Source and Formation of the Arsenic in Ground Water in Hanoi , Vietnam
Do Van Binh
Hanoi University of Mining and Geology
Abstract: The problem of Arsenic source in groundwater has yet been addressed thoroughly. From the results of the analysis of samples, this article gives the statement that the Arsenic in groundwater of Quaternary sediments in Hanoi has mainly natural source, with the impact of man-made factors (industrial waste water, use of crop protection products, etc.). The article also explains the formation of Arsenic in groundwater in Hanoi area is closely related to the reduction by two main mechanisms, reducing mechanism of oxyhydroxit (Fe3 + OHAs) As liberation by microorganisms and reducing mechanism of As adsorbed on iron oxide or oxyhydroxit replaced by bicarbonate. The process of oxidation of minerals containing As is needed to be researched more.
1. Source of arsenic (As) in groundwater
As in groundwater has two sources: natural and artificial. Natural origin due to the natural process of birth: process of oxidization, the process of elimination, the biochemical process. Artificial origin due to the human activities of birth: burning fossil fuels, emissions, waste water containing As,use of plant protection drugs, pesticides, irrigation,industrial waste ...
On the basis of analysis of As in the samples:river water, lake water, waste water, groundwater,soil it can be stated that As in groundwater of Quaternary sediments in Hanoi area has the natural origin or geological origin.
Waste water is discharged from many sources:domestic, industrial, medical, craft, cultural services, human and animal excretion, etc. Among the total of 245 samples (Nguyen Van Dan and Tong Ngoc Thanh 2001) waste water were analysed, therea are 204 samples with As content of less than 0.05 mg/l, accounting for 83%. Only 41 samples with high As content higher than 0.05 mg/l, accounting for 17%. Highest value of As in the samples of groundwater analysed in To Lich River and Kim Nguu River is higher than 0.05 mg/ l, but the average value of the samples is low(<0.05 mg/l).
The result of As analysis in samples of the Red River and Nhue River shows that As content is quite low. Of the 90 analysis results of Red River water samples, no sample of As exceeds the value of 0.05 mg / l, accounting for 0%. Of the 130 analysis results of water samples of Nhue River,only 4 samples of As exceeds the value of 0.05 mg/ l, accounting for 3%. Total number of samples with As content higher than 0.05 mg/l in both the Red River and Nhue River is 4/220 samples,accounting for 1.8%.
As content in water in many ponds and lakes in the whole of Hanoi is quite low. Of the 42 samples analyzed only one sample with As content higher than 0.05 mg / l (Phu Ninh Lake), the remaining samples with low As content, below the level of 0.05 mg / l.
Irrigated area covers an area of growing plants in suburban districts. Analysis results of samples in the boreholes P44, P54, P67 and P79 in the irrigated area show low levels of As in water, less than 0.05 mg / l. A small amount of As has source from man-made activities, in some samples of waste water, the high content of As is found.
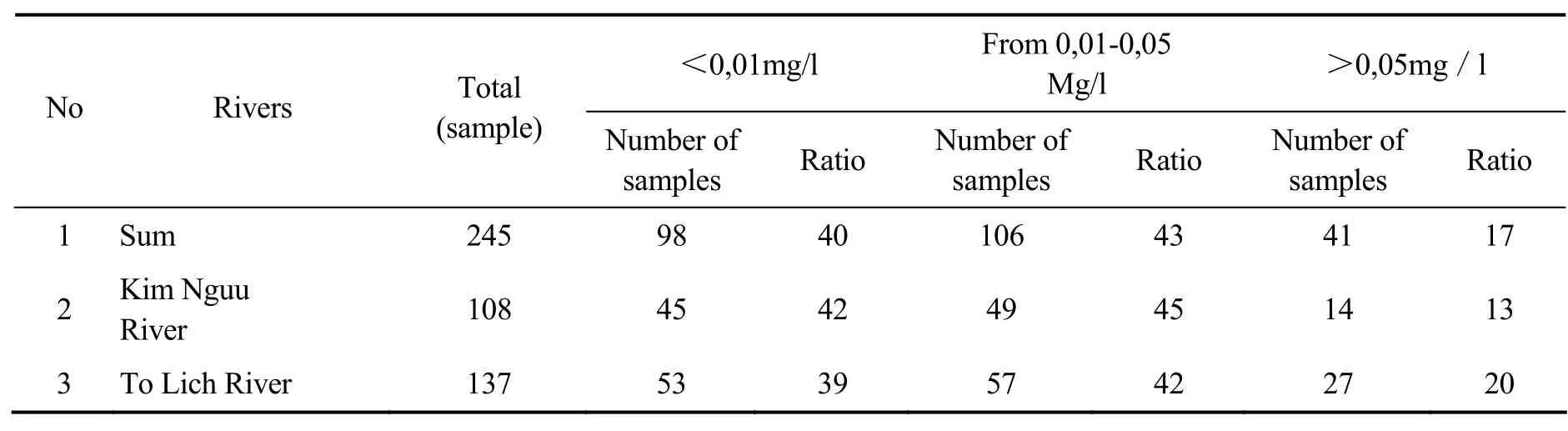
Table 1 Analysis results of As in water of Kim Nguu River and To Lich River (Bibudhendra Sarkar 2002)
Comments: The highest value of As in the water samples analyzed in To Lich River and Kim Nguu is higher than 0.05 mg / l, but the average value of the samples is low. Compared to the limit of 0.05 mg / l, the measured values of the samples meet the demand of waste water quality.
