min under nitrogen atmosphere with the flow rate of 50 mL/min as the protective gas.Then the evolved gases along a gas transmission line were guided into the gas detection cavity of infrared spectrometer while they were detected from 4000 cm-1to 450 cm-1.
Calculation for melting point and purity
Linear relationship between the temperatures (Ts)in DSC melting curve and the inverses of relative DSC peak area (1/F)was obtained using Van't Hoff Equation[8].

where T0is the melting point of pure substance,R is the gas constant,ΔHfis the enthalpy of melting.
According to the DSC curve,by substituting Tsand 1/F into Eq. (1),the linear correlation coefficient r,the impurity content x and the melting point of pure substance were obtained by the linear least square method with Tsvs 1/F.
Methodology of thermal analysis kinetics
Coats-Redfern method[9]and Achar method[10]were used to computationally process the data of TG and DTG,which were thermal analysis kinetics methods and shown as next.
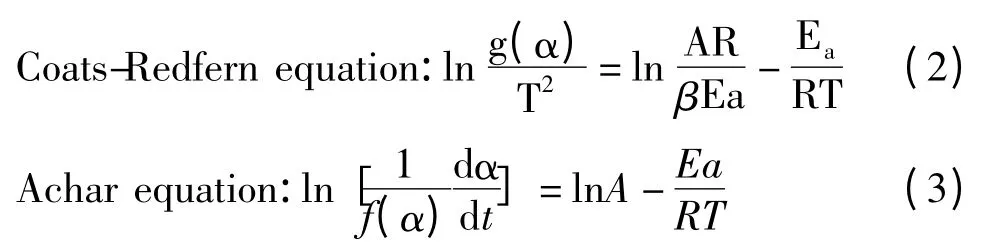
Where β is the heating rate,Eaapparent activation energy,A pre-exponential factor,R gas constant,T Kelvin Temperature,α conversion percentage,α = (winitialwt)/(winitial-wfinal),winitial(orfinal)initial (or final)mass percentage of each stage decomposition,f(α)differential mechanism function and g(α)integral mechanism function.
According to TG-DTG curves at the same heating rate,by substituting data from the TG-DTG curves into Eq.(2)and Eq(3),the linear regression between ln[g(α)/T2](ln[(dα/dt)/f(α)])and 1/T can be established by the linear least square method. From the values of the slope a and the intercept b for the plots,Eaand A can be calculated,respectively.
Molecular simulation
Chemical structural formula of genipin was drawn by Chemdraw and then structurally optimized with the minimum energy by Chem3D.Molecular model was put into GAMESS (Software of Quantum Chemistry)and its calculation of atomic charges was carried out with B3LYP (Density Functional Theory calculation)at a 6-31G level[11,12].Structural formula of geniposide was computationally processed in the same way.
Results and Discussion
Melting point and purity of genipin and geniposide
As shown in Fig.1,the DSC melting curve of standards at the heating rate of 0.5 ℃/min showed the melting of genipin started from 116.83 ℃to 121.52 ℃while the peak temperature was 120.70 ℃and the enthalpy of melting ΔH was 30.34 kJ/mol. Tested with the same conditions,geniposide melted after 153.47 ℃and ended up at 162.54 ℃while the DSC peak temperature was 161. 00 ℃ and the enthalpy of melting ΔH was 24.58 kJ/mol.
