Effects of Tannic Acid on Active Aluminum Species Distribution in Various Tea Soils
You-Jian Su,Wan-You Liao,Ye-Jun Wang,Yong-Li Zhang,Yi Luo,Shan-Guo Hu
Tea Research Institute,Academy of Agricultural Science of Anhui Province,Qimen 245600,China.
1.Introduction
A variety of organic acids in tea garden,which has long been known[1].Tea in the growth process will produce secondary metabolites such as polyphenolic compounds in the form of secretions or litter into the soils[2].Studies have shown that secondary metabolites through a variety of metabolic pathways,although not directly affect in plant development,but they are in the entire tea garden ecosystem functionality is extremely important[3-5].The main phenolic compounds in tea litter with hydroxy-aromatic ring and other functional groups of a class of compounds with tannic acid[6].In the regulation of tea-the interaction between the soil,especially in organic matter dynamics and nutrient cycling and other aspects play an important role[7]with closely related to soil mineralized[8,9].Soil dissolution aluminum and aluminum ions chemical forms by soil their material composition,pH,electrolytes,and a variety of factors such as temperature[10,11].Organic acid is an important part of tea garden soil material.The crystalline state of organic acid can change the surface characteristics of aluminous minerals,while changing the aluminum oxide formation,thus affecting the surface active mechanism and pollution,further influence its environmental significance[12-14].With an organic acid ions added to the acidic soil can form complexes with aluminum,thus affecting the aluminum in the soil and the environment in the chemical behavior[15].The domestic research on tannins are mainly focused on its effects on plant physiology and as drug development application and so on[16-20].Research on the effect of less activity in the tea garden soil aluminum species distribution and soil pH value,and some research only mentioned the effect of tannic acid on activity of aluminum is a complex reaction,the concentration range of influence and the specific process is not clear the influence of aluminum by tannic acid.This research chooses Jiangxi and Yunnan in two different locations in the tea garden soil as the research object,in different soil pH environmental conditions,to the tannic acid in soil solution by adding different concentration,after 4 weeks of culture,changes of morphology and content analysis of soil active aluminum,change and soil buffer capacity determination of soil pH the mechanism of the effect of tannic acid,may be on the activity of aluminum in tea garden soil,in order to understand the influence of content of tannic acid in tea leaf litter on soil environment.
2.Materials and methods
2.1.The soil conditions
The tested soils are derived from the tea gardens in Yunnan province city of Simao region and Jiangxi province Nanchang County Huang Ma Xiang Jiangxi Sericulture Institute of tea orchard.Yunnan Puer tea garden in Simao is located at 22°47'23″N,100°58'33″E,as the monsoon climate region in low latitude plateau,between the annual rainfall of 1 490~1 580 mm,annual mean temperature of 18~20℃;wet season,soil type is mainly plateau laterite,the main varieties of tea leaf Yunnan population,tea planting period of about 30 years,tea garden soil fertility level is not high.The garden of Jiangxi Huang Ma Nanchang County Xiang Jiangxi sericulture Tea Research Institute(28°32'55″N,115°56'21″E)located in the lower reaches of Yangtze River,which belongs to the subtropical monsoon climate,annual rainfall in 1 375~1 550 mm,annual average temperature of 17.5 ℃,soil type is red soil,the main plant tea varieties for Qimen content(Camellia sinensis),moderate levels of soil fertility,field management level is good.Soil sampling points in accordance with the"S"-shaped distribution,Yunnan Pu'er tea garden located sampling points 165,Nanchang tea set sampling points 124,earth were divided into 0~20 cm,20~40 cm layers,soil location close to the tea dripping along from fertilization ditch 5 ~10 cm.Samples were air-dried,remove plant debris,grated over 2 mm sieve and stored for use.The main garden soil chemical properties are shown in Table1.
