Afforestation effects on soil microbial abundance,microbial biomass carbon and enzyme activity in dunes of Horqin Sandy Land,northeastern China
ShaoKun Wang ,XueYong Zhao ,TongHui Zhang ,YuQiang Li ,Jie Lian ,2,WenDa Huang ,2,JianYing Yun
1.Naiman Desertification Research Station,Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
2.University of Chinese Academy of Sciences,Beijing 100039,China
1 Introduction
Horqin Sandy Land is one of the most severely desertified sandy land in the semiarid agro-pastoral zone in northern China (Wanget al.,2004).Increased anthropogenic disturbances have converted the original landscape of tree-scattered grasslands into mobile and semi-mobile dunes in Horqin Sandy Land (Zhaoet al.,2003).Implementation of the policy of fencing and planting grass and trees has restored some parts of the degraded sandy land in recent years(Zhaoet al.,2010).Pinus sylvestrisvar.mongolicaLitv.is resistant to drought,cold and poor soil nutrients,which originally grew in Daxinganlin and Hulun Buir Grasslands of China,and it became one of the most important tree species in protecting soil erosion from wind and sand and to conserve soil and water in northern China (Jiao,2001;Yiet al.,2006).Populus simoniiCarrière is one of the most common tree species in Horqin Sandy Land,which is also well resistant to drought,cold and poor soil nutrients,and it plays a key role in regulating climate,resisting wind and sand and conserving water and soil (Huet al.,2007).Numerous studies have been performed on these species in relation to their growth,community stability,transpiration,vegetation structure,microenvironment improvement,curst features,soil nutrient and soil respiration (Zenget al.,1996;Guoet al.,2004;Yiet al.,2006;Guoet al.,2007;Huanget al.,2008;Jianget al.,2011;Liet al.,2011).However,little research has been accomplished in relation to afforestation effects on soil microbial features in arid and semi-arid regions.
Soil microbial properties are biological indicators to assess soil quality,which are closely related to soil fertility(Pajareset al.,2010;Jinet al.,2011;Liuet al.,2012).Microorganisms take part in the processes of litter decomposition,soil humus formation and soil nutrient circulation (Li,1996).Microbial abundance and microbial biomass carbon regulate soil environment and soil carbon storage (Jiaet al.,2010),while soil enzymes indicate material cycling and energy flow in terrestrial ecosystems (Burns,1978).
Here,we investigated soil microbial abundance,microbial biomass and enzyme activity inP.sylvestrisandP.simoniiforests,which were originally mobile dunes,to analyze the restoration ability of these species in sandy mobile dunes,to provide an ecological theory for estimating afforestation effects in a sandy land ecosystem.
2 Material and methods
2.1 Study area
The study area is located in Naiman County in the middle part of Horqin Sandy Land,China (42°55′N,120°42′E;360 m a.s.l.).The climate in this area is temperate,semiarid continental monsoonal,receiving an average annual precipitation of 360 mm,with more than 75% in the growing season from June to September.The annual mean open-pan evaporation is around 1,935 mm.Annual mean wind velocity ranges from 3.2-4.1 m/s,and the dominant wind is southwest to south in summer and autumn and northwest in winter and spring.The zonal soil is sandy chestnut,which is sandy in texture,light yellow in color and loose in structure,making it vulnerable to wind erosion.The landscape is covered by mobile,semi-mobile and fixed dunes,and interdune lowlands as well as open grassland.The vegetation is dominated byCaragana microphyllaLam.,Artemisia halodendronTurcz.,Melissitus ruthenicus(L.) Latsch.,Cleistogenes squarrosa(Trin.) Keng,Lespedeza davurica(Laxm.)Schindl.,Setaria viridis(L.) P.Beauv.,Pennisetum centraasiaticumTzvelev,andAgriophyllum squarrosumMoq.(Zhaoet al.,2003).
Since the 1970s,numerous artificial forests have been established in different sizes and ages,and the dominant tree and shrub species areUlmus pumilaL.,Pinus sylvestrisvar.mongolica,Populus simoniiandCaragana microphylla(Liuet al.,1996).In the study area,the height ofP.sylvestrisandP.simoniiis 6-8 m and 7-11 m,respectively,and the canopy density ofP.sylvestrisandP.simoniiforests is 60%-80%and 50%-70%,respectively.