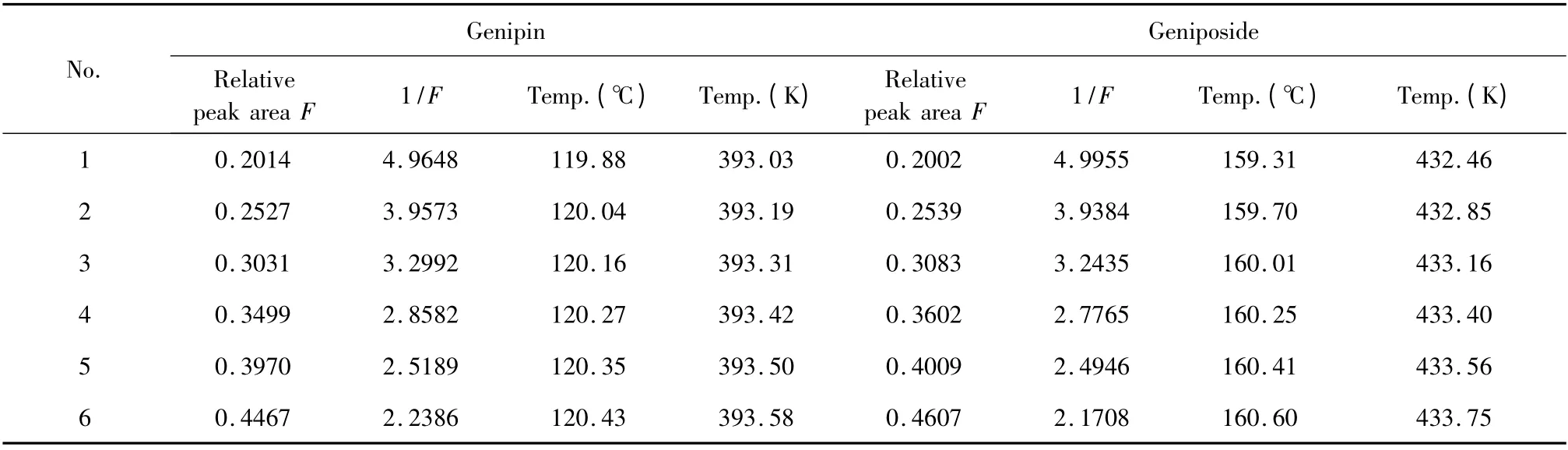
Table 1 Melting temperatures and relative peak area in DSC melting curve at 0.5 ℃/min
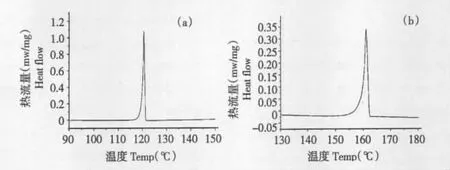
Fig.1 DSC melting curves of genipin (a)and geniposide (b)at 0.5 ℃/min
By substituting picked out datas of melting temperatures Tsand the relative DSC peak area F (0.1 <F <0.5,shown in Table 1)into Van't Hoff equation,the linear regression equations between Tsand 1/F were Y=-0.1993X +394.00 with linearly dependent coefficient r =0.9936 (for genipin)and Y =-0. 4550X +434.68 with r = 0. 9956 (for geniposide)were obtained.
From the intercept b of regression equations,the melting point of pure substance T0for genipin was obtained,which was 394.00 K or 120.85 ℃. According to the slope a =(R·T02·x)/ΔHf,the impurity content x was calculated,which was 0. 0047. Hence,the purity of the standard was 99.53%.In the same way,the melting point of geniposide was 434.68 K or 161.53 ℃while the purity of the standard was 99.29%.
Thermal decomposition mechanism
As shown in Fig.3(a),thermal decomposition of genipin was analyzed and it was found that the curves of TG and DTG remained the same levels and straight before 151℃.It showed that without water sorption,the powder did not undergo decomposition while the mass percentage was constant at 100%.After 151 ℃,the mass