2.2.Trials processing settings
From different sites and different soil original air dried soil samples from each of 15 kg,content and distribution of physicochemical properties and active aluminum first determine the basis of the average is divided into 4 parts,according to the buffer capacity of soil sample,by adding different volume to soil in 0.01 mol/L HCl and 0.02 mol/L Ca(OH)2will be treated as pH respectively to 3.0,3.5,4.0,4.5 samples,training to air dry state after about 4 weeks,then from each soil cultivation of sampling in 15 copies,each 200 g,and then to which were added 0.4,2,4,8,12,mmol/kg tannin acid as 3 parallel,4 weeks of cultured,the natural air dry at room temperature,grinding through a 40 mesh sieve.The final determination of changes in soil of different forms of active aluminum content and pH value after cultured.
2.3.Items and methods of measurement
Soil pH with Sartorius PB-10 pH was measured(water/soil ratio of 2.5∶1),organic carbon and nitrogen analyzer with(multi N/C 2001 TOC)determination of cation exchange capacity(CEC)was measured using NH4Ac exchange method[21],available nitrogen diffusion method using alkaline hydrolysis,phosphorus and potassium was measured by ICP-OES,soil buffer capacity was measured using an automatic potentiometric titrator,modification of different forms of soil active al extraction method on the basis of the reference to Wu Chunhua's method[22],Using KCl(1 mol/L),HCl(1 mol/L),NH4AC(1 mol/L pH4.8),NaOH(0.5 mol/L)four kinds of chemical extraction solvent extraction different forms of activity in soil aluminum.Weigh the dried soil sample into 4 parts each 1g plastic bottle,were added to 50mL four kinds of extraction solvent,extraction of different forms of aluminum,the first in a thermostatic shaker 30 min(25℃),and then to 3000 r·min-1was centrifuged for 10 min at medium speed quantitative filter paper filter clearing fluid,then microporous membrane(pore size 0.45 μm)filter,prepare for the test solution[23].Pipette NaOH(0.5 mol/L),HCl(1 mol/L),KCl(1 mol/L),NH4AC(1 mol/L pH4.8)extraction of the test solution 0.25,0.5,2.0,1.0 mL in four 25 mL volumetric flask,add a small amount of distilled water,adjusted to pH 5.6,plus 0.05%aluminum reagent(Rose tricarboxylic acid ammonium)5 ml,shake,set the volume let stand 30 min,with 755 type spectrophotometer at the wavelength of 520 nm colorimetry,calculation of various aluminum content by subtraction.Determination of pH buffer capacity,taken 11 glass beaker,followed by No.,each beaker test soil samples weighed 4 g,1 to 5,then added 0.5,1.0,2.0,3.0,4.0 ml beaker of 0.1 mol/L HCl;6 ~ on the 11th and then added to the beaker HCl equivalent,such as the concentration of NaOH,No.6 beaker not add acid,CO2-free distilled water added to make the total volume of each beaker 20.0 ml,shake,place 72 h daily intermittent shaking 3 ~4 times,measured pH.At pH 4-7 and pH as the vertical axis,the amount of acid added to the abscissa,the establishment of linear equations to obtain pHBC[24].Titration curves of the soil pH jump range can be approximated as a straight line,the acid,the amount of alkali linearly related soil pH,slope,b represents a unit quantity of added acid,base induced variation of soil pH(b=ΔpH/ΔC),ΔC for the added acid(or base)of the amount,b greater the absolute value,indicating that the soil buffering capacity worse[25,26].

Table1.Main chemical properties of the soils in the experiment.
