Loss of Function of Ferredoxin2 Can Promote Flowering in Arabidopsis thaliana
LIU Bing,WEI Haixuan,SU Jianbin,ZHANG Yang,WANG Jinfa,WANG Hongbin ,FENG Dongru
(State Key Laboratory of Biocontrol//Guangdong Key Laboratory of Plant Resources,School of Life Sciences,Sun Yat-sen University,Guangzhou 510275,China)
Flowering is the turning point from the vegetative stage to the reproductive stage in the growth process of plants.In Arabidopsis thaliana,the flowering time is regulated by many different pathways:gibberellin,autonomous,vernalization,and light-dependent pathways[1-2].
Plants utilize light as the signal to determine the timing of flowering.In Arabidopsis thaliana,there are a lot of various photoreceptors to monitor light quality and quantity.These photoreceptors can perceive light to regulate the flowering time[3].In Arabidopsis,these photoreceptors include the red/far-red light-absorbing phytochromes(PHYs)(PHYA to PHYE)[3-5]and the blue/UV-A light-absorbing cryptochromes(CRYs)(CRY1 and CRY2)[6-9],phytochromes and cryptochromes work together to involve in regulating flowering[5,10]. In the previous studies, we have known the far-red light can promote flowering[5,11,12]and the far-red light photoreceptor mutant——phyA mutant displays a late-flowering phenotype when grown both under long-day and short-day conditions[10].In contrast,red light can delay flowering[5,11-13]and the red light photoreceptor mutant——phyB mutant displays an early-flowering phenotype when grown both under long-day and short-day conditions[10].Moreover,the phyA phyB double mutant also displays an early-flowering phenotype when grown under long-day and short-day conditions[10].So it could be concluded that the impairment in the physiology function of phytochromes can promote flowering in the overall effect.
In plants,to sense and harvest the light signals,the photoreceptors must maintain some structures under the participation of the chromophores.In plants,open chain tetrapyrroles can function as the chromophores of light-sensing phytochromes[14].To synthesize tetrapyrrole chromophores,the heme is first linearized by heme oxygenase to form the open-chain tetrapyrrole intermediate ——biliverdin IXα (BV).BV is subsequently reduced by phytochromobilin(PФB)synthase ——HY2,which is a ferredoxin-dependent bilin reductase to produce the PФB chromophore for light-sensing phytochromes[15-16].Therefore HY2 plays very important role in the formation of phytochromes and the physiology function of phytochromes.Moreover,the hy2 mutant also displays the early-flowering phenotype[17],this result further supports the conclusion that the impairment of the physiology function of phytochromes can promote flowering in the overall effect.
Ferredoxins(Fds)are the major donor systems for electrons to many various receptor systems in plastids.In photosynthetic organisms,Fds play roles not only in electron transfer system of photosynthesis but also in many redox reactions mediated by several oxidoreductases,such as sulfite reductase,nitrite reductase,glutamate synthase,and ferredoxin:thioredoxin reductase[18].In Arabidopsis thaliana,the PФB synthase ——HY2 has been shown to require ferredoxins(Fds)as the electron donors for reduction reaction[15-16].
Here,we found that a Ds-T-DNA insertion line of Arabidopsis thaliana for the gene encoding the most major ferredoxin(Fd2,At1g60950)can promote flowering in the process of growth both under long-day and short-day conditions.We show in this report that the loss of AtFd2functioncan promote flowering,AtFd2 can interact with AtHY2 in the chloroplast,and the loss of AtFd2 function can impair the responses mediated by phytochromes.These results implicate that the loss of AtFd2 can promote flowering by impairing the physiology function of phytochromes.
1 Materials and methods
1.1 Plant materials
For all experiments shown in this work,Arabidopsis thaliana plants of the ecotype Noessen WT and Fd2-KO were sent by Renate Scheibe and determined as described[17].
1.2 Flowering phenotype studies
The Noessen WT and Fd2-KO Arabidopsis thaliana seeds were germinated on soil and grown under long-day conditions(16h light/8h dark)or short-day conditions(8 h light/16 h dark)illuminated by white light at a fluence rate of 120μmol/m2/s.The number of days from germination to bolting,the number of rosette leaves when bolting,and the number of cauline leaves at maturity were scored.