2.2 Experimental design
2.2.1 Soil sampling
Three sites of around 20-year-oldP.sylvestris(PSM)andP.simonii(PSC) mature forests were chosen near Naiman Desertification Research Station CAS as study sites.Three mobile dunes (vegetation coverage less than 10%)were chosen as a control (CK).Soil physicochemical characteristics in PSM,PSC and CK are presented in Table 1.Five quadrats (1m×1m) were randomly set as sampling plots in each site.In all the quadrats,soil cores were collected to a depth of 0-20 cm,and a pooled sample was made by mixing five sub-samples from five locations in each quadrat.Every pooled sample was sieved (<2 mm) to remove rocks and plant material,and stored separately in ziplock bags.All samples were stored at 4 °C for the examination of microbial abundance (bacteria,actinomycetes,fungi,cellulose decomposers and azotobacters),microbial biomass carbon and enzyme (dehydrogenase,peroxidase,protease,urease and cellobiohydrolase) activity.
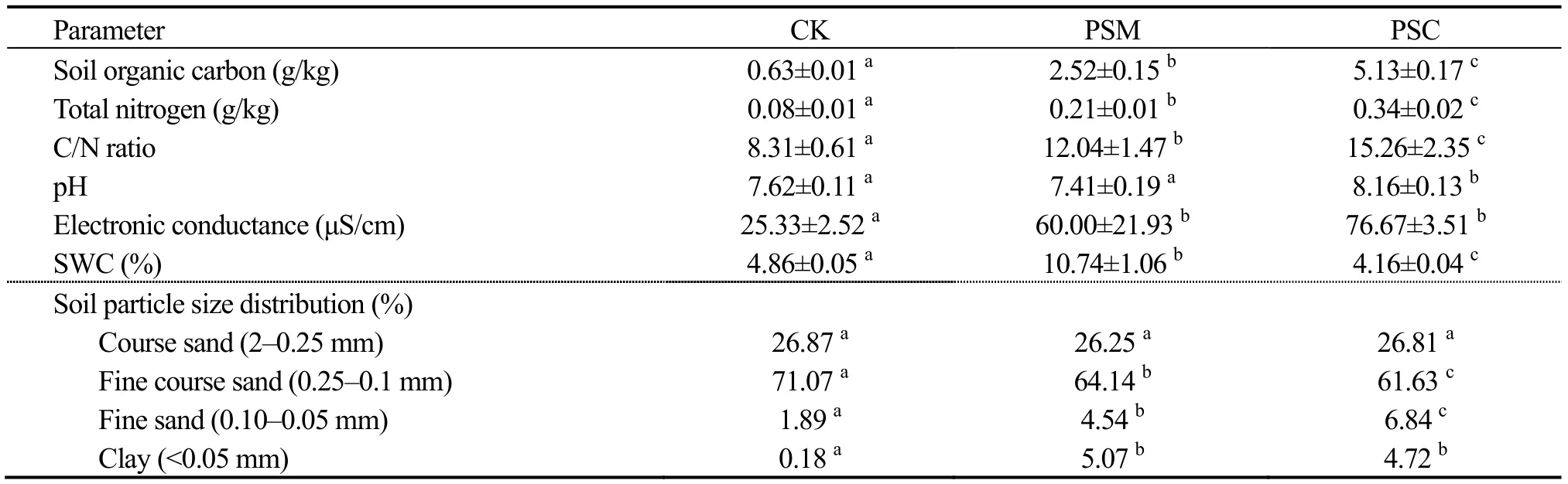
Table 1 Soil characteristics of the studied sites
2.2.2 Analysis methods
Culturable soil microbial abundance were determined as colony-forming unites (CFU) by the pour plate method (Xu and Zheng,1986).Nutrient agar (for bacteria),modified Gause’s synthetic agar (for actinomycetes),rose bengal agar(for fungi),modified Ashby N-free agar (for azotobacters)and Hutchinson agar (for cellulose decomposers) mediums were used to culture microbial groups.Bacteria,actinomycete and fungi counts were conducted after incubation of the plates at 28-30 °C for 3-5 days.Azotobacter and cellulose decomposer counts were conducted after incubation at 28-30 °C for 15-20 days under aerobic conditions.Microbial biomass carbon (MBC) was assessed by using the chloroform fumigation-extraction method (Vanceet al.,1987).Soil enzyme activity were assayed by the methods of Guan (1986).Dehydrogenase activity (DEHa) was assessed by the TTC method.Peroxidase activity (PERa) was assessed by the potassium permanganate titration method.Protease activity (PROa) was assessed by the gelatin hydrolyzation method.Urease activity (UREa) was assessed by the nesslerization colorimetric analysis.Cellobiohydrolase activity (CELa) was assessed by the anthrone colorimetric analysis.
