Decreased klotho expression and elevated blood phosphate level in presenilin PS1/PS2 conditional double knockout mice
WANG Dong,ZHAI Wen-zhu,SU Jing-jing,MENG Bo,ZHAO Zheng,HU Jin-feng
(Key Laboratory of Brain Functional Genomics,Ministry of Education,Shanghai Key Laboratory of Brain Functional Genomics,East China Normal University,Shanghai 200062,China)
0 Introduction
The klotho protein has been characterized as an anti-aging factor[1].Klotho deficiency in mice causes penetrant phenotypes resembling human premature aging syndromes,such as a short life span,growth retardation,infertility,ectopic calcification,cognitive impairment,premature thymic involution,and neurodegeneration[1-3].In contrast,klotho overexpression extends the mouse lifespan by 20%~30%[4].Klotho protein,which has two forms(a TypeⅠtransmembrane form and a secreted form),is predominantly expressed only in the kidney and brain,but has distinct functions in the whole body[1,5,6].Klotho is critical in maintaining the phosphate and calcium homeostasis,via its interaction with fibroblast growth factor 23(FGF23)[7],transient receptor potential vanilloid 5(TRPV5)[8,9],and Na+,K+-ATPase[10].It also represses insulin/IGF-1signaling[4],enhancing the expression level of antioxidant enzymes to protect cells and tissues from oxidative stress[11].Most recent reports documented that klotho also serves as an antiinflammatory modulator[12-15].
Presenilin PS-1and PS-2are subunits ofγ-secretase,which is one of the key modulators of amyloid precursor protein(APP)shedding.The PS1/PS2conditional double knockout(PS cDKO)mice(forebrain-specific knockout of PS1and whole-body knockout of PS2)develops multiple age-dependent phenotypes that mimic human Alzheimer’s disease(AD),agrievous aging-related neurodegenerative disease.These phenotypes include brain atrophy,synaptic dysfunction,hyperphosphorylation of tau,cognitive impairment,and increased inflammatory responses[16-19].Recently,some phenotypes in PS cDKO mice were also found to expand from CNS to periphery,such as increased oxidative stress[20]and inflammation[19].Notably,we recently demonstrated that a promising derivative of xanomeline(a muscarinic M1/M4agonist)that could effectively ameliorate several neurodegenerative phenotypes of PS cDKO mice[21].
Klotho level has been previously found to decline in an age-associated manner in animals[22]and human[23].Decreased klotho expression has also been found both in human patients with chronic renal failure(CRF)[24]and in diabetes animal models[15].Whether klotho also participates in the syndromes of PS cDKO mice has so far not been elucidated.In the present study,using PS cDKO mice as an ideal existing aging-related disease model,we analyzed the renal and brain klotho level and several klotho-involved phenotypes,such as the blood level of calcium and inorganic phosphate,as well as kidney function,to explore whether klotho is involved in the phenotypes of PS cDKO mice.
1 Materials and methods
1.1 Animals
The PS cDKO mice were obtained as previously described[16,21].Female mice of 3-month-and 12-month-old that carried the transgene Cre,fPS1/fPS1,and PS2-/-were used as PS cDKO mice while their age-matched littermates(without Cre,fPS1/+and PS2+/+or fPS1/+and PS2+/-)served as control mice.All mice were raised under SPF conditions(12-hour light/dark cycle,20~26℃,and 40%~70%humidity).
1.2 Blood testing
After an overnight fast,mice from each group were anesthetized with chloral hydrate,and freshly heparinized blood was withdrawn from the retro-orbital sinus.The blood samples were then centrifuged at 3 500r/min for 15min at 4℃and plasma were carefully collected and stored immediately at-80℃until use.The calcium,inorganic phosphate,blood urine nitrogen(BUN)and creatinine(CRE)concentrations of the plasma were determined with commercial kits(Phosphate:Shanghai Fosun Long March Medical Science Co.,China;Calcium,BUN;and CRE:Wako,Japan)using an automated analyzer(Hitachi-7020,Japan),according to manufacturer’s instructions.Blood samples of the same age were collected and assayed in the same batch.