1.3 BIFC study
Plasmid HY2-YN was made by cloning the AtHY2 fragment without the stop codon from Arabidopsis cDNA with the primers 5'-AGTCGACATGGCTTTATCAATGGAGT-3'and 5'-TTACTCGAGGCCGATAAATTGTCCT-3'and inserting into the pUC-SPYNE(YN)multiple cloning sites(MCS)with SalⅠand XhoⅠ.
Plasmid Fd2-YC was made by cloning the AtFd2 fragment without the stop codon from Arabidopsis cDNA withtheprimers5'-TAGGATCCATGGCTTCCACTGCTCT-3'and5'-GCCCTCGAGAACAATGTCTTCTTCTT-3'and inserting into the pUC-SPYCE(YC)multiple cloning sites(MCS)with BamHⅠand XhoⅠ.
Plasmids bZIP63-YN and bZIP63-YC were made by cloning the bZIP63 fragment without the stop codon from Arabidopsis cDNA and inserting into the pUCSPYNE and pUC-SPYCE multiple cloning sites(MCS)with BamHⅠand XhoⅠ.
Construct pairs of bZIP63-YN and bZIP63-YC,HY2-YN and Fd2-YC,pUC-SPYCE(YC)and HY2-YN,pUC-SPYNE(YN)and Fd2-YC,YN and YC were transiently expressed in rice protoplasts isolated from 9-day-old rice seedlings.Fluorescence was observed using an Olympus fluorescent microscope and visualized with Olympus DP2-BSW software.
1.4 Hypocotyl Measurements
Arabidopsis thaliana seeds were surface-sterilized in 50%bleach for 15 min and washed five times with sterile ddH2O before plating on a 1?2 MS plate with 0.8%(w)Agar.The seeds were vernalized in the dark at 4℃ for 4 days.Germination was induced with a 4 hr white light treatment.The seeds were followed grown at 23℃ in a LED chamber(Percival Scientific,Perry,IA)under dark and certain light conditions(red or far-red light)for 6 days.For both photograph and hypocotyl length measurements,6-day-old seedlings were photographed using a camera,and hypocotyl length was measured using ruler.
2 Results
2.1 Loss of AtFd2 can promote flowering in Arabidopsis thaliana
We found that the Fd2-KO mutant flowered earlier than wild type both under long-day(16h light/8h dark)and short-day(8 h light/16h dark)conditions(Figure 1A and 2A).In order to further support the phenotype,we chose three most common physiological indexes(the number of days from sowing to bolting,the number of rosette leaves when bolting,and the number of cauline leaves at maturity)to score.These physiological indexes could reflect the period of flowering,generally speaking,the numerial number was smaller,the flowering was earlier.The results showed that the number of days from sowing to bolting,the number of rosette leaves,and cauline leaves of Fd2-KO mutant were all smaller than that of wild type(Figure 1B-D and Figure 2B-D).So we confirmed that the loss of AtFd2 can promote flowering in Arabidopsis thaliana,and the loss of AtFd2 mayinvolve in the regulation of flowering in Arabidopsis thaliana through some pathway.