percentage began to decrease while the weightlessness increased gradually with two stages. According to the experimental mass loss of 42.04% and atomic charges of genipin,the first stage started from 151 ℃to 271 ℃while this weightlessness could be explained with cracking of C10-O11,C2-C3,O4-C5 and C12-O17 in the molecular structure with the theoretical mass loss of 41.59%.The second stage began at 271℃and ended up at 598 ℃with a mass loss of 28.72%,explained with cracking of C1-C2 and C1-C7 with the theoretical mass loss of 29.22%.
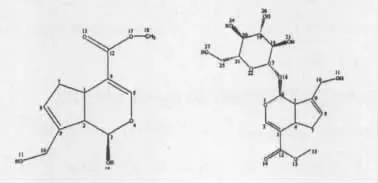
Fig.2 Molecular structure of genipin (a)and geniposide (b)
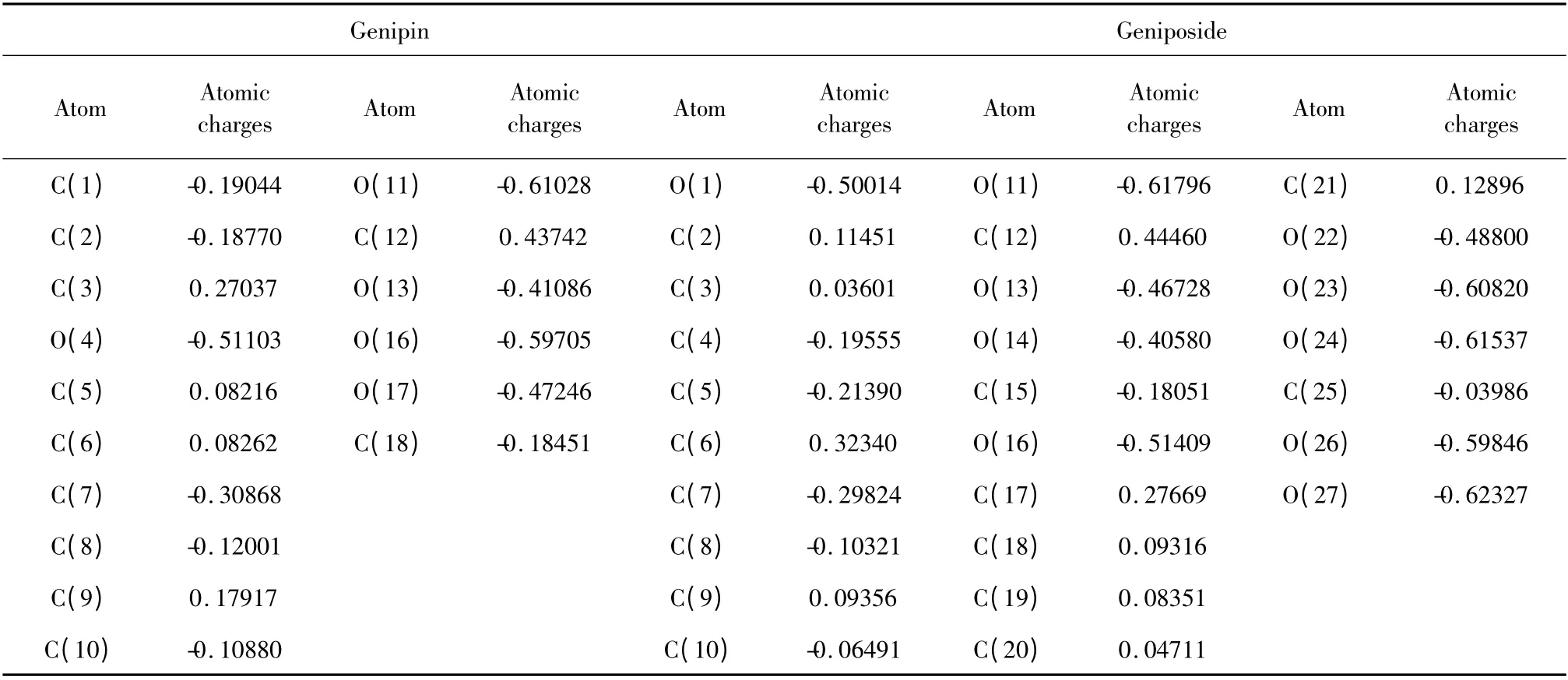
Table 2 Atomic charges of genipin and geniposide without hydrogens
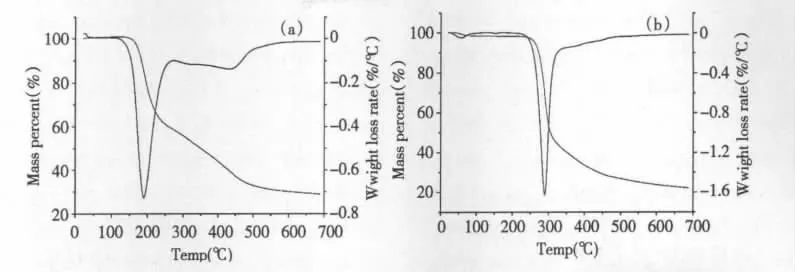
Fig.3 TG and DTG curves of genipin (a)and geniposide (b)at 10℃/min
As shown in Fig.3(b),from 29 ℃to 110 ℃geniposide lost weight of 1.76% adsorbed water while from 110℃the curves of TG and DTG remained at the same levels and straight before 213 ℃,which showed the powder did not undergo decomposition. According to the experimental mass loss of 59. 65% and atomic charges,thermal decomposition began at 213 ℃ and ended up at 361 ℃,explained with cracking of C6-O16(glucosidic bond ),O1-C6,C5-C6 and C12-O13 with the theoretical mass loss of 57.47%.