The use of plant protection drugs, chemicals for the preservatives of wood, paints, cosmetics ...will provide a certain amount of As to groundwater,especially on the uppermost aquifer (qh). Of the more than 200 types of plant protection drugs being used, almost 100% of the drugs contain As.Excessive drug use will gradually accumulate in the soil and leach into groundwater.
From the analysis of the As content in water of river, pond, lake, waste water, irrigation...compared to the content of As in groundwater regulated in the standards and regulations of Vietnam, it can be stated that As in groundwater of the Quaternary Aquifer in Hanoi has mainly natural origin (origin of geology, Do Van Binh 2007). Fig. 1 shows the points have taken samples.
Fig. 1 The points have take samples
2. The formation of As in water
The formation and form of existence of As in water is a complex process, dependent synthesis of the geochemical process, hydro-geochemistry,microorganisms, reducing environment or oxidization ... The formation of As from the soil into the water is shown in its geochemical process in the environment (Fig. 2)
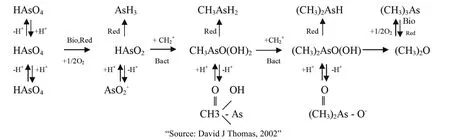
Fig. 2 Summary of environmental geochemical of As.
In the groundwater environment, depending on the values of pH and Eh that main form of existence of As also changes following to the rules shown in Fig. 3.