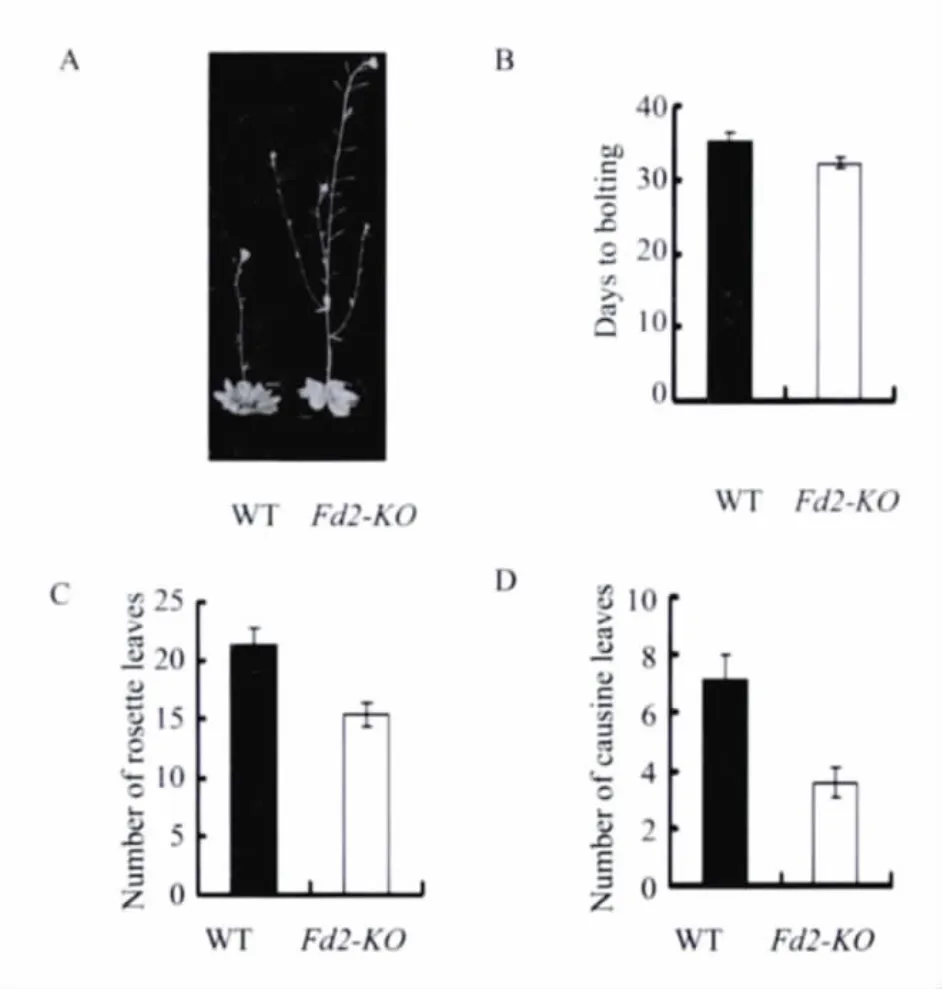
Fig.1 Phenotypes of the Noessen WT and Fd2-KO Arabidopsis plants under long-day conditions
2.2 AtFd2 interacts with AtHY2 in the chloroplast
In order to further confirm whether AtFd2 interacts with AtHY2,we created the plasmid for expression of AtHY2 fused to the N-terminal yellow fluorescence protein(YN)under control of the 35S-CaMV promoter and the plasmid for expression of AtFd2 fused to the C-terminal yellow fluorescence protein(YC)under control of the 35S-CaMV promoter.And we transiently expressed HY2-YN and Fd2-YC together in the rice protoplasts.The fluorescence emission of YFP was monitored in rice protoplasts using an Olympus fluorescence microscope.We found that co-expression of the HY2-YN and Fd2-YC fusion proteins in rice green tissue protoplasts produced obvious YFP signals in the chloro-plast(Figure 3D).This result is in agreement with several previous studies that AtHY2 and AtFd2 are localized to the chloroplast.And the result confirms that AtHY2 physically interacts with AtFd2.As the negative controls,co-expression of HY2-YN with empty pUC-SPYCE(YC)vectors,emptypUC-SPYNE(YN)with Fd2-YC vectors,or two empty vectors pUC-SPYNE(YN)with pUC-SPYCE(YC)produced no BiFC fluorescence(Figure 3A-C).As a positive control,the bZIP63-YN and bZIP63-YC fusion proteins in rice green tissue protoplasts produced obvious YFP signals in the nucleus(Figure 3E).