3.Results
3.1.Different soil pH buffering capacity analysis
As can be seen from Table2,two tea different layers of soil pH buffering capacity was not significantly different,0 ~20 cm soil pH buffering capacity(pHBC)slightly higher than the 20 ~40 cm soil,Yunnan Pu'er tea garden soil pHBC greater than in Nanchang,Jiangxi.Soil organic matter has a great influence on the pHBC,the higher organic matter content,the higher pHBC.According to table 1 can see,Jiangxi Nanchang Yunnan Pu'er tea and tea garden soil,organic matter content in the soil layer cm 0 ~20 than 20 ~40 cm layer of soil is higher,the surface soil of pHBC was significantly higher than the underlying soil.Soil organic matter containing active functional groups such as carboxyl rich,(-COOH)and phenolic hydroxyl(-OH),when the existence of these groups to anions,they can pass through the association reaction H+external source acid buffer.Tannic acid can be from a variety of minerals include kaolinite dissolution of aluminum and other cations,thereby promoting mineral weathering[27,28],Can promote aluminum short-range(50 h)dissolved organic anions,multi nuclear surface complex formation on the mineral surface and on the aluminum short-range dissolution inhibition[29].Rich in organic matter significantly enhanced the buffering action of soil,clay content higher buffer capacity is large.An important factor in soil pH also affect pH buffer capacity[30],the higher the pH value,the greater the value of soil pHBC.

Table2.Regression equations and correlation coefficients of liner portion of the pH buffer capacity.
3.2.Effect of tannic acid in the tea garden soil forms and contents of active aluminum
Exchangeable aluminum in tea garden soil is the main cause of acid aluminum species,which arise directly from the hydrolysis of H+on garden soil pH of the most obvious[31].From Figure 1 and Figure 2 can be seen that Yunnan and in Jiangxi tea garden soil after treated with different concentrations of tannic acid exchangeable Al,content of the show,pH3.0 >pH3.5 >pH4.0>pH4.5.It can be seen from Figure 1,the concentration in the 0 ~2.0 mmol/kg adding tannic acid,different pH soil exchangeable Al content is relatively high;tannic acid concentration greater than 4.0 mmol/kg,the exchangeable aluminum content of a sharp decline,dropping from 58%to 82%.

Fig.1.Effects of tannic acid on exchangeable Al content in soils of YN tea garden.
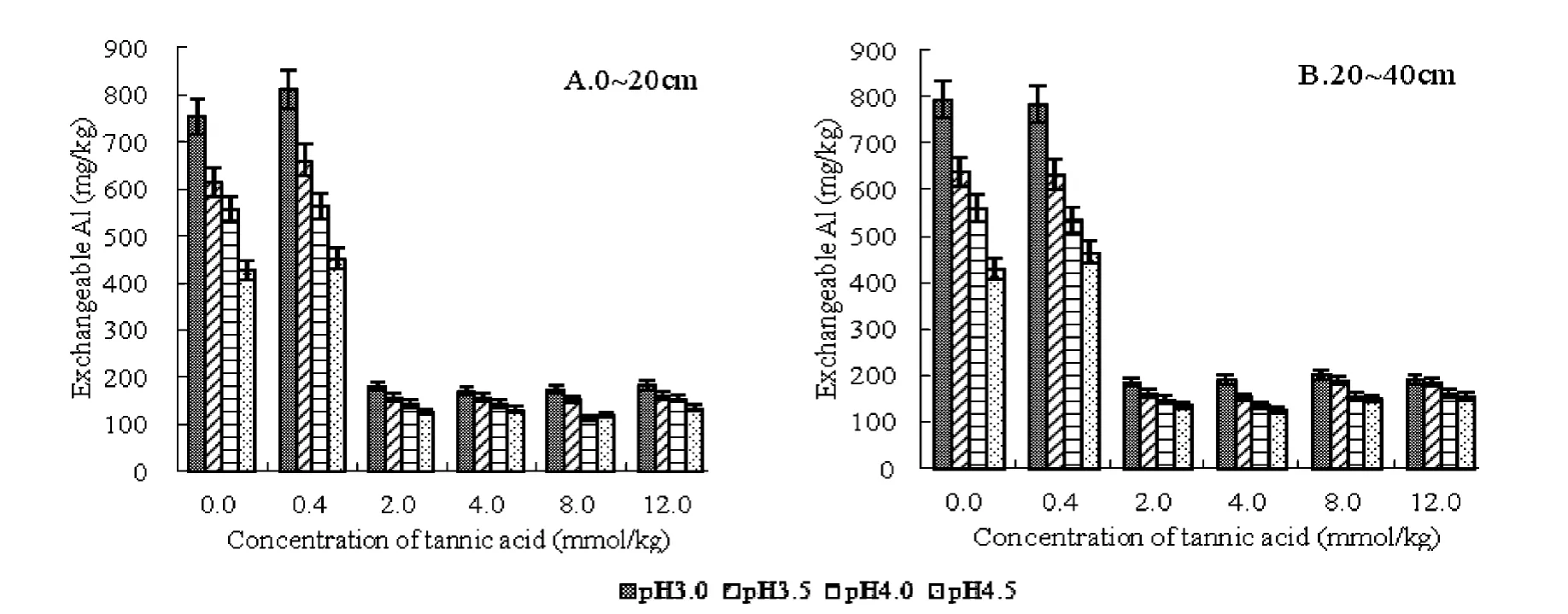
Fig.2.Effects of tannic acid on exchangeable Al content in soils of JX tea garden.