2.2.3 Data analyses
Data were analyzed and described by Microsoft Excel,SPSS 17.0 and Origin 8.0 for Windows.Values were presented as mean ± SE,and significant differences among treatment values were calculated by one-way analysis of variance (ANOVA).Least significant difference (LSD) tests were performed to evaluate differences among individual treatments.Correlations between microbial abundance,microbial biomass carbon and enzymatic activity were analyzed using Pearson’s 2-tailed tests.
3 Results
3.1 Effects of afforestation on soil microbial abundance in sandy land
Soil microbial abundance was significantly higher in afforested soils than that in mobile dune soils.Abundance of soil bacteria,actinomycetes,fungi,cellulose decomposers and azotobacters fell in the order of PSM>PSC>CK (Table 2).Total microbial abundance in PSM and PSC forest soil was 50.16 and 72.48 times of that in CK,respectively,and the differences were significant among PSM,PSC and CK.Microbial abundance were 73.63 and 63.76 times for bacteria,58.06 and 28.38 times for actinomycetes,421.75 and 291.68 times for fungi,24.56 and 12.81 times for cellulose decomposers higher in PSC and PSM forest soils than that in CK,respectively.Azotobacter abundance was 32 and 43 per gram dry soil in PSM and PSC forest soil,respectively,and no azotobacter was detected in mobile dune.Soil microbial abundance was higher in PSC than that in PSM,and their difference was insignificant for bacterial abundance(p>0.05),while the difference was significant for the other four kinds of microbial groups (p<0.05).
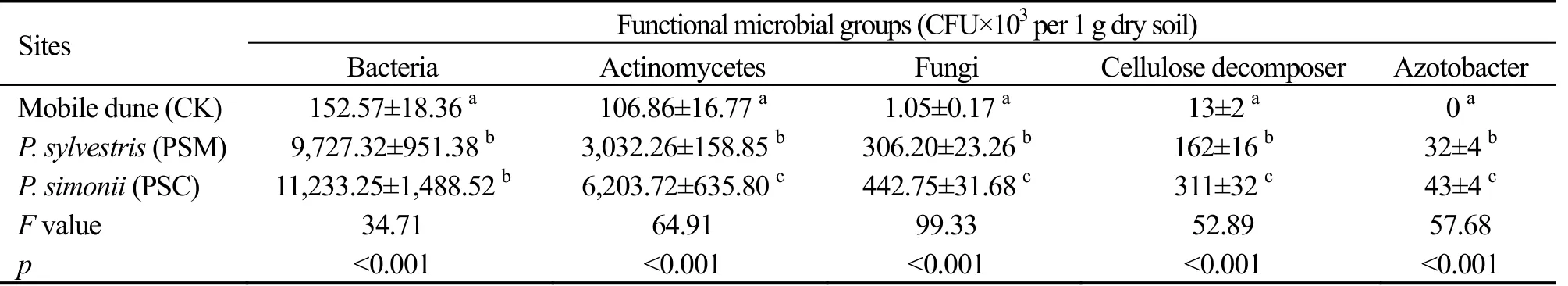
Table 2 Characteristics of the abundance of soil microbial groups in different artificial forests
3.2 Effects of afforestation on soil microbial biomass carbon in sandy land
From Figure 1 it is clear that soil microbial biomass carbon was significantly higher in afforested soils than that in mobile dune soils,and was 23.76 and 33.34 times in PSM and PSC forest soils than that in mobile dune soils (CK),respectively.Microbial biomass carbon was higher in PSC than that in PSM,but the difference was insignificant (p>0.05).