1.3 Protein extraction and Western blotting
All mice were deeply anesthetized and then sacrificed.Kidneys and the left brain hemispheres were quickly dissected out,snap frozen in liquid nitrogen,and stored at-80℃until protein extraction was performed.Tissues were homogenized and lysed in a lysis buffer(Beyotime,China)containing 1mM PMSF,followed by centrifugation at approximately 11 500r/min at 4℃for 5min.Western blotting procedures were previously described in a preceding paper[21].Protein concentrations were determined by a BCA method(Beyotime,China).Equal amounts of protein(40μg)were loaded to SDS-PAGE,resolved,and then transferred onto nitrocellulose membranes(Millipore,USA).The blots were incubated for one hour at room temperature first with 5%non-fat milk,then primary antibodies,and subsequently secondary antibodies.The protein bands were visualized with an ECL kit(CWBIO,China)and analyzed with a Quantity One GelDoc XR gel imaging system(Bio-Rad,USA).Relative protein levels were normalized toβ-actin and expressed as a percentage of control mice.Antibodies were:anti-klotho(1∶1 000;R&D,USA),anti-β-actin(1∶1 000;Cell Signaling Technology,USA),and proper HRP-conjugated secondary antibodies(1∶5 000;Kangchen,China).
1.4 Statistics
All data were analyzed using GraphPad Prism 5.01(GraphPad Software,USA)and were expressed as means±SEM.Unpaired Student’s t-test was used for individual comparisons.Statistical significance was considered at p<0.05.
2 Results
2.1 Significantly reduced renal klotho expression in 12-month-old PS cDKO mice
Compared with age-matched control mice,a significant reduce in renal klotho level was found in 12-month-old female PS cDKO mice(see Fig.1Band D;n=3,p<0.05),while there was no significant difference in renal klotho level between PS cDKO and control mice at 3months of age(see Fig.1Aand C).
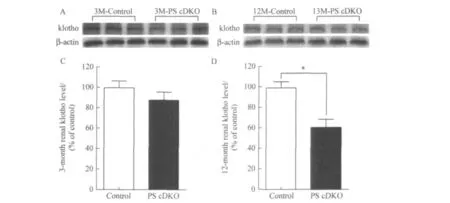
Fig.1 Renal klotho level of 3-month-old(A and C)and 12-month-old(B and D)female PS cDKO or control mice(error bars show means+SEM,*p<0.05,n=3)
2.2 Significantly reduced brain klotho level in 3-month-old PS cDKO mice
In brains,as early as 3months of age,decreased klotho level was observed in PS cDKO mice,compared with control mice of the same age(see Fig.2;n=3,p<0.05).

Fig.2 Brain klotho level of 3-month-old female PS cDKO or control mice(error bars show means+SEM,*p<0.05,n=3)
2.3 Elevated blood phosphate and slightly reduced blood calcium in 12-month-old PS cDKO mice
We further analyzed the effects that may be caused by renal klotho downregulation.Considering klotho as a key regulator of calcium and phosphate homeostasis,we first tested the blood calcium and inorganic phosphate level.Plasma levels of calcium and phosphate didn’t differ between PS cDKO and control mice at 3months of age(see Fig.3).However,at the age of 12months,slightly reduced plasma calcium((2.93±0.03)vs·(3.08±0.07)mg·dL-1in control mice,p<0.05,n=5~9;see Fig.3A)and significantly elevated plasma phosphorus((2.06±0.17)vs·(1.46±0.17)mmol·L-1in control mice,p<0.05,n=5~9;see Fig.3B)were observed in PS cDKO mice.

Fig.3 Plasma calcium(A)and inorganic phosphate(B)level of 3-month-and 12-month-old female PS cDKO or control mice(error bars show means+SEM,*p<0.05,n=5~9)
2.4 No significant sign of kidney dysfunction in PS cDKO mice
As klotho expression is downregulated in patients with CRF and is regarded as an early biomarker for CRF[24,25],we tested the kidney function of PS cDKO mice by measuring the levels of blood urine nitrogen(BUN)and creatinine(CRE),two markers reflecting renal function.As shown in Fig.4,no difference in BUN between 3-month-old PS cDKO and control mice were observed,while at the age of 12months,a tiny increase in BUN in PS cDKO mice was detected((12.16±0.26)vs·(10.90±0.19)mg·dL-1in control mice,p<0.01,n=5~7;see Fig.4A).However,the level of blood CRE,a more sensitive indicator of impaired kidney function,didn’t differ between PS cDKO and control mice,at age of both 3and 12months(see Fig.4B),indicating no obvious sign of renal dysfunction in PS cDKO mice.
3 Discussion
Klotho is an anti-aging factor that elicits collective effects in aging process and agingrelated disorders.Age-associated decline of klotho expression in brain and periphery has been found in animal models and human subjects[22,23].Suppressed renal klotho level is also observed in CRF animal models and human patients[24,26].Multiple observations on klotho deficient mice indicated that klotho may be involved in neurodegeneration.For instance,klotho deficient mice displayed reduced Purkinje cells[1]and mesencephalic dopaminergic neurons[27],as well as impaired cognitive functions[2].Mild but not serve neurodegeneration was observed in histological investigations[3],possibly due to the short life span of klotho deficient mice(only about 60.7days)[1].In addition,recently,it was also found that klotho gene expression is regulated by soluble amyloid precursor proteinβ(sAPPβ)[28].