Hydroxyl aluminum Al(OH)2+Al(OH)+2is a form of the smallest proportion of active aluminum,which accounts for about 2% ~5%amount of active aluminum.As you can see from Figure 3,tannic acid concentration 0 mmol/kg,with the increase of soil pH,first increased and then decreased trend of hydroxyl ground aluminum content in the upper soil layer and the lower soil is decreased gradually trend.Tannic acid with concentration of 0.4 mmol/kg,the differences in content of hydroxyl aluminum in the lower soil and upper soil.Concentration of tannic acid is 2.0 mmol/kg,the upper soil hydroxyl aluminum content from 178.8 mg/kg increased to 409.7 mg/kg,showing a rising trend.Analysis the Figure 4,the concentration of tannic acid is added from 0.4 mmol/kg rise to 2.0 mmol/kg,hydroxyl aluminum decreased significantly,and then remaining at a relatively balanced state.Comparing Figures 3 and 4 can be seen in Yunnan tea soil layers 0~20 cm hydroxyl aluminum content higher than 20 ~40 cm soil layer,while the performance of garden soil in Jiangxi opposite.

Fig.3.Effects of tannic acid on hydroxyl Al content in soils of YN tea garden.

Fig.4.Effects of tannic acid on hydroxyl Al content in soils of JX tea garden.
Acid-soluble inorganic aluminum Al(OH)03accounted for 18%to 33%of the total activity of the aluminum with an average of about 25%.From Figure 5 and Figure 6 can see,tannic acid content is the content of 2.0 ~4.0 mmol/kg two tea garden soil acid soluble inorganic aluminum rapidly decreased at a concentration of 4.0 mmol/kg reached the lowest value.Jiangxi garden soil acid-soluble inorganic aluminum content is slightly higher than the garden soil in Yunnan.In different layers,the acid-soluble inorganic aluminum content in the content is basically the same.Acid soluble inorganic aluminum is a special form of precipitation between aluminum and soluble aluminum,which also determines the sensitivity to soil pH is more significant,because of the increase of H+concentration in the soil,will be dissolved to the switching state of aluminum and aluminum hydroxide ground state;while the H+concentration decreases,can make changes to alum precipitated direction.

Fig.5.Effects of tannic acid on acid-soluble Al content in soils of YN tea garden.

Fig.6.Effects of tannic acid on acid-soluble Al content in soils of JX tea garden.
Humic aluminum Al-HA is relatively stable in soil active aluminum shape,its content and the organic group content of the soil is closely related.Under different pH conditions(Figure 7,figure 8),the tannic acid treatment,the morphology of Al-HA in various forms of aluminum in the highest,accounting for 51% ~70%of the total reactive aluminum,an average of about 63%,far higher than other forms.Tannin concentration from 2 mmol/kg to 4 mmol/kg,Al-HA content decreased rapidly in form,8 mmol/kg dropped to the lowest.The tannic acid addition is 0.4 ~2.0 mmol/kg,with the increase of the pH value,the content of humic acid aluminum increase gradually.Comparison of Yunnan and Jiangxi tea garden soil,can be seen that the humic aluminum in the soil of tea garden in Yunnan are significantly higher than the content of Jiangxi tea garden,the former is nearly 2 times of the latter.