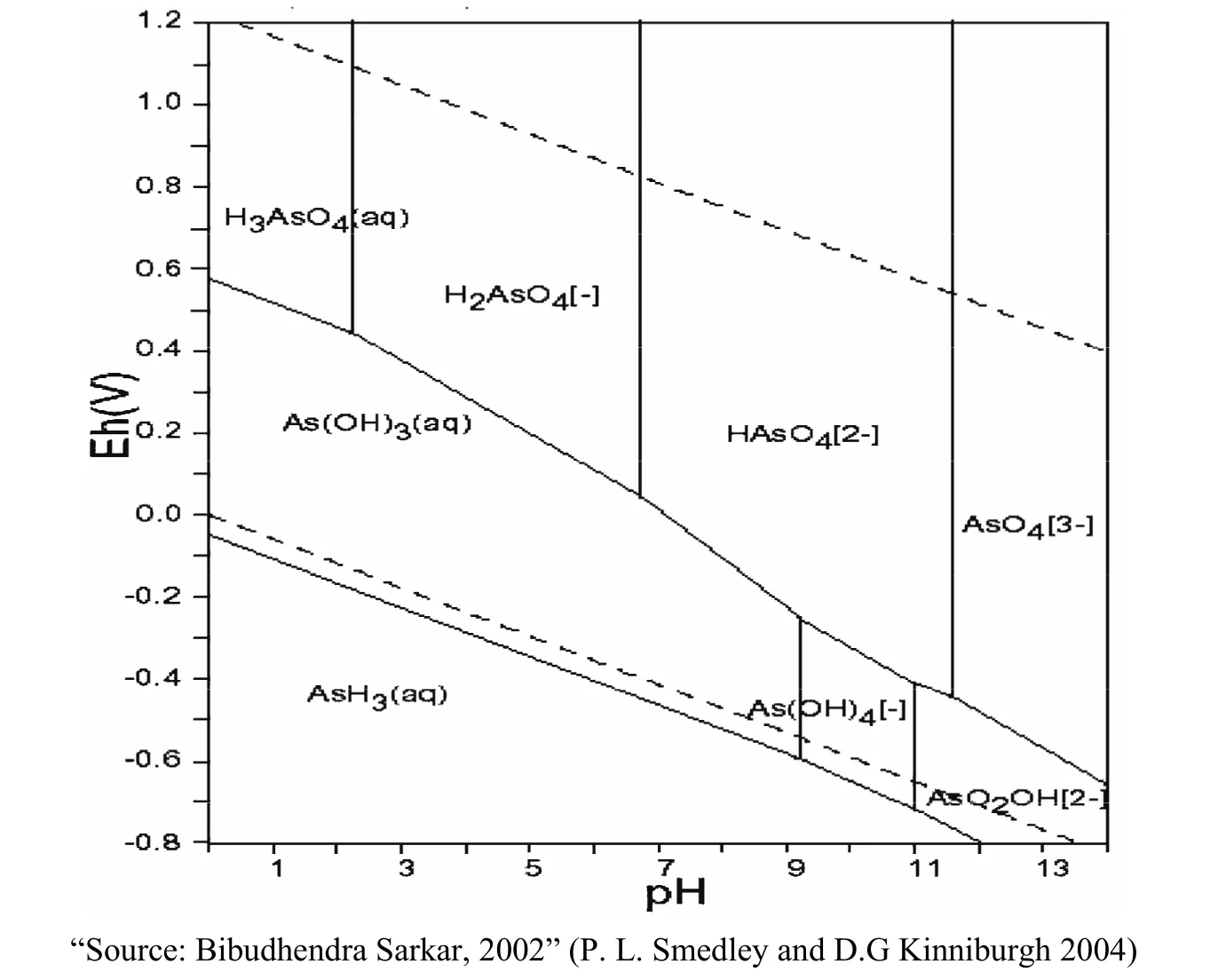
Fig. 3 Forms of existence of As depending on the pH and Eh
From Fig. 2 we see that As (+5) exists quite commonly in the upper half of the graph, i.e. in the oxidized conditions (Eh> 0), whereas in reducing conditions (Eh<0), As (+3) dominates (the lower half of the graph). PH value was found that when pH <6.5, As mainly exists in the form ofand when pH> 6.5, As mainly exists in the form of. When doing research on As, in addition to directly determine As in the different types of ion using the methods of analysing samples, it can also indirectly know the existence of As based on the value of Eh and pH measured in the field.
Besides the role of pH and the oxidization, the geochemical transformation of As moving from soil into water has the very important role of microorganisms (Dang Duc Nhan 2009). So based on the type of sediment we can guess the level of abundance of As in soil and water in that environment.
3. As in groundwater in the Hanoi area is formed by the reducing
Groundwater in Quaternary sediment aquifers in Hanoi is mainly water of bicarbonate - calcium or bicarbonate-calcium-magnesium (Fig. 4), a small number of samples is type of bicarbonate -calcium, sodium. Figures 4a and Figure 4b show the water in the results of sample analysis in Hanoi in the rainy season and dry season.