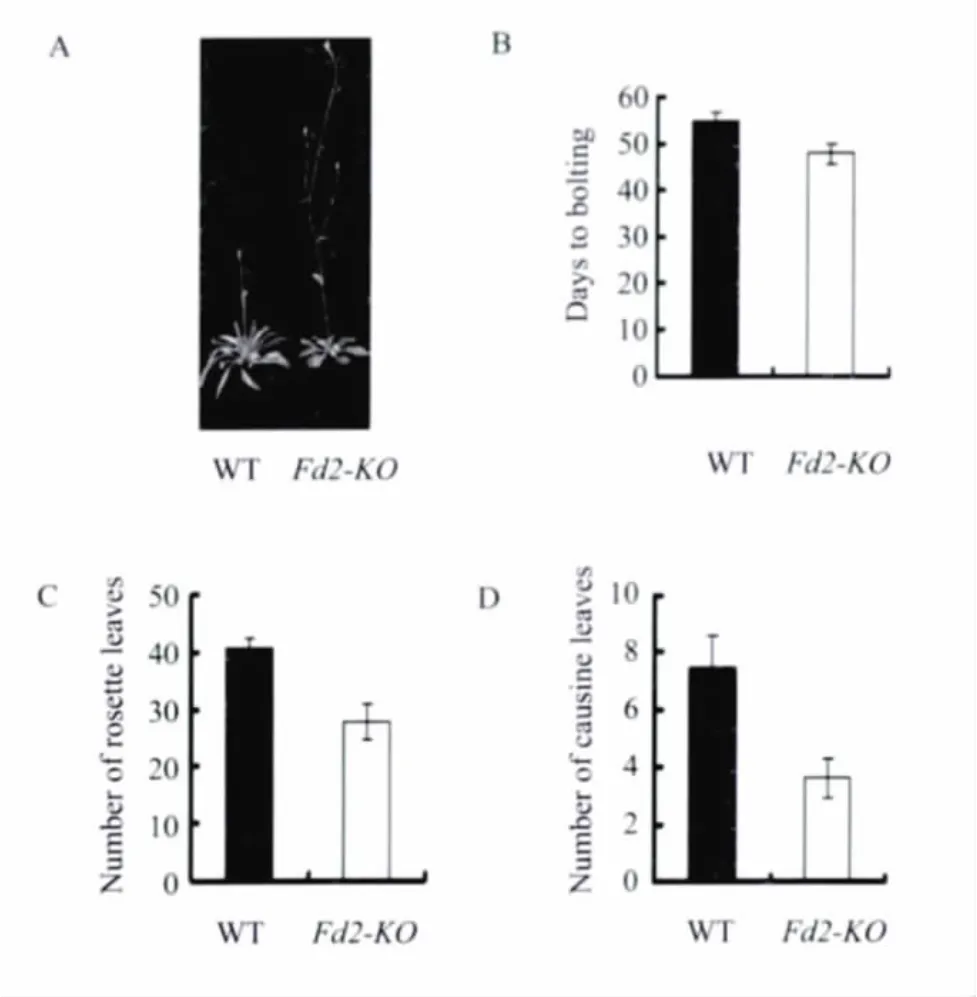
Fig.2 Phenotypes of the Noessen WT and Fd2-KO Arabidopsis plants under short-day conditions
2.3 Fd2-KO mutants are impaired in the responses mediated by phytochromes
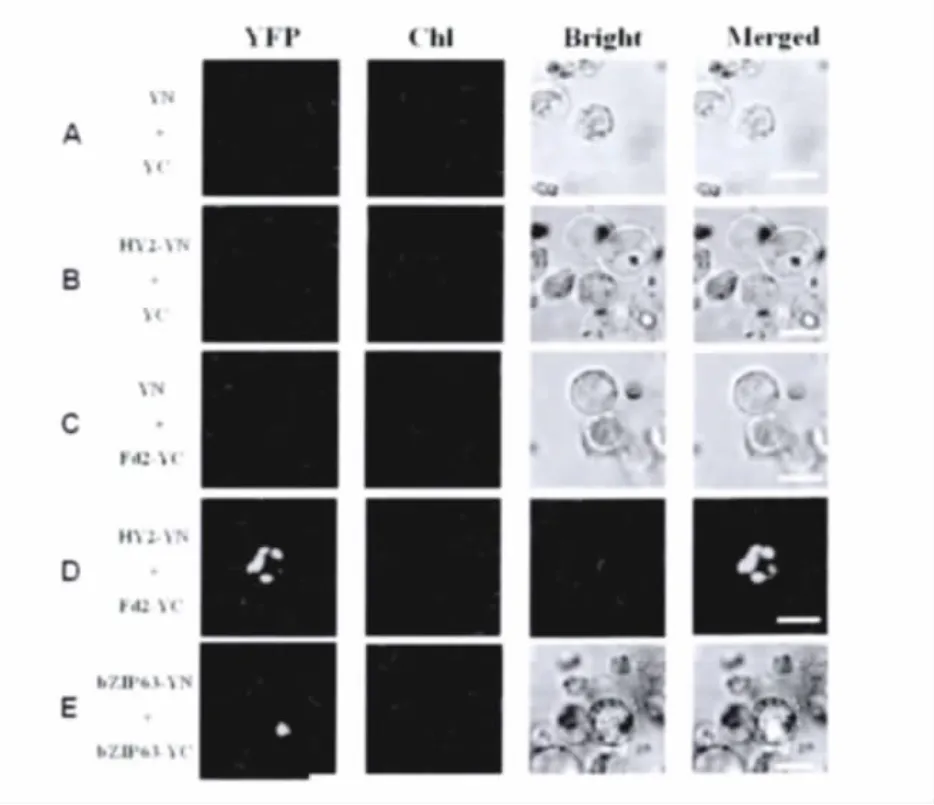
Fig.3 AtFd2 interacts with AtHY2 in the chloroplast
In order to study whether Fd2-KO Mutants promote flowering by impairing the responses to both red light and far-red light mediated by phytochromes,we first examined the response to red light mediated by phytochromes in Fd2-KO seedlings by measurement of hypocotyl lengths of 6-day-old seedlings compared to wild type grown under continuous red light.In dark conditons,Fd2-KO seedlings had shorter hypocotyl lengths than wild type since the loss of AtFd2 inhibited the normal growth of seedling.But the gap of the hypocotyl length between Fd2-KO seedlings and wild type seedlings reduced with the increasement of the red light intensity(Figure 4A).Moreover,Fd2-KO seedlings had longer hypocotyl length than wild type seedlings under high red light intensity(Figure 4B).