Fig.7.Effects of tannic acid on humic-acid Al content in soils of YN tea garden.

Fig.8.Effects of tannic acid on humic-acid Al content in soils of JX tea garden.
3.3.Effect of tannic acid in tea garden soil pH value
Figure 9-A shows that when the amount of tannic acid is added in 0.4 mmol/kg,each soil pH value reached the maximum,and the tannin concentration 0.4 ~4.0 mmol/kg section,soil pH decreased slowly,then with the tannic acid concentration increases,the soil pH decreases significantly.In figure 9-B,pH4.0 and pH4.5 treatment,tannin acid concentration 0 ~2.0 mmol/kg,soil pH value was decreased,in the 2.0 ~4.0 mmol/kg segment,the pH value of soil basic balance.Different concentrations of tannic acid soil,the soil 0 ~20 cm and 20~40 cm soil layers soil pH variation trend,but at the same pH soil,tannic acid on the impact of the lower soil pH is greater than the upper,because the upper soil organic matter content is high,the soil pH changes have certain buffer.

Fig.9.Effects of tannic acid on soil pH value of YN tea garden.
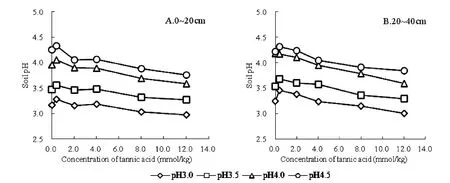
Fig.10.Effects of tannic acid on soil pH value of JX tea garden.
3.4.Different forms of active aluminum garden soil and soil pH,pHBC correlation analysis
In different soil layers,various forms of active aluminum,pH and buffering capacity of soil correlation analysis(Table3)show that the upper layer(0~20 cm)of aluminum in soil exchangeable Al3+and between pH and Al-HA showed a significant negative correlation(P <0.01),but not with pHBC was a significant negative correlation(P < 0.01).Hydroxyl aluminum Al(OH)2+,Al(OH)02and pH and Al-HA between is a significant positive correlation(P <0.05),with pHBC is a significant negative correlation(P <0.05).Acid-soluble aluminum Al(OH)03and between pH and Al-HA weak negative correlation with pHBC positive correlation between weakly.pH and pHBC had very significant positive correlation,the correlation coefficient is 0.852(P <0.01).Comprehensive analysis shows that the effects of different forms of active aluminum in tea garden soil are affected by pHBC and pH changes mainly in the upper soil layer.

Table3.Pearson correlation coefficient between active aluminum species distribution in different pH buffer capacity and pH.
4.Discussion
Different organic acids on the different influences of the system release of aluminum in the various chemical balance.The balance comprises aluminum,aluminum dissolution equilibrium adsorption desorption equilibrium,organic acid,organic acid adsorption desorption equilibrium dissociation equilibrium and aluminum and organic acid complex dissociation equilibrium[32].Increased activity of aluminum in soil of low concentration of tannic acid treated was mainly due to the organic acid cause soil acidification,thus the activation of aluminum in soil,resulting in the low concentration range,with tannic acid concentration increases,increase the content of active aluminum in the soil.In addition,the tannic acid and humic acid may also occur competitive complex of aluminum,so also exists between the organically complex Al and humic acid aluminum into each other[33].Therefore,in this study,showing a low concentration of tannic acid can increase the content of aluminum soil activity,while high concentration of tannic acid can decrease the active aluminum content.This study shows that,although the tannic acid can influence the Al adsorption through the adsorption of tannins,but the aluminum complexation is strong,the complexation of Al content in soil solution increased humic acid aluminum,the adsorption desorption equilibrium towards desorption mobile direction,the total final result is to increase the soluble aluminum,exchange aluminum reduction.