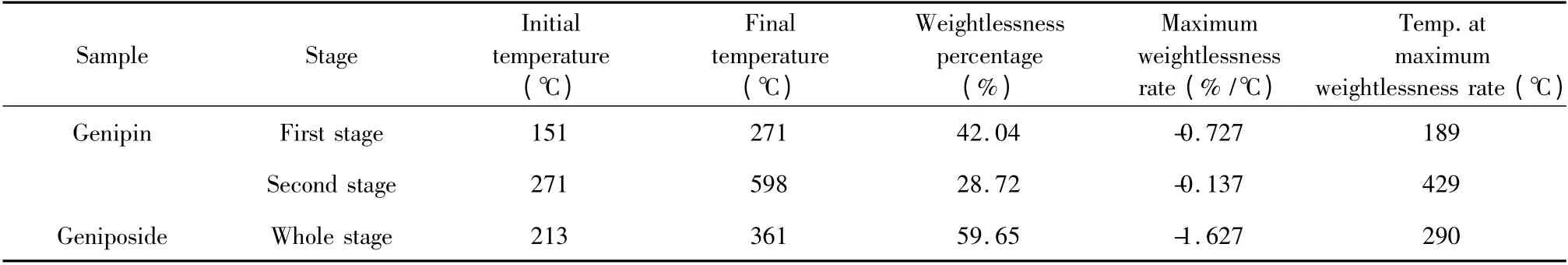
Table 3 Characteristic parameters in the every-stage thermal decomposition of genipin and geniposide
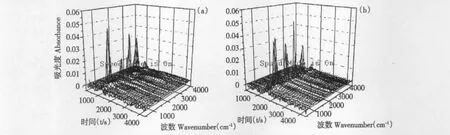
Fig.4 Three dimensional infrared spectrum of evolved gases from the thermal decomposition of genipin(a)and geniposide(b)
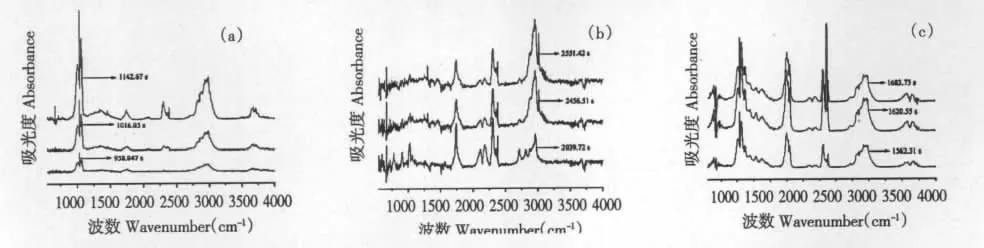
Fig.5 Infrared spectrum of evolved gases from the thermal decomposition of genipin at first stage (a),at second stage(b)and geniposide (c)at different time
As shown in Fig.4(a),three dimensional infrared spectrum of evolved gases from the thermal decomposition of genipin was obtained while the spectrums at 958.047 s,1016.05s and 1142.67 s were related to the first stage.After analysis and presented in Table 4,it was found that wave number,vibration type and corresponding functional group at the three time were consistent and the absorbance increased with the time,which revealed they were gases decomposed from the same stage. Three groups of ester,alcoholic hydroxyl and methyl occurred and verified the cracking of C12-O17 and C10-O11,after which the corresponding methyl ester and methyl alcohol were generated.It was also verified that the cracking of C2-C3 and O4-C5 resulted in the generation of CO2. As shown in Fig.5(b)and presented in Table 4,the groups of evolved gases at second stage were analyzed by the spectrums at 2039.72 s,2456.51 s and 2551.42 s.The previous deduction of cracking of C1-C2 and C1-C7 was verified while the cracked groups generated terminal olefine,ester group,carboxyl and aldehyde group. In addition,a little CO2and acetylenic bond were detected and related to the minor decarboxylation and dehydrogenation.
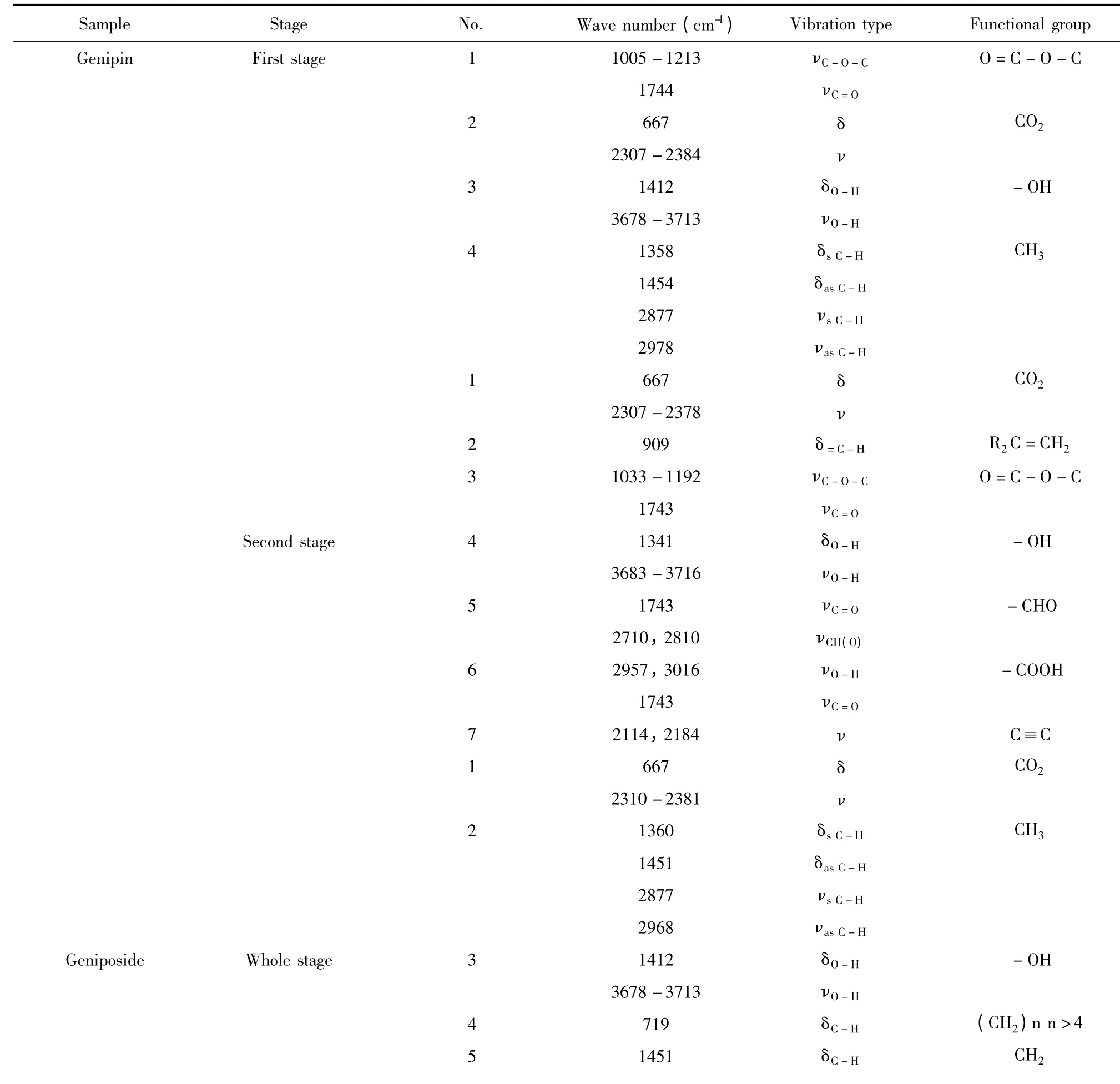