3.3 Effects of afforestation on soil enzyme activity in sandy land
Soil enzyme activity in mobile dunes obviously increased after afforestation.Dehydrogenase (DEH),peroxidase (PER),protease (PRO),urease (URE) and cellobiohydrolase (CEL) activities were significantly higher in afforested soils than those in mobile dune soils.From Figure 2,it was found that soil enzyme activity,including DEH,PER,PRO,URE and CEL activities in PSM and PSC forest soils were 19.00 and 27.54,4.78 and 9.89,4.05 and 8.67,29.93 and 37.46,9.66 and 13.42 times higher than that in mobile dune (CK) soils,respectively.Soil DEH,PER and PRO activities were significantly higher in PSC forest soils than that in PSM forest soils (p<0.05) (Figures 2a,2b,2c).Soil URE and CEL activities were higher in PSC forest soils than that in PSM forest soils,but the differences were insignificant between the two artificial forests (p>0.05) (Figures 2d,2e).
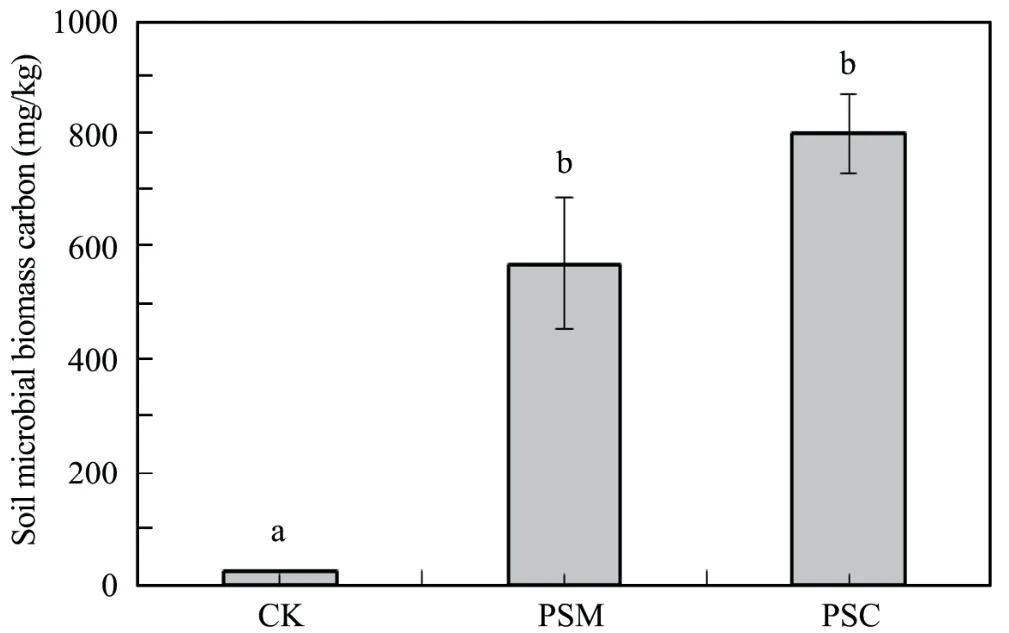
Figure 1 Soil microbial biomass carbon in different artificial forests.Values (mean ± SE) with different letters are significantly different at p<0.05

Figure 2 Soil enzyme activity in different artificial forests.Values (mean ± SE) with different letters are significantly different at p<0.05 for each enzyme activity
3.4 Correlations among soil microbial abundance,microbial biomass and enzyme activity in artificial forests
Pearson’s correlations among soil microbial abundance,microbial biomass and enzyme activity in afforested soils are presented in Table 3.Results show that soil total microbial abundance was significantly positive with bacteria,actinomycetes,fungi,cellulose decomposers abundance,and microbial biomass carbon (p<0.01),while showing insignificant positive relations with dehydrogenase,peroxidase,urease and cellobiohydrolase (p>0.05).Bacteria abundance shows a significant positive relations with other microbial group abundance and microbial biomass carbon (p<0.05),while the correlations were insignificant with soil enzyme activity (p>0.05).Actinomycete abundance shows a significant positive relation with all microbial abundance,microbial biomass and enzyme activity measured in our experiment.Fungi,cellulose decomposer,and azotobacter abundance show similar correlations with other parameters:they are significantly and positively correlated with other microbial group abundance,microbial biomass carbon,dehydrogenase,peroxidase and protease (p<0.05),and positively but insignificantly correlated with peroxidase and protease (p>0.05).Soil microbial biomass carbon was significantly and positively correlated with microbial abundance (p<0.05),and positively but insignificantly correlated with the five kinds of enzyme activities (p>0.05).Soil dehydrogenase and peroxidase activities show similar correlations with other parameters we measured:it shows significant and positive correlation between dehydrogenase and peroxidase activities (p<0.01),and they are significantly and positively correlated with actinomycete,fungi and cellulose decomposer abundance (p<0.05),while their correlations were insignificant with protease,urease and cellobiohydrolase activities (p>0.05),respectively.Protease activity was significantly correlated with microbial abundance (p<0.05),except for bacteria abundance(p>0.05),and it was significantly and positively correlated with urease and cellobiohydrolase activities (p<0.01).Correlation between urease and cellobiohydrolase activities was significantly positive (p<0.01).