5.Conclusion
As can be seen in this study of tannic acid on different parts of the garden soil in active aluminum content of the inflection point is not the same.Tannins low concentration can improve the soil content of various forms of aluminum,but with the increase in the amount of tannic acid,the various forms of aluminum content are inhibited,with an increase in soil pH,high concentrations tannic acid on the inhibition of the release of active aluminum increased.Added amount of tannic acid concentration in the 0 ~0.4 mmol/kg,the soil pH increased significantly,after they continued to decline,soil pH and tannin concentration at this stage,in line with equation:YpH=(0.04CDN+3.82(P <0.01).In tannic acid concentration reaches 8.0 ~12.0 mmol/kg,soil pH value will not change.pH and tannic acid on the total amount of active aluminum garden soil was mutual weakening effect relationship.
1.Ding Y Z,Li Z A,Zou B.Low-molecular-weight organic acids and their ecological roles in soil.Soil,2005,37(3):243-250.
2.Binkley D,Giardina C.Why do tree species affect soils?The warp and woof of tree-soil interactions.Biogeochemistry,1998,42:89-106.
3.Kraus TEC,Dahlgren R A,Zasoski R J.Tannins in nutrient dynamics of forest ecosystems:A review.Plant and Soil,2003,256:41-66.
4.Lin Y M,Liu J W,Xiang P,et al.Tannin dynamics of propagules and leaves of Kandelia candel and Bruguiera gymnorrhiza in the Jiulong River Estuary,Fu jian,China.Biogeochemistry,2006,78:343-359.
5.Lin Y M,Liu J W,Xiang P,et al.Tannins and nitrogen dynamics in mangrove leaves at different age and decay stages(Jiulong River Estuary,China).Hydrobiologia,2007,583:285-295.
6.Waterman P G,Moles.Analysis of Phenolic Plant Metabolites.Oxford:Black Well Scientific Publications,1994,pp238-239.
7.Northup R R,Dahlgren R A,John G M.Polyphenols as regulators of plant-litter-soil interactions in northern California's pygmy forest:A positive feedback.Biogeochemistry,1998,42:189-220.
8.Kraus T E,Zasoski R J,Dahlgren R A.Fertility and pH effects on polyphenols and condensed tannin contents in foliage and roots.Plant and Soil,2004,262(1/2):95-109.
9.Zhong Z K,Wang R C,Jiang B.Ecological feedback significance of polyphenols in organic layer of forest soil.Chinese Journal of Applied Ecology,2003,14(3):341-344.
10.Xu R K.Organic acids on the dissolution of aluminum and species distribution of aluminum in acidic soils.Soil,1998,(4):214-217.
11.Yu T R,Ji G L.Electrochemical Methods of Soil and Water Research.Beijing:Science Press,1991,pp348-382.
12.Huang P M.Soil mineral-organic matter-microorganism interactions:Fundamentals and impacts.Advances in Agronomy,2004,82:391-472.
13.Yu G,Saha UK,Kozak L M,Huang P M.Kinetics of cadmium adsorption on aluminum precipitation products formed under the influence of tannate[J].Geochim.Cosmochim.Acta,2006,70(20):5134-5145.
14.Hu Y F,Xu R K,Dynes JJ,Blyth RIR,Yu G,Kozak LM,Huang P M.Coordination nature of aluminum(oxy)hydroxides formed under the influence of tannic acid studied by X-ray absorption spectroscopy.Geochim.Cosmochim.Acta,2008,72(8):1959-1969.
15.Qin R J,Chen F X.Low molecular weight organic acid ions to reduce the role of soil aluminum toxicity.Soil and Fertilizer,1996,5:12-14.
16.Jin X,Lv C L,Sun S H,et al.Research progress on extraction of secondary metabolites of trees.Forest Pest and Disease,2008,27(1):31-34.