Table 4 Infrared spectral analysis of evolved gases from the thermal decomposition of genipin and geniposide

2844νs C-H 2923νas C-H 6 1746νC=O-CHO 2715,2818νCH(O)7 2085-2187νC≡C
As shown in Fig.4(b),three dimensional infrared spectrum of evolved gases geniposide was obtained while the spectrums at 1562.31s,1620.55s and 1683.73s were related to the single stage of thermal decomposition.(CH2)n(n >4),CH2and alcoholic hydroxyl groups occurred and verified the cracking of C6-O16(glucosidic bond)and a little acetylenic bond was related to the minor dehydroxylation of glucose ring. It was also verified that the cracking of O1-C6,C5-C6 and C12-O13 resulted in the generation of CO2,methyl and aldehyde groups.
Thermal decomposition kinetics
Inference for thermal decomposition mechanism function The DTG curves of genipin after 598 ℃(and geniposide after 361 ℃)remained level,which showed the thermal decomposition was not apparent so that only the fast pyrolysis stages of genipin and geniposide were analyzed by thermal analysis kinetics methods.
Firstly,Coats-Redfern method and Achar method were used to calculate the kinetic parameters. The conversion percentages αi(from 0.1 to 0.9 at an interval of 0. 05)were substituted into the forty common solid mechanism functions[13]so that the values of differential and integral mechanism functions (f(αi)and g(αi))were obtained.Then the datas of f(αi),g(αi),corresponding Kelvin temperature Tiand rate of conversion(dα/dt)iwere substituted into Eq.(2)and Eq(3)so that linear regression between ln[g(αi)/Ti2](or ln[(dα/dt)i/f(αi)])and 1/Tiwas established by the least square method. Then from the slope a and intercept b of the regression equation,the apparent activation energy Eaand natural logarithm of pre-exponential factor lnA were calculated.