Table 3 Correlations among soil microbial abundance,microbial biomass carbon and enzymatic activity in artificial forests
4 Discussion
4.1 Microbial abundance
Microbial abundance reflects microorganism function and activity in a soil environment (Yao and Huang,2006).In our study,soil bacteria,actinomycetes and fungi abundance were significantly higher in afforested soil than those in mobile dune.The main reason is that vegetation coverage is much lower,and plant species is much less in mobile dune than that in an artificial forest,and soil organic matter becomes the limiting factor for microorganism growth in mobile dunes (Jinet al.,2011).After planting trees in mobile dunes,in addition to sequestration of dust,tree roots and root secretion alter the soil environment by increasing soil organic matter,and soil organic matter ensures microorganism growth and regeneration (Yanget al.,2005).Microorganism activity feeds back to forest trees by providing available nutrients for the trees through decomposition and nitrogen fixation (Liuet al.,2005).
Fungi and azotobatcer abundance were greatly changed from mobile dune soils to forest soils.Fungi abundance in forest soil was hundreds times more than that in mobile dune,which could be explained by the fact that fungi are more specific in decomposing cellulose,hemicellulose and lignin(Liuet al.,2005).There is little vegetation in mobile dunes,and lower soil nutrients restrict fungi growth.Afforestation increased litter input,and the litter increased cellulose,hemicellulose and lignin content in forest soils.Therefore,fungi abundance in forest soils is significantly higher than that in mobile dune soil.We did not detect azotobacters in mobile dunes.This indicates that nitrogen fixing capacity is extremely low in mobile dunes,and the soil environment is not suitable for both plants and microorganism growth in mobile dunes.We did detect azotobacters in afforested soil,which was originally mobile dune.This result could be explained by two reasons.Firstly,azotobacters can be distinguished by three basic kinds:authigenic,symbiotic and endogenous azotobacters,and symbiotic and endogenous azotobacters were more effective than authigenic azotobacter (Elmerichet al.,1997).The joint effect of nitrogen fixation between azotobacters and plants could reinforce plant competitive ability for more nutrients and space (Menget al.,2011).Secondly,after planting trees,tree roots would bring some symbiotic and endogenous azotobacters into the soil,and root secretion could provide nutrients for microorganism growth,and microorganisms in turn help the growth of trees(Wanget al.,2012).
4.2 Relationship among soil microbial attributes in artificial forests
Our results show that correlations were positively significant between the abundance of any two microbial groups and the correlations were significantly positive between soil microbial biomass carbon and microbial abundance.Cooperation among soil microbial groups ensured soil chemical reactions going smoothly.Therefore,correlations among the microbial basic groups show a significantly positive healthful ecosystem (Yao and Huang,2006).Also,microbial biomass carbon is dominated by microbial species and abundance,which indicates that microbial abundance and microbial biomass carbon are significantly correlated (Guo and Zhu,1997),which is in accordance with our research in different types of sandy dunes in Horqin Sandy Land (Wanget al.,2008).