17.Gao L J,Cui J H,Liu F Y,et al.Plant secondary metabolites application and development.Bulletin of Biology,2004,39(7):15-17.
18.Li H P,Wang Z G,Yang M S,et al.The relation between tannin and phenol constituents and resistance to Anoplophora glabripennis of various poplar tree species.Journal of Agricultural University of Hebei,2003,26(1):36-39.
19.Zhang L P,Sun C X,Li J Q,et al.The present conditions and development trend of plant polyphenols research.Scientia Silvae Sinicae,2005,41(6):157-162.
20.Lv C M,Fan H Y,Jiang H,et al.Research advances on synthesis of secondary metabolities by plant cell culture.Journal of Yunnan Agricultural University,2007,22(1):1-7.
21.Lu R K.Methods for Chemical Analysis of Soil.Version 3.Beijing:China Agricultural Science and Technology Press,2000,pp126-129.
22.Wu C H,Yu W,Yin J Y,et al.Study on the leaching of active aluminum from sludge and the distribution of aluminum species.Environmental Chemistry,2001,20(3):262-264.
23.Li X Y.Soil Chemistry.Beijing:Higher Education Press,2001,pp217-218.
24.Cheng J M,Hu G L,Pan G X.New method for evaluating buffering capacity and equilibrium pH of paddy soil with simulation parameter.Journal of Agro-environmental Science,2004,23(3):569-573.
25.Zhang Y C,Wang J D,Shen M X,et al.Effects of long-term fertilization on soil acidification in Taihu Lake region China.Acta Pedologica Sinica,2010,47(3):465-471.
26.Shen Y,Yi Y L,Zhang D G,et al.Research on pH buffer capacity and acidification rate of arable brown soil.Journal of Soil and Water Conservation,2012,26(1):95-100.
27.Tan K H.Degradation of soil minerals by organic acids.In:Huang P M,Schnitzer M.Eds.Interactions of Soil Minerals with Natural Organics and Microbes;Soil Science Society of American,Madison,WI,1996,pp1-28.
28.Vance G F,Stevenson F J,Sikora F J.Environmental chemistry of aluminum-organic complexes.In:Sposito G.ed.The Environmental Chemistry of Aluminum(Second edition);CRC Press,Inc.,1996,pp170-176.
29.Stumm W,Wieland E.Dissolution of oxide and silicate minerals:Rates depend on surface speciation.In:Stumm W.Ed.Aquqtic Chemical Kinetics;Wiley,New York,1990,pp367-400.
30.Pan G X.Improved acid-base titration curve method used to study the response of soil to acid rain.Journal of Nanjing Agricultural University,1991,14(4):128-132.
31.Yang S Q,Zhang A P,Yang Z L,et al.Change of orchard soil pH in the Loess Plateau.Chinese Journal of Eco-Agriculture,2010,18(6):1385-1387.
32.Xu R K,Ji G L,Jiang X.Effect of low-molecular-weight organic acids on aluminum release from kaolinite.Acta Pedologica Sinica,2002,39(3):334-339.
33.Yu J,Yu Y C,Fang L,et al.Effects of low-molecular-weight organic acids on the pH and the form of aluminum of forest soils.Journal of Fujian College of Forestry,2005,25(3):243-246.
- 茶叶的其它文章
- Stability of Tea Catechins and Antioxidant Properties of Green Tea Extracts as Affected by Boiling-treatment
- The 30-Day Oral Administration Studies of Liposoluble Tea Polyphenols in Rats
- Oxidative Stability of Green Tea Extract-Enriched Rice Bran Oil During Storage
- Analysis of the Volatile Chemicals of Longjing Tea from Different Production Locations Using Electronic Nose
- A Study on Physiological Character of Fresh Tea Leaves in Different Cold-Resistant Varieties
- Analysis of Volatile Compounds of Jinmudan Oolong Tea by Different Wrapping-Twisting