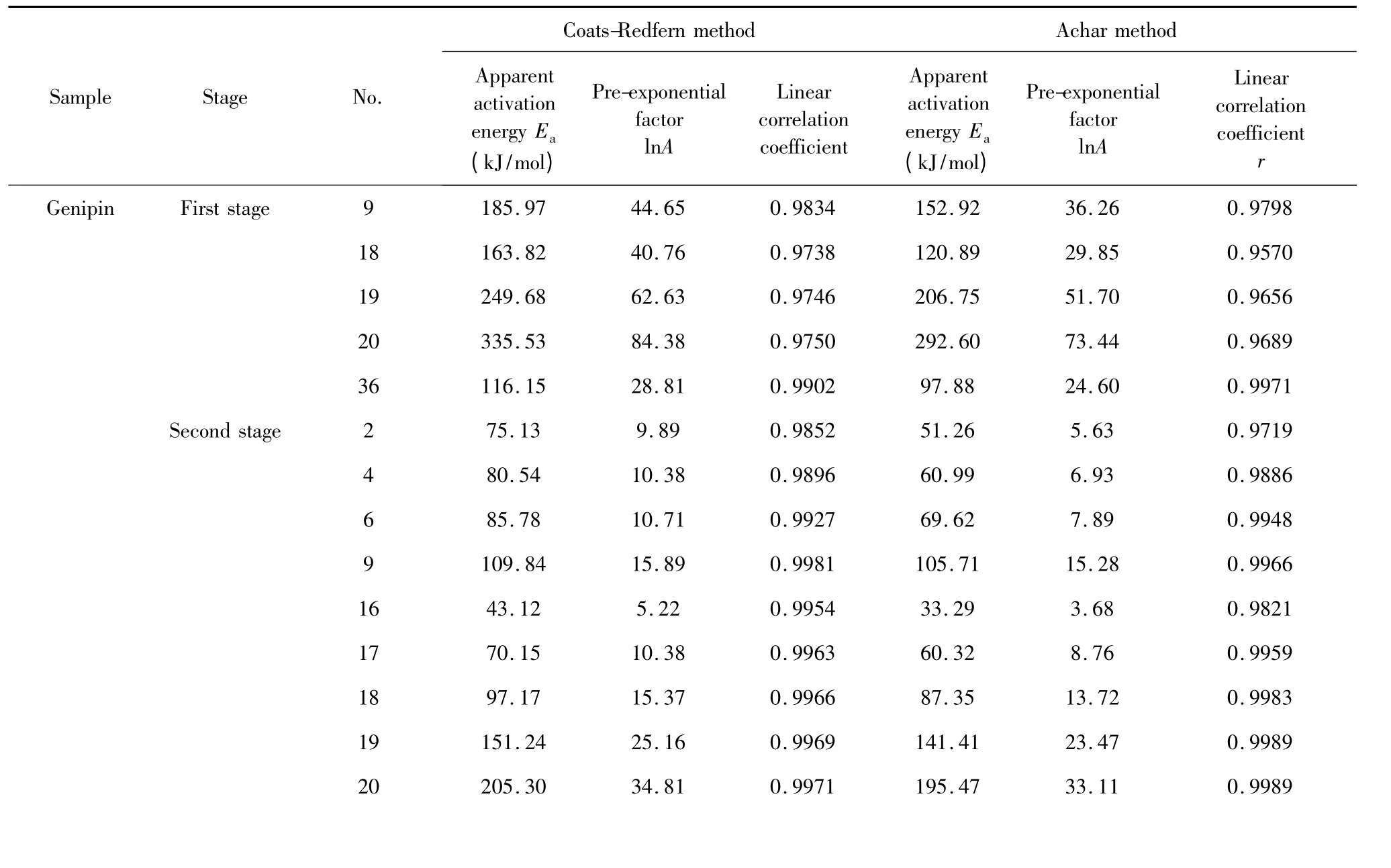
Table 5 Linearly dependent kinetic parameters by Achar and Coats-Redfern methods
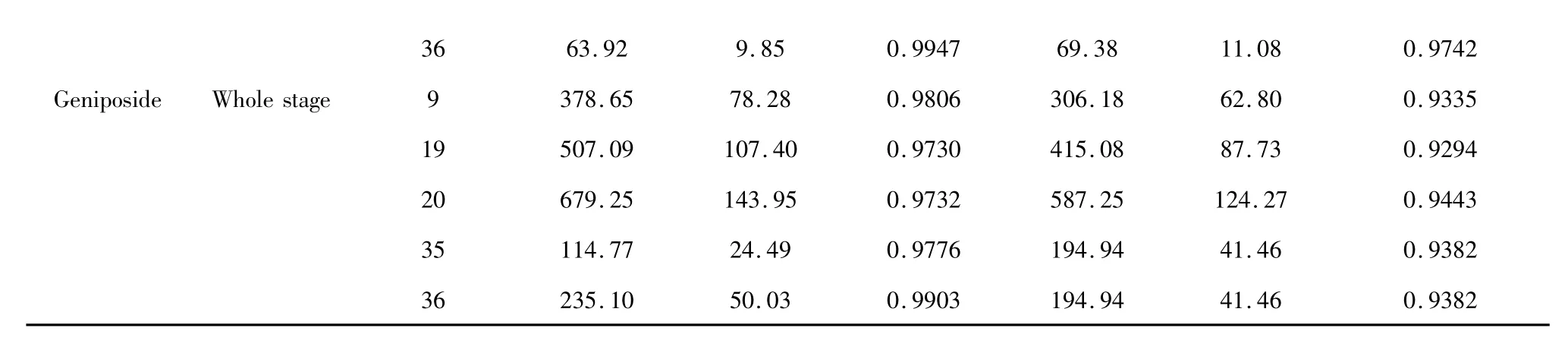
3663.929.850.994769.3811.080.9742 GeniposideWhole stage9378.6578.280.9806306.1862.800.9335 19507.09107.400.9730415.0887.730.9294 20679.25143.950.9732587.25124.270.9443 35114.7724.490.9776194.9441.460.9382 36235.1050.030.9903194.9441.460.9382
Presented in Table 5,after comparison for the first stage of genipin,the linear correlation coefficients r of regression equation substituted with mechanism functions of No.9,18,19,20,36 and 37 were above 0. 95,while similarly for the second stage there were ten groups of mechanism functions substituted into Coats-Redfern equation and Achar equation producing the linear correlation coefficient r above 0.95,of which the No. were 2,4,6,9,16,17,18,19,20 and 36.For the whole stage of geniposide,there were five groups with coefficient r above 0.93,of which the No. were 9,19,20,35 and 36.Kinetic parameters
By comparison of calculation presented in Table 5,it was found that for the first stage of genipin the values of apparent activation energy Eaand natural logarithm of pre-exponential factor lnA with substitution of No.36 mechanism function into Coats-Redfern equation and Achar equation were close to each other and the