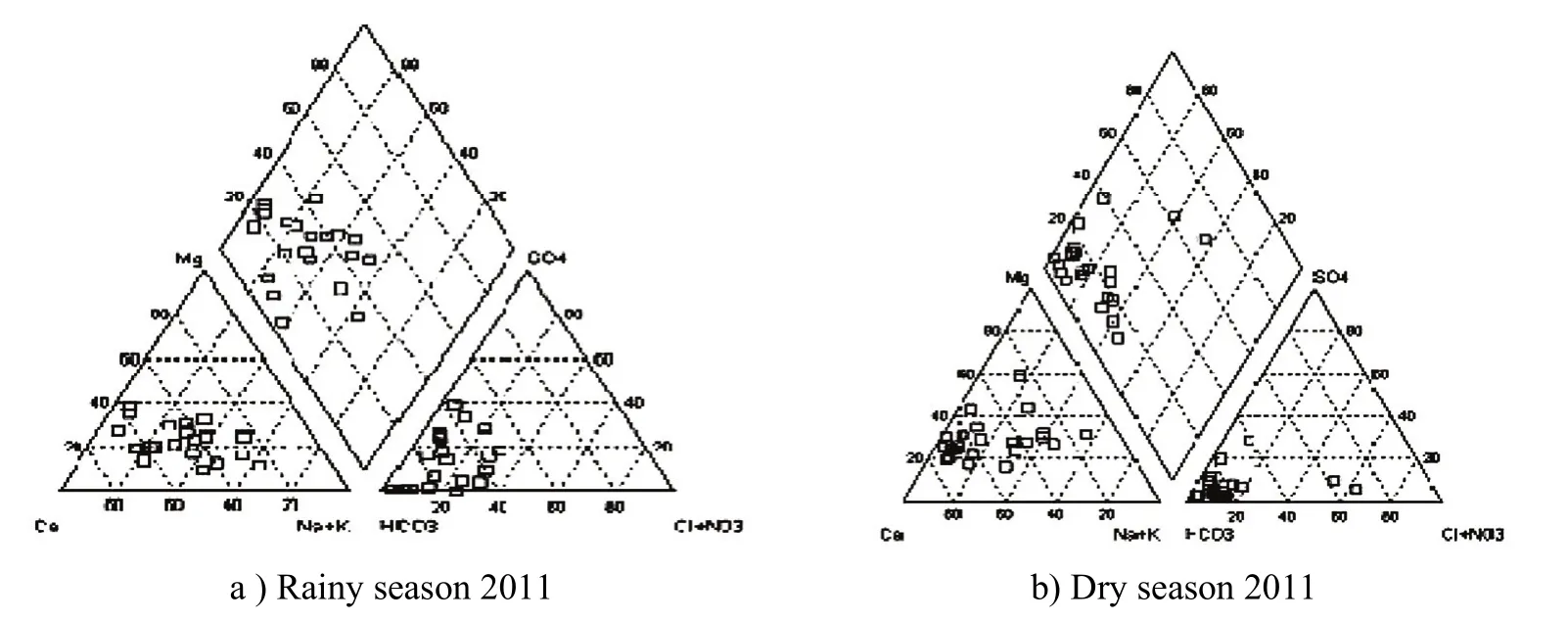
Fig. 4 Pipe Graph shows water in Hanoi in the rainy season and dry season
Pipe graph of the groundwater samples in Hanoi area was built on the basis of the results of the analysis of the chemical components of water samples in the seasons, in which Figure a is rainy season and Figure b is the dry season. All the samples shown on the graph shows the concentration of them in the form of calcium bicarbonate, magnesium. Pipe Graph also shows the nature of groundwater in Hanoi area was built on the basis of the results of analysis of the chemical compositions of water in the dry season of 2010.
Correlation between the content of As in groundwater and the content of bicarbonate HCO3-(or also known as alkalinity) is quite closely related (R2=0,8). Figure 5 shows that close correlation.