Fig.4 Blood urine nitrogen(BUN)(A)and plasma creatinine(CRE)(B)of 3-month-and 12-month-old female PS cDKO or control mice(error bars show means+SEM,**p<0.01,n=5~7)
PS cDKO mice have a series of AD-like phenotypes that makes these mice a useful model for age-related degeneration disorders.Several therapeutic strategies,including calorie restriction(CR)[29]and environment enrichment(EE)[30],were found to be beneficial in this model.We recently identified a promising derivative of xanomeline(a muscarinic M1/M4agonist)that effectively ameliorated several phenotypes of PS cDKO mice[21].Herein,using PS cDKO mice as an age-related disease model,we tested the hypothesis that klotho may be involved in the phenotypes of PS cDKO mice.Compared with control mice,decline in klotho level was observed both in brain and in kidney,at the age of 3and 12months,respectively,indicating apossible role of klotho in the disorders of PS cDKO mice.Further analysis revealed a significant elevation in plasma phosphorus and slight reduction in plasma calcium in 12-month-old PS cDKO mice,however,no significant signs of renal dysfunction were detected.
The blood phosphate level is mainly regulated by vitamin D and parathyroid hormone(PTH)to maintain a balance between dietary intake,mobilization from bone,and excretion into urine[31].The active vitamin D(1,25-dihydroxyvitamin D3)increases the blood level of calcium and phosphate,while PTH,on the other hand,only increases blood calcium level with little effects on blood phosphate.The membrane form of klotho acts as a coactivator of FGF23[7,32],a bone-derived hormone that binds to FGF receptor 1.With the indispensable help of klotho,the FGF23signaling is activated to suppress vitamin D synthesis and downregulate phosphate reabsorption in kidney.Accordingly,klotho deficient mice have higher blood phosphate,calcium,and 1,25-dihydroxyvitamin D3levels[33].Besides FGF23pathway,klotho(secreted form)also interacts with ion channels to regulate transepithelial calcium transport:①it activates the TRPV5to increase calcium(re)absorption in the kidney and intestine[8];②it also binds to Na+,K+-ATPase and promotes its surface recruitment in response to a low extracellular calcium solution[10].Recently,translocation-caused klotho overexpression in a female patient resulted in hypophosphatemic rickets and hyperparathyroidism[34].In contrast,hyperphosphatemia is commonly found in CRF patients[35],accompanied by hyperparathyroidism.In PS cDKO mice,declined renal klotho expression may also be responsible for the excess blood phosphate.The blood calcium of aged PS cDKO mice,however,was decreased,which was different from klotho deficient mice,possibly due to some other mechanisms.It seemed that such a moderate decline in klotho level and impaired phosphate metabolism were not sufficient to further elicit renal dysfunction in PS cDKO mice.Even though,as klotho is a hormonal factor with multiple distinct functions in the whole body,klotho insufficiency may contribute to some other phenotypes of PS cDKO mice.
Klotho is also a suppressor of oxidative stress.It improves the expression of antioxidant enzymes to enhance resistance to oxidative stress,via suppressing insulin/IGF-1signaling[11]or activating cAMP-dependent pathway[36].Therefore,increased oxidative stress level was observed in the brains of klotho deficient mice[2].Interestingly,in PS cDKO mice,increased oxidative stress was also found,as revealed by enhanced lipid peroxidation both in brain and in periphery[20,37].The declined klotho level may be involved in such an elevation of oxidative stress.
It should be noted that klotho expression declined earlier in brain(at 3months of age)than in kidney(at 12months of age),while the elevation of oxidative stress occurred as early as 2to 4months of age,both in brain and in periphery[20,37].Considering these,it is possible that declined klotho level may contribute to the enhanced lipid peroxidation in cortex,but not in periphery.In the periphery of PS cDKO mice,only PS2is knocked out.The PS2homozygous deficient mice,however,develops little obvious phenotypes,except for age-dependent mild pulmonary fibrosis and hemorrhage[38].Therefore,the declined renal klotho level may not be the consequence of PS2knockout.The lack of klotho-involved phenotypes in PS2deficient mice favors the possibility that the renal klotho decline may also be caused by PS cDKO.