linear correlation coefficients r approached 1.Similarly for the second stage of genipin and whole stage of geniposide,No.9 and No.36 were substituted into two kinetic calculation methods to obtain the most consistent results.
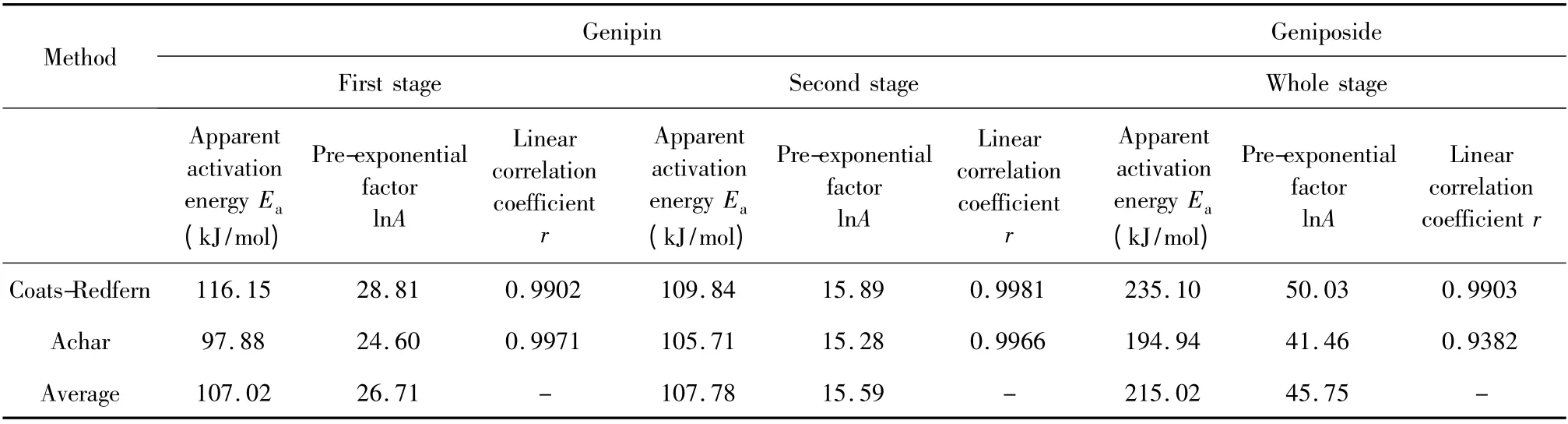
Table 6 Kinetic datas for the thermal decomposition of genipin and geniposide
It can be certain that the most probable mechanisms of the thermal decomposition of genipin at first stage,at second stage and geniposide were Chemical Reaction,Three-Dimension Diffusion,Chemical Reaction,corresponding with Reaction Order equation,Z.-L.-T.equation and Reaction Order equation,respectively.The differential mechanism functions f(α)were (1-α)2,3/2(1-α)4/3[(1-α)-1/3-1]-1and (1-α)2,respectively while the integral mechanism functions g(α)were (1-α)-1-1,[(1-α)-1/3-1]2and (1-α)-1-1 respectively.The apparent activation energy Eawere 107. 02 kJ/mol,107.78 kJ/mol and 215.02 kJ/mol,respectively while the natural logarithm of pre-exponential factor lnA were 26.71,15.59 and 45.75,respectively.These three stages of kinetic datas were compared and found that apparent activation energy Eaof two stages of genipin were not different but much lower than geniposide,which theoretically revealed the geniposide connected with glycosidic bond had stronger thermal stability than genipin.