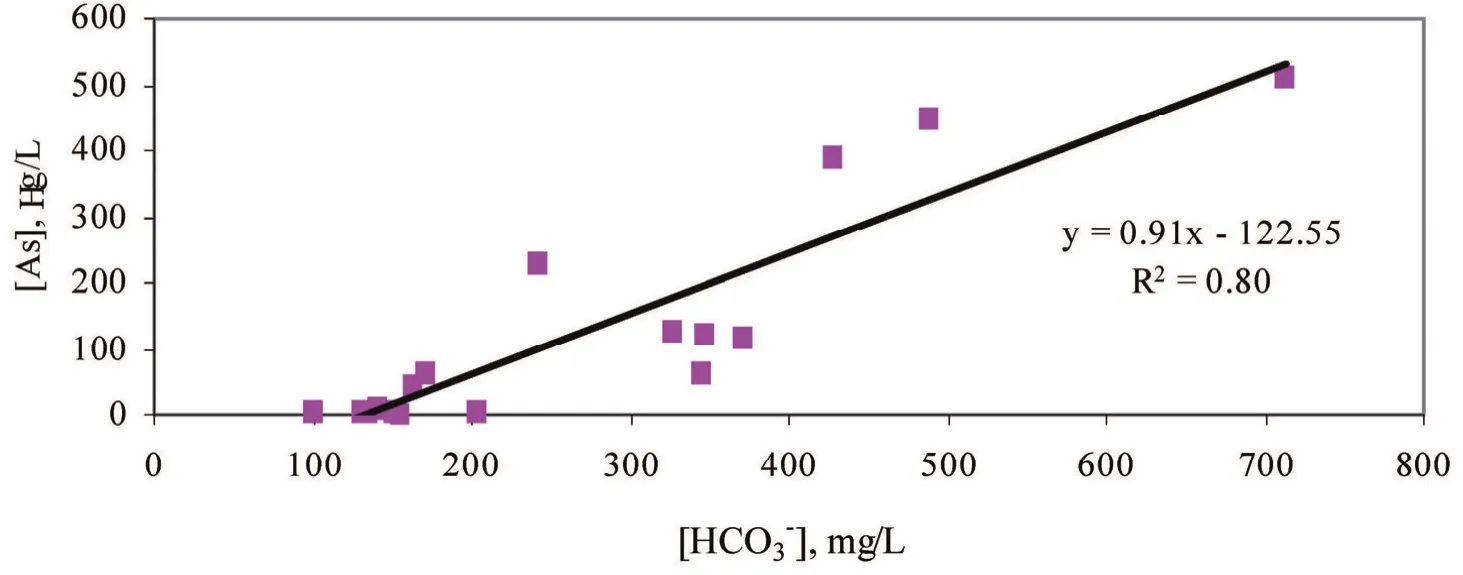
Fig. 5. The relationship between bicarbonate and As in groundwater samples (9/2010)
From the results above, it can be said that As in groundwater in Hanoi (old city) was formed by 2 main reduction mechanism:
reduction oxyhydroxit mechanisms(Fe3+OOHAs) liberate As caused by microorganisms:
Sand and soil always contain an amount of adsorption As (Ravenscroft et al. 2001). In the process of adsorption, As is "absorbed" and stay close to the iron mineral particles with organic compounds. The process of accumulation of As and the materials containing it happens during the long geological process of the formation of the delta. In the sedimentary environment, in the absence of oxygen under the influence of microbial activity, the iron complexes (containing As adsorbed) will convert from chemotherapy +3 into+2 chemotherapy disolving into water and releasing As . That process occurs in the following steps:
Organic compounds (NOM - Natural Oganic Mass) are decomposed by microbial activities or by the respiration of roots releasing CO2and water by the equation:
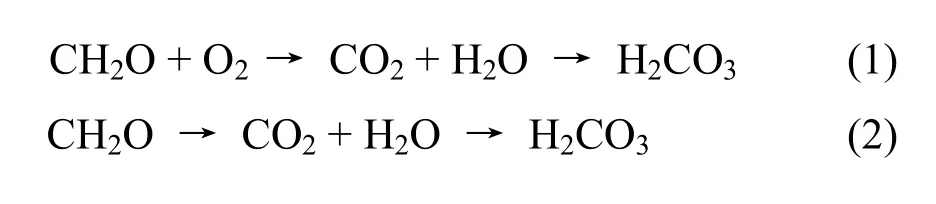
Where H2CO3is the symbol of the process of hydration of carbon dioxide.
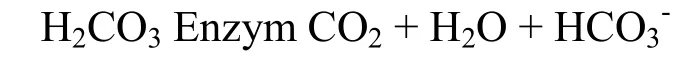
The iron complex chemotherapy +3 will be melted to create chemotherapy +2 according to the reaction:
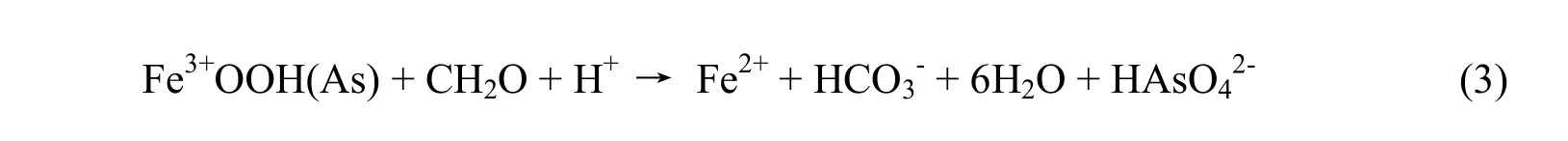
Thus, to get the reaction (3) there must be Fe3+OOHAs, microorganisms (found in organic material of mud clay layers) and moisture conditions, a favorable environment.