Quite recently,several reports revealed that klotho also served as an anti-inflammation factor[12-15].Both in endothelial cells[12]and in diabetic kidneys[15],klotho inhibits inflammatory response by suppressing tumor necrosis factor(TNF)-α-induced NF-κB activation and subsequent formation of inflammatory cytokines.Meanwhile,renal klotho is negatively regulated by inflammation and downregulated by TNF-αand TNF-like weak inducer of apoptosis(TWEAK)via NF-κB signaling[14].It is also reported that membrane form of klotho suppressed retinoic acid-inducible gene I(RIG-I)mediated inflammation by inhibiting interleukin-6(IL-6)and IL-8expression[13].In PS cDKO mice,increased inflammatory responses were found both in brain and in periphery,beginning at 3and 6months of age,respectively[18,19].As revealed by antibody array analysis,in 9-month-old PS cDKO mice,TNF-αlevel was elevated in both cortex and serum,by 15.7and 2.8folds,respectively,compared with control mice[19].Collectively,it could be inferred that in PS cDKO mice,increased inflammatory response may decrease klotho expression;and the decreased klotho level may further drive inflammation.The detailed relationship between klotho and oxidative stress and inflammation remains to be elucidated.
[1]KURO-O M,MATSUMURA Y,AIZAWA H,et al.Mutation of the mouse klotho gene leads to a syndrome resembling ageing[J].Nature,1997,390(6655):45-51.
[2]NAGAI T,YAMADA K,KIM H C,et al.Cognition impairment in the genetic model of aging klotho gene mutant mice:a role of oxidative stress[J].Faseb J,2003,17(1):50-52.
[3]SHIOZAKI M,YOSHIMURA K,SHIBATA M,et al.Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice[J].Neuroscience,2008,152(4):924-941.
[4]KUROSU H,YAMAMOTO M,CLARK J D,et al.Suppression of aging in mice by the hormone klotho[J].Science,2005,309(5742):1829-1833.
[5]MATSUMURA Y,AIZAWA H,SHIRAKI-IIDA T,et al.Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein[J].Biochemical and Biophysical Research Communications,1998,242(3):626-630.
[6]SHIRAKI-IIDA T,AIZAWA H,MATSUMURA Y,et al.Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein[J].FEBS Letters,1998,424(1-2):6-10.
[7]URAKAWA I,YAMAZAKI Y,SHIMADA T,et al.Klotho converts canonical FGF receptor into a specific receptor for FGF23[J].Nature,2006,444(7120):770-774.
[8]CHANG Q,HOEFS S,VAN DER KEMP A W,et al.The beta-glucuronidase klotho hydrolyzes and activates the TRPV5channel[J].Science,2005,310(5747):490-493.
[9]CHA S K,ORTEGA B,KUROSU H,et al.Removal of sialic acid involving klotho causes cell-surface retention of TRPV5channel via binding to galectin-1[J].Proc Natl Acad Sci USA,2008,105(28):9805-9810.
[10]IMURA A,TSUJI Y,MURATA M,et al.alpha-Klotho as a regulator of calcium homeostasis[J].Science,2007,316(5831):1615-1618.
[11]YAMAMOTO M,CLARK J D,PASTOR J V,et al.Regulation of oxidative stress by the anti-aging hormone klotho[J].Journal of Biological Chemistry,2005,280(45):38029-38034.
[12]MAEKAWA Y,ISHIKAWA K,YASUDA O,et al.Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation[J].Endocrine,2009,35(3):341-346.
[13]LIU F,WU S,REN H,et al.Klotho suppresses RIG-I-mediated senescence-associated inflammation[J].Nature Cell Biology,2011,13(3):254-262.
[14]MORENO J A,IZQUIERDO M C,SANCHEZ-NINO M D,et al.The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB[J].Journal of the American Society of Nephrology,2011,22(7):1315-1325.
[15]ZHAO Y,BANERJEE S,DEY N,et al.Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA(serine)536phosphorylation[J].Diabetes,2011,60(7):1907-1916.
[16]FENG R,WANG H,WANG J,et al.Forebrain degeneration and ventricle enlargement caused by double knockout of Alzheimer’s presenilin-1and presenilin-2[J].Proc Natl Acad Sci USA,2004,101(21):8162-8167.
[17]SAURA C A,CHOI S Y,BEGLOPOULOS V,et al.Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration[J].Neuron,2004,42(1):23-36.
[18]BEGLOPOULOS V,SUN X,SAURA C A,et al.Reduced beta-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice[J].Journal of Biological Chemistry,2004,279(45):46907-46914.