Shelflives of genipin and geniposide
According to the values of Eaand lnA of genipin and geniposide at the fast pyrolysis stages,the reaction rate constant k at the corresponding temperature Tcwas calculated as the formula k = Ae-Ea/RTcwhile the negative logarithm of rate constant pk was also obtained.On the basis of the value of pk and shelflife of drug[14],if the value of pk is below 7.5,its shelflives are 1.5-2 years.If pk is below 11 and above 7. 5,its shelflives are 3 years.If pk is above 10.5,its shelflives are 4-5 years.By substituting Eaand lnA into the formula k =Ae-Ea/RTc,the values of pk of genipin and geniposide at room temperature (25 ℃)were calculated,which were 7.15 and 17. 80,respectively. So it was inferred that the shelflives of them were 1.5-2 years and 4-5 years,respectively.
Conclusions
Genipin and geniposide were non-isothermally analyzed by differential scanning calorimetry and the linear regression was established by Van't Hoff equation to obtain the melting point and purity,which were 99.53%and 99. 29% and the melting points were120. 85 ℃and 161.53 ℃,respectively.
The thermal decomposition of genipin began at 151℃with two stages while the decomposition of geniposide started from 213 ℃to 361 ℃with one single stage.The deduction by quantum chemistry method and infrared spectral analysis of the evolved gases could verify the experiment weightlessness data,which explained thermal decomposition mechanism on the molecular level.
The most probable mechanisms of the thermal decomposition of genipin at first stage,at second stage and geniposide were Chemical Reaction,Three-Dimension Diffusion,Chemical Reaction,corresponding with Reaction Order equation,Z.-L.-T. equation and Reaction Order equation,respectively. Apparent activation energy Eaof two stages of genipin were much lower than geniposide,which theoretically revealed the geniposide had stronger thermal stability than genipin.
According to the values of Eaand lnA of genipin and geniposide at the fast pyrolysis stages,it was inferred that the shelflives of them were 1. 5-2 years and 4-5 years,respectively.
1 Huo L,Su J,Shen J,et al.Studies on the microbial transformation of geniposide.Nat Prod Res Dev(天然产物研究与开发),2008,20:70-73.
2 Yang YS,Zhang T,Yu SC.Research progress and pharmacological value of genipin. Chin Trad Patent Med,2011,33:130-133.
3 Kim ES,Jeong CS,Moon A.Genipin,a constituent of Gardenia jasminoides Ellis,induces apoptosis and inhibits invasion in MDA-MB-231 breast cancer cells. Oncol Rep,2012,27:567-572.
4 Tian JS,Cui YL,Hu LM,et al. Antidepressant-like effect of genipin in mice.Neurosci Lett,2010,479:236-239.
5 Duan LH,Jin B,Gao LJ,et al. Genipin-crosslinked soybean protein/chitosan hydrogels for controlled release.J Chem Ind Eng,2012,63:962-969.
6 Liu YH,Li J,Lin MT,et al. Modern research and development of geniposide in gardenia.J Chin Pharm,2012,47:406-409.
7 Hsu HY,Yang JJ,Lin SY,et al. Comparisons of geniposidic acid and geniposide on antitumor and radioprotection after sublethal irradiation.Cancer Lett,1997,113(1-2):31-37.
8 Liu ZH. Analytical Chemistry Handbook-eighth Volume of Thermal Analysis. Beijing:Chemical Industry Press,2000.154-155.
9 Coasts AW,Redfern JP. Kinetic parameters from thermogravimetric data.Nature,1964,201:68-69.
10 Rotaru A,Constantinescu C,Anca M,et al. Matrix assisted pulsed laser evaporation of zinc benzoate for ZnO thin films and non-isothermal decomposition kinetics. Thermochim Acta,2010,498(1-2):81-91.
11 Xu WY,Wang XJ,Jin NR. Mechanism and kinetics of thermal decomposition for 2-(4-carboxylphenyl)-5-amino-6-hydroxybenzoxazole.Bull Sci Tech,2008,24:747-751.
12 Zeng YX,Wang C,Wang BQ. Thermodynamic function and crystal structure of hydrocortisone --Analysis by density functional theory.Chem Res,2012,23:47-52.
13 Hu ZR,Shi QZ. Thermal Analysis Kinetics. Beijing:Science Press,2001.127-131.
14 Xu F.Calorimetry and thermal analysis studies on thermodynamic properties of drugs.Dalin:Dalian Institute of Chemical Physics,Graduate School of Chinese Academy of Sciences,PhD.2005.