Enzyme activity is important to indicate soil potentiality and to catalyze a complicated chain of chemical and biological reactions for litter decomposition,nutrient cycling,and soil structure formation.Soil enzymes can be recognized as oxidoreductases and hydrolases according to different pathways of catalytic reaction (Guan,1986).Alvearet al.(2005)and Madejónet al.(2007) demonstrated that soil enzymes work together to catalyze the reaction in soil formation and mineralization.Soil enzymes affect each other in biological reactions,and show a close relation with each other in the soil environment (Alvearet al.,2005;Madejónet al.,2007).Our results were different in which the correlation between the two oxidoreductases (dehydrogenase and peroxidase)activities was significantly positive,and correlations among the three hydrolases (protease,urease and cellobiohydrolase)activities were significantly positive.Correlations between Oxidoreductases and hydrolases activities were positive but not significant.
4.3 Soil microbial biomass ratio (Cmic:C ratio) in artificial forests
Soil microbial biomass ratio is the ratio between soil microbial biomass carbon and soil organic carbon (Cmic:C ratio)(Weiet al.,2008).This ratio is an important indicator for soil carbon pool,and it is used to indicate organic carbon stability and effectivity,and organic matter mineralization potential.The higher the ratio,the more soil organic matter is mineralized which transforms more soil organic matter into microbial biomass carbon (Xuet al.,2010).Soil Cmic:C ratio in Table 4 shows that the ratio inP.sylvestrisandP.simoniiforest soils were significantly higher than that in mobile dune soils (CK) (p<0.05).After planting trees in mobile dunes for about 20 years,soil Cmic:C ratio was significantly increased by 5.15 times (P.sylvestris) and 3.01 times (P.simonii) more than that in mobile dune soil.Planting trees in mobile dunes can increase available soil nutrients,thus improving the living environment for soil microorganisms.These plantings reinforce soil nutrient stability and effectivity by increasing microbial abundance and activity in sandy lands.Soil Cmic:C ratio was 34.79% higher inP.sylvestrisforest soil than that inP.simoniiforest soil.Although microbial biomass carbon is lower inP.sylvestrisforest soil than that inP.simoniiforest soil,soil microbial carbon ratio appeared different.The reason may be explained by different mineralization ability of microbes ofP.sylvestrisandP.simonii.Water use efficiency ofP.sylvestrisandP.simoniitreespecies differ significantly,and transpiration inP.sylvestrisis about twice of that inP.simonii(Chang and Zhao,1990;Huet al.,2007).In recent years,rainfall has been decreasing and underground water level has declined in Horqin Sandy Land,thus available water has significantly decreased in sandy lands.Pinus sylvestrisis more effective thanP.simoniiin water use,thus there is more available water forP.sylvestris,which promotes tree and soil microorganism growth and fasters soil organic matter mineralization.Pinus sylvestrisandP.simoniiare both effective tree species in fixing mobile dunes and improving soil fertility in Horqin Sandy Land.Considering water use uptake efficiency and availability for trees,P.sylvestrisis more efficient,stable and sustainable thanP.simonii.Therefore,soil microbial biomass carbon ratio (Cmic:C ratio) is an efficient indicator to estimate soil stability and availability in artificial forest.

Table 4 Cmic:C ratio in artificial forest
5 Conclusion
After plantingP.sylvestrisandP.simoniitress for about 20 years in mobile dunes in Horqin Sandy Land,soil properties were significantly improved.Afforestation increased soil microbial abundance,microbial biomass carbon and enzyme activity in sandy dunes.Soil microbial abundance,microbial biomass carbon and enzyme activity were higher inP.simoniiforest soils than that inP.sylvestrisforest soils.Planting suitable trees in mobile dunes not only accelerates mobile dune fixation,but also improves soil physicochemical properties and microbiological activity.P.sylvestrisandP.simoniiare suitable tree species in fixing mobile dunes and improving soil fertility in Horqin Sandy Land.Soil microbial biomass carbon ratio (Cmic:C ratio) is an efficient indicator to estimate soil stability and availability in artificial forest.Based on an overall consideration of available soil nutrients,water use efficiency,plantation stability and sustainability,P.sylvestrisis better thanP.simoniiin fixing mobile dunes in sandy lands.
This paper was financially supported by the National Science and Technology Support Program (2011BAC07B02),Young Scientists Foundation of Chinese Academy of Sciences (CAS) (Y251951001) and National Natural Science Foundation of China (41171414 and 31170413) from Cold and Arid Regions Environmental and Engineering Research Institute,CAS.We express our sincere thanks to the anonymous reviewers for their valuable comments and suggestions on this manuscript,and to all the members of Naiman Desertification Research Station,CAS,for their help in field and laboratory work.