The response to far-red light mediated by phytochromes was also examined in Fd2-KO seedlings by measurement of hypocotyl lengths of 6-day-old seedlings grown under continuous far-red light compared to wild type.The result was similar with the result of red light(Figure 5A and 5B).These results indicate that Fd2-KO Mutants are impaired in the responses mediated by phytochromes and implicate that the loss of At-Fd2 may promote flowering by impairing the physiology function of phytochromes.
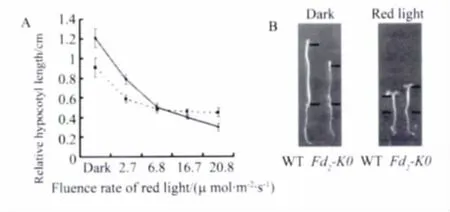
Fig.4 Phenotypes of the Noessen WT and Fd2-KO Arabidopsis seedlings under red light conditions.
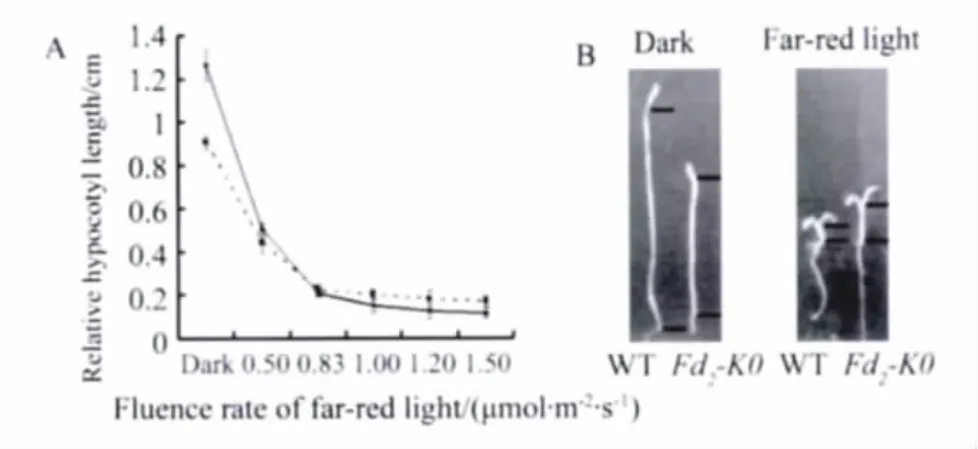
Fig.5 Phenotypes of the Noessen WT and Fd2-KO Arabidopsis seedlings under far-red light conditions.
3 Discussion
Based on the phenotype of Fd2-KO mutants,which flowered earlier than wild type both under longday and short-day conditons,we proposed the possible pathway AtFd2 regulates in flowering.
The previous studies have shown that phytochromes are involved in the regulation of flowering.The far-red light photoreceptor-PHYA and the red light photoreceptor-PHYB are the most important phytochromes in Arabidopsis thaliana.Based on the effect of far-red/red light and the phenotype of phyA、phyB、phyAphyB mutants[5,10-13],we may come to the conclusion that the impairment of the physiology function of phytochromes can promote flowering in the overall effect.