Mechanism of reduction of As adsorbed on iron oxide or oxyhydroxit replaced by bicarbonate
As appeared in groundwater is due to the replacement of it by other ions which directlyexisting in complexes of iron. This phenomenon is explained as follows: As in the river water has a thermo dynamical balance with bicarbonate content. When the water level is lowered, river water will supplement to groundwater. The new balance between iron oxide or iron hydrit with the bicarbonate will be set.Bicarbonate source in this case is generated mainly due to dissolution of calcite or Mg-calcite which are very common components in unconsolidated sediments in the delta. Process of giving born tois represented by the reactions (4); (5); (6);(7); (8); (9) below:
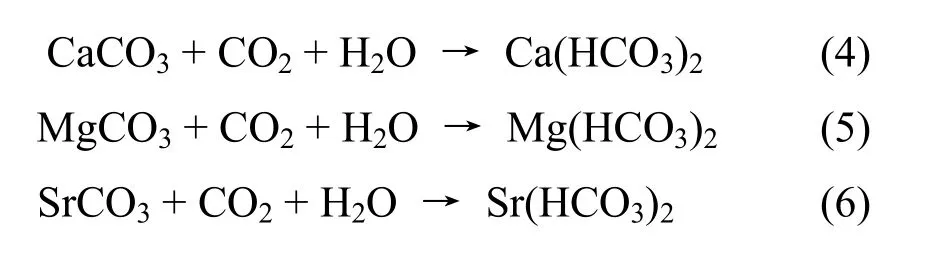
On the right side, substances are dissolved ones,so they dissociate and createby the equation
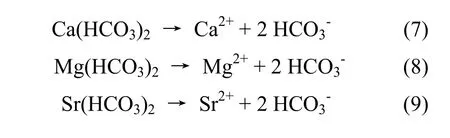
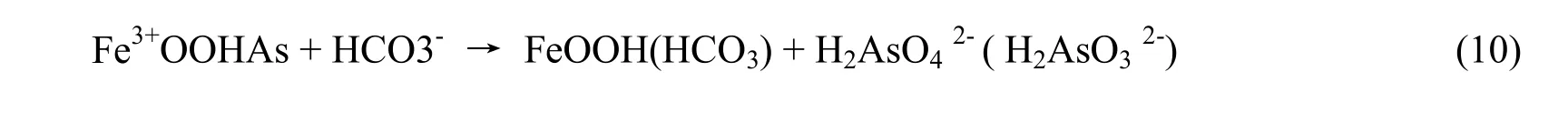
4. As in groundwater formed by oxidization of minerals
In nature there are thousands of minerals containing As. Particularly the group of hydroarsenat and arsenat have 213 minerals, the group of sulfurarsenat has 73 minerals ... Minerals containing As content with different rates, usually from a few mg / kg to several hundred mg / kg. In soil, there are fragments of minerals containing As with high content. The process of oxidizating Arsenopyrite ore, a mineral containing As is shown in equation (11a) and (11b):
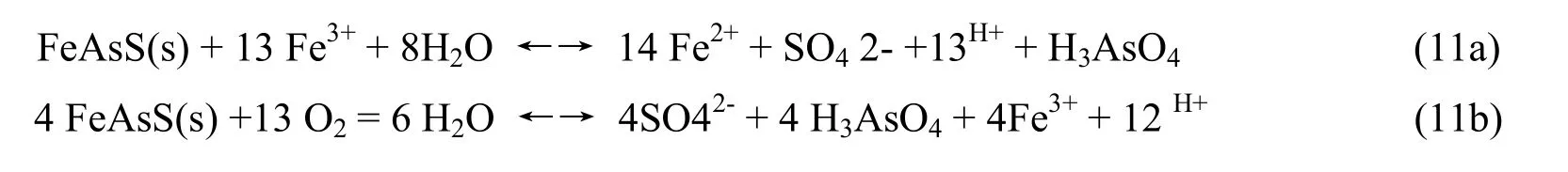
Arsenic acid is a weak acid so it is easier to dissociate according to the following equation:
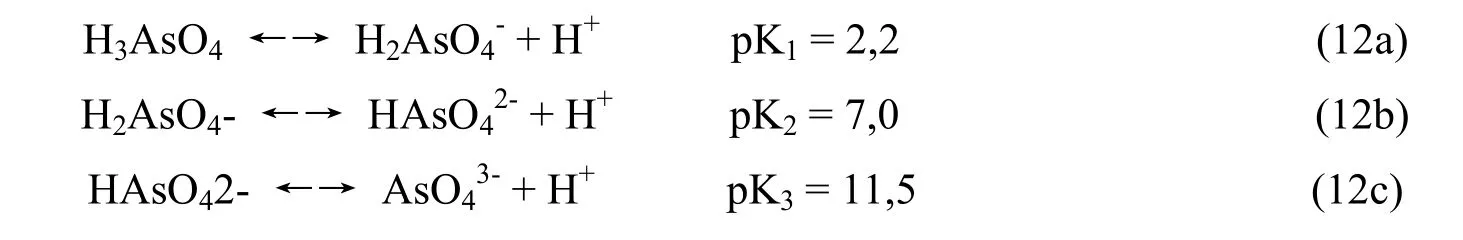
Arsenate acid H3AsO3is also a weak acid so it is easier to dissociate according to the Equation13 (a, b, c)
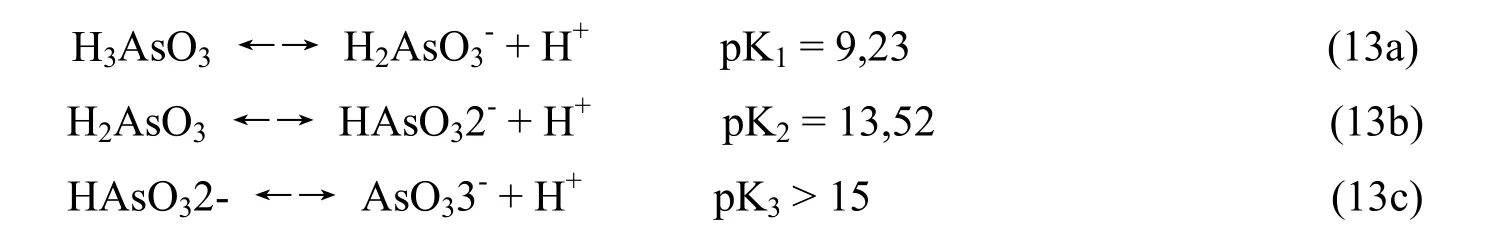
Therefore in the water there always exist two forms of As with chemotherapy (+3) and (+5). The rate of As (+3) and As (+5), depending on pH conditions and oxidation - redox environmet. The researches of scientists around the world have proved that in condition of pH = 7 As exists 50%in the form ofand 50% in the form of( Bibudhendra Sarkar 2002; P. L.Smedley and D.G Kinniburgh 2004). In the natural groundwater environment As (+3) exists primarily in the form of neutral, iearseno acid. (Le Van Cat 2005).
For Delta region, there are not many documents about the mineral composition of unconsolidated sediments As is formed from ore or not, and in what level should be further investigated.
Below are a few pictures describing the release of arsenic from the iron (Fig. 6).
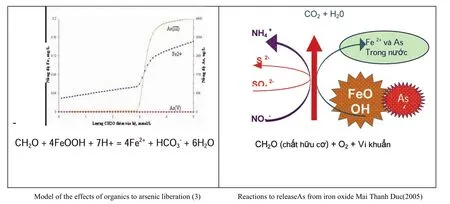
Fig. 6 Arsenic release from iron
- 地下水科学与工程(英文版)的其它文章
- Study on Ecological Environment and Sustainable Land Use Based on Satellite Remote Sensing
- Stable Isotope Composition of Rainfall, Surface Water and Groundwater along the Yellow River
- Organic Contamination of Soil and Goundwater in the Piedimont Plain of the Taihang Mountains
- Comparison of Three Brine Migration Models in Groudwater
- Effect of Farmyard Manure Application on Dissolution of Carbonate Rocks and Its Eco-environmental Impact
- Groundwater quality Management in China