Alvear M,Rosas A,Rouanet JL,Borie F,2005.Effects of three soil tillage systems on some biological activities in an Ultisol from southern Chile.Soil and Tillage Research,82(2):195-202.
Burns RG,1978.Soil Enzymes.Academic Press,London-New York-San Francisco.
Chang XL,Zhao WZ,1990.Study on moisture physiology ofPinus sylvestrisvar.mongolicaLitv andPopulus simoniiCarr.and water condition of woodland.Journal of Desert Research,10(4):18-24.
Elmerich C,Kondorosi A,Newton WE,Elmerich C,1997.Biological nitrogen fixation for the 21st Century.Springer,Dordrecht.
Guan SY,1986.Soil Enzymes and the Research Approaches.China Agriculture Press,Beijing,China.
Guo JX,Zhu TC,1997.Study on numbers and biomass of soil microorganism inAneurolepidiumChinese grassland.Acta Ecologica Sinica,17(1):78-82.
Guo R,Wang XK,Liu K,Yang F,2004.Carbon and nitrogen pool in forest soil underPinus sylvestrisvar.mongolica.Soils,36(2):192-196.
Guo YR,Zhao HL,Zhao XY,Zuo XA,Luo YY,2007.Crust development and its influences on soil physicochemical properties in artificial forest of Horqin Sand Land.Journal of Desert Research,27(6):1000-1006.
Hu ZH,Wang DL,Hu QY,2007.Study on growth and transpiration ofPinus sylvestrisvar.mongolica,Pinus fabulae formisandPopulus simoniiin North Shanxi Province.Journal of Shanxi Agricultur University(Natural Science Edition),27(3):245-249.
Huang G,Zhao XY,Su YG,Yue G,2008.Assessment on the effects ofPinus sylvestrisvar.mongolicaplantation on microenvironment improvement in the Horqin Sandy Land.Arid Zone Research,25(2):212-218.
Jia GM,Liu BR,Wang G,Zhang B,2010.The microbial biomass and activity in soil with shrub (Caragana korshinskiiK.) plantation in the semi-arid loess plateau in China.European Journal of Soil Biology,46(1):6-10.
Jiang L,Yang W,Yao Y,Lu Q,Du M,Wang L,2011.Vegetation recovery in Mongolian Pine Sand-fixing Belts in response to boundary effect of forest-grassland.Journal of Desert Research,31(2):372-378.
Jiao SR,2001.Afforestation technology of needle-leaved trees in Keerqin Sandy Land.Inner Mongolia Forestry Science &Technology,(3):31-36.
Jin ZZ,Lei JQ,Xu XW,Li SY,Fan JL,Peng HQ,2011.Diversity of soil microbe at different sites in shelterbelt of moving sand area.Journal of Desert Research,31(6):1430-1436.
Li FD,1996.Soil Microbiology.Chinese Agriculture Press,Beijing,China.
Li YQ,Zhao XY,Liu XP,Shang W,Feng J,Su N,2011.Soil carbon sequestration in sand fixation plantation ofPinus sylvestrisvar.mongolicaand response of soil respiration to drought and wet condition.Journal of Desert Research,31(2):282-287.
Liu X,Zhao H,Zhao A,1996.Sand Environment and Vegetation in Horqin Sandy Land.Science Press,Beijing.
Liu YL,Xiong YH,Xie SX,Ding GJ,2005.A study on soil microflora of planting eleven years’ Masson pine.Journal of Mountain Agriculture and Biology,24(3):199-204.
Liu YM,Li XR,He MZ,Jia RL,Li XJ,Zhang ZS,2012.Effect of biological soil crusts on soil microbial biomass carbon content.Journal of Desert Research,32(3):669-673.
Liu ZX,Zhu TH,Zhuang J,2005.Research advances in root exudates and rhizosphere microorganisms of forest trees.World Forestry Research,18(6):25-31.
Madejón E,Moreno F,Murillo JM,Pelegrín F,2007.Soil biochemical response to long-term conservation tillage under semi-arid Mediterranean conditions.Soil and Tillage Research,94(2):346-352.