And we learned that one of the most important enzymes in the synthesis of chromophore for light-sensing phytochromes ——HY2,which is ferredoxin-dependent and requires ferredoxins(Fds)as the electron donors to perform itsnormalphysiologicalfunction[15-16].Moreover,the hy2 mutant also flowered early[18],this result further supported the conclusion that the impairment of the physiology function of phytochromes can promote flowering in the overall effect.
AtFd2 is the most important ferredoxin in Arabidopsis thaliana,so we guessed that the loss of AtFd2 may promote flowering by impairing the physiology function of phytochromes.
We demonstrated that AtFd2 can interact with AtHY2 in chloroplast.Though the previous studies have proven that AtHY2 requires AtFd2 as the electron donors through the biochemical and structural studies,our results first prove that AtFd2 can interact with AtHY2 in vivo through BIFC.The results further support that AtHY2 is ferredoxin-dependent and may require AtFd2 as the electron donors for double bond reductions.
In conclusion,all the results implicated that the loss of AtFd2 may promote flowering by impairing the physiology function of phytochromes.However,because of the difference between the physiology progress mediated by phytochromes and the flowering progress,we can identify the genetic regulation relationship between AtFd2 and phytochromes to further confirm our conclusions in the following research.
[1]JAEGER K E,GRAF F.The control of flowering in time and space[J].J Exp Bot,2006,57(13):3415 -3418.
[2]KOMEDA Y.Genetic regulation of time to flower in Arabidopsis thaliana[J].Annu Rev Plant Biol,2004,55:521-535.
[3]QUAIL P H.Phytochrome photosensory signalling networks[J].Nat Rev Mol Cell Biol,2002,3(2):85 - 93.
[4]CHEN M,GALVAO R M,et al.Arabidopsis HEMERA/pTAC12 initiates photomorphogenesis by phytochromes[J].Cell,2010,141(7):1230 -1240.
[5]ENDO M,NAGATANI A.Flowering regulation by tissue specific functions of photoreceptors[J].Plant Signal Behav,2008,3(1):47-48.
[6]BRIGGS W R,CHRISTIE J M.Phototropins 1 and 2:versatile plant blue-light receptors[J].Trends Plant Sci,2002,7(5):204-210.
[7]CASHMORE A R,JARILLO J A.Cryptochromes:blue light receptors for plants and animals[J].Science,1999,284(5415):760-765.
[8]LI Q H,YANG H Q.Cryptochrome signaling in plants[J].Photochem Photobiol,2007,83(1):94 -101.
[9]LIU L J,ZHANG Y C.COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis[J].Plant Cell,2008,20(2):292-306.
[10]CERDAN P D,CHORY J.Regulation of flowering time by light quality[J].Nature,2003,423(6942):881 -885.
[11]JANG S,MARCHAL V.Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response[J].EMBO J,2008,27(8):1277-1288.
[12]KANG C Y,LIAN H L.Cryptochromes,phytochromes,and COP1 regulate light-controlled stomatal development in Arabidopsis[J].Plant Cell,2009,21(9):2624 -2641.
[13]REED J W,NAGPAL P.Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development[J].Plant Cell,1993,5(2):147 -157.
[14]MOGLICH A,YANG X.Structure and function of plant photoreceptors[J].Annu Rev Plant Biol,2010,61:21-47.
[15]CHIU F Y,CHEN Y R.Electrostatic interaction of phytochromobilin synthase and ferredoxin for biosynthesis of phytochrome chromophore[J].J Biol Chem,2010,285(7):5056-5065.
[16]KOHCHI T,MUKOUGAWA K.The Arabidopsis HY2 gene encodes phytochromobilin synthase,a ferredoxindependent biliverdin reductase[J].Plant Cell,2001,13(2):425-436.
[17]VOSS I,KOELMANN M.Knockout of major leaf ferredoxin reveals new redox-regulatory adaptations in Arabidopsis thaliana[J].Physiol Plant,2008,133(3):584-598.
[18]JEONG S,CLARK S E.Photoperiod regulates flower meristem development in Arabidopsis thaliana[J].Genetics,2005,169(2):907 -915.