[19]JIANG X,ZHANG D,SHI J,et al.Increased inflammatory response both in brain and in periphery in presenilin 1and presenilin 2conditional double knock-out mice[J].Journal of Alzheimers Disease,2009,18(3):515-523.
[20]ZHU M,GU F,SHI J,et al.Increased oxidative stress and astrogliosis responses in conditional double-knockout mice of Alzheimer-like presenilin-1and presenilin-2[J].Free Radical Biology and Medicine,2008,45(10):1493-1499.
[21]WANG D,YANG L,SU J,et al.Attenuation of neurodegenerative phenotypes in Alzheimer-like presenilin 1/presenilin 2conditional double knockout mice by EUK1001,apromising derivative of xanomeline[J].Biochemical and Biophysical Research Communications,2011,410(2):229-234.
[22]DUCE J A,PODVIN S,HOLLANDER W,et al.Gene profile analysis implicates klotho as an important contributor to aging changes in brain white matter of the rhesus monkey[J].Glia,2008,56(1):106-117.
[23]YAMAZAKI Y,IMURA A,URAKAWA I,et al.Establishment of sandwich ELISA for soluble alpha-klotho measurement:Age-dependent change of soluble alpha-klotho levels in healthy subjects[J].Biochemical and Biophysical Research Communications,2010,398(3):513-518.
[24]KOH N,FUJIMORI T,NISHIGUCHI S,et al.Severely reduced production of klotho in human chronic renal failure kidney[J].Biochemical and Biophysical Research Communications,2001,280(4):1015-1020.
[25]HU M C,KURO-O M,MOE O W.Secreted klotho and chronic kidney disease[J].Advances in Experimental Medicine and Biology,2012,728:126-157.
[26]SUGIURA H,YOSHIDA T,TSUCHIYA K,et al.Klotho reduces apoptosis in experimental ischaemic acute renal failure[J].Nephrology Dialysis Transplantation,2005,20(12):2636-2645.
[27]KOSAKAI A,ITO D,NIHEI Y,et al.Degeneration of mesencephalic dopaminergic neurons in klotho mouse related to vitamin D exposure[J].Brain Research,2011,1382:109-117.
[28]LI H,WANG B,WANG Z,et al.Soluble amyloid precursor protein(APP)regulates transthyretin and Klotho gene expression without rescuing the essential function of APP[J].Proc Natl Acad Sci USA,2010,107(40):17362-17367.
[29]WU P,SHEN Q,DONG S,et al.Calorie restriction ameliorates neurodegenerative phenotypes in forebrainspecific presenilin-1and presenilin-2double knockout mice[J].Neurobiology of Aging,2008,29(10):1502-1511.
[30]DONG S,LI C,WU P,et al.Environment enrichment rescues the neurodegenerative phenotypes in presenilinsdeficient mice[J].European Journal of Neuroscience,2007,26(1):101-112.
[31]BERNDT T,KUMAR R.Phosphatonins and the regulation of phosphate homeostasis[J].Annual Review of Physiology,2007,69:341-359.
[32]KUROSU H,OGAWA Y,MIYOSHI M,et al.Regulation of fibroblast growth factor-23signaling by klotho[J].Journal of Biological Chemistry,2006,281(10):6120-6123.
[33]TSUJIKAWA H,KUROTAKI Y,FUJIMORI T,et al.Klotho,agene related to a syndrome resembling human premature aging,functions in a negative regulatory circuit of vitamin D endocrine system[J].Molecular Endocrinology,2003,17(12):2393-2403.
[34]BROWNSTEIN C A,ADLER F,NELSON-WILLIAMS C,et al.A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism[J].Proc Natl Acad Sci USA,2008,105(9):3455-3460.
[35]KESTENBAUM B,SAMPSON J N,RUDSER K D,et al.Serum phosphate levels and mortality risk among people with chronic kidney disease[J].Journal of the American Society of Nephrology,2005,16(2):520-528.
[36]RAKUGI H,MATSUKAWA N,ISHIKAWA K,et al.Anti-oxidative effect of klotho on endothelial cells through cAMP activation[J].Endocrine,2007,31(1):82-87.
[37]GU F,ZHU M,SHI J,et al.Enhanced oxidative stress is an early event during development of Alzheimer-like pathologies in presenilin conditional knock-out mice[J].Neuroscience Letters,2008,440(1):44-48.
[38]HERREMAN A,HARTMANN D,ANNAERT D,et al.Presenilin 2deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1deficiency[J].Proc Natl Acad Sci USA,1999,96(21):11872-11877.