Meng XF,Long XH,Kang J,Wang XQ,Liu ZP,2011.Isolation and identification of endogenic nitrogen-fixing bacteria in the roots of Jerusalem artichoke (Helianthus tuberosus).Acta Prataculturae Sinica,20(6):157-163.
Pajares S,Gallardo JF,Masciandaro G,Ceccanti B,Etchevers JD,2010.Enzyme activity as an indicator of soil quality changes in degraded cultivated Acrisols in the Mexican Trans-volcanic Belt.Land Degradation&Development,22(3):373-381.
Vance ED,Brookes PC,Jenkinson DS,1987.An extraction method for measuring soil microbial biomass C.Soil Biology and Biochemistry,19(6):703-707.
Wang SK,Zhao XY,Zhang TH,Tang X,Wang XY,Liu ZG,2012.Impact of different shrubs on rhizospheric microorganism in Horqin Sandy Land.Journal of Arid Land Resources and Environment,26(5):140-144.
Wang SK,Zhao XY,Zuo XA,Zhao W,2008.Characteristics of microbe flora in different dunes during plant germination period in Horqin Sandy Land.Journal of Desert Research,28(4):696-700.
Wang T,Wu W,Xue X,Sun QW,Zhang WM,Han ZW,2004.Spatial-temporal changes of sandy desertified land during last 5 decades in northern China.Acta Geographica Sinica,59(2):203-212.
Wei TF,Ren YL,Zeng H,He JS,2008.Effects of throughfall manipulation on dynamics of soil microbial biomass carbon and microbial quotient in aPinus sylvetrisvar.mongolicaplantation.Acta Scientiarun Naturalium Universitatis Pekinensis,(4):52-59.
Xu GH,Zheng HY,1986.A Manual of Soil Microbial Analysis Method.Agriculture Press,Beijing,China.
Xu SX,Guo DF,Zhang LH,2010.Effects of moving to soil microbial biomass carbon and organic carbon mineralization in grassland ecosystem.Resources of Environment and Development,3:21-23.
Yang T,Xu H,Liu H,Fang DH,Zhu JJ,2005.Soil nutrient,microorganism and enzyme activity inPinuse sylvestrisplantations.Journal of Soil and Water Conservation,19(3):50-53.
Yao HY,Huang CY,2006.Soil Microbial Ecology and Their Research Approaches.Science Press,Beijng,China.
Yi XY,Zhao HL,Cui JY,Li YQ,Zuo XA,Zhou H,2006.Growth of small areaPinus sylvestrisvar.mongolicaartificial forest under different densities in Horqin Sandy Land,North of China.Acta Ecologica Sinica,4:1200-1206.
Zeng DH,Jiang FQ,Fan ZP,Zhu JJ,1996.Stability of Mongolian pine plantations on sandy soil.Chinese Journal of Applied Ecology,7:337-343.
Zhao HL,Zhao XY,Zhang TH,Wu W,2003.Desertification Processes and Its Restoration Mechanisms in the Horqin Sand Land.Ocean Press,Beijing.
Zhao XY,Wang SK,Luo YY,Huang WD,Lian J,2010.Is desertification reversion sustainable in Northern China?—A case study in Naiman County,part of typical agro-pastoral transitional zone in Inner-Mongolia,China.Global Environmental Research,14:63-70.
 Sciences in Cold and Arid Regions2013年2期
Sciences in Cold and Arid Regions2013年2期
- Sciences in Cold and Arid Regions的其它文章
- Amount and temperature effects responsible for precipitation isotope variation in the southern slope of Himalayas
- Seasonal changes in the relationship between species richness and community biomass in grassland under grazing and exclosure,Horqin Sandy Land,northern China
- The effects of extreme rainfall events on carbon release from biological soil crusts covered soil in fixed sand dunes in the Tengger Desert,northern China
- Probabilistic modeling of soil moisture dynamics in a revegetated desert area
- Effects of shrubs and precipitation on spatial-temporal variability of soil temperature in microhabitats induced by desert shrubs
- OSL chronology and paleoclimatic implications of paleodunes in the middle and southwestern Qaidam Basin,Qinghai-Tibetan Plateau
