烟酰胺腺嘌呤二核苷酸磷酸与纳米氧化铝和勃姆石的作用
李 丽 汤勇铮 李卉卉 杨绪杰 陆天虹 杨小弟*,,
(1教育部软化学与功能材料重点实验室,南京理工大学化工学院,南京 210094)
(2江苏省新型动力电池重点实验室,南京师范大学化学与材料科学学院,南京 210097)
(3南京晓庄学院生物化工与环境工程学院,南京 211171)
0 Introduction
With the development of science and technology,nanoparticles have been widely used in many fields.Due to the unique properties and small size,the engineered aluminum(hydr)oxides have a wide range of applications,such as coatings and abrasives and additives in the fields of composites and heat transferenhancing nanofuluids[1].However,researches on the potential health risks of exposure to nanoparticles lag behind the rapid development of nanotechnology.Now we have been facing an increasing incidence of neurodegenerative diseases, such as Alzheimers disease,Huntingtons disease and Parkinsons disease.Although the exact etiology is uncertain,environmental pollutants,including aluminum and nanoparticles,may be the important risk factors for these diseases[2-6].In the past years,it has been recognized that Al3+has strong toxicity,meanwhile it may forms Al2O3and AlOOH under weak alkaline and alkaline conditions.Moreover,various speciation of aluminum have also been confirmed to have different toxicities[7-9].Recently,it has been found that the toxicity of nanosized corundum is more than that of micron-sized corundum to organisms[10-11].Pakrashi et al.[12]reported that Al2O3demonstrated a mortality rate of 57%to Bacillus subtilis,36%to Escherichia coli,and 70%to Pseudomonas fluorescents at low concentration(20 μg·mL-1)level using 1 g·L-1NaCl as the medium.And it has been confirmed that nano-sized boehmite is regarded as having the pulmonary toxicity.[12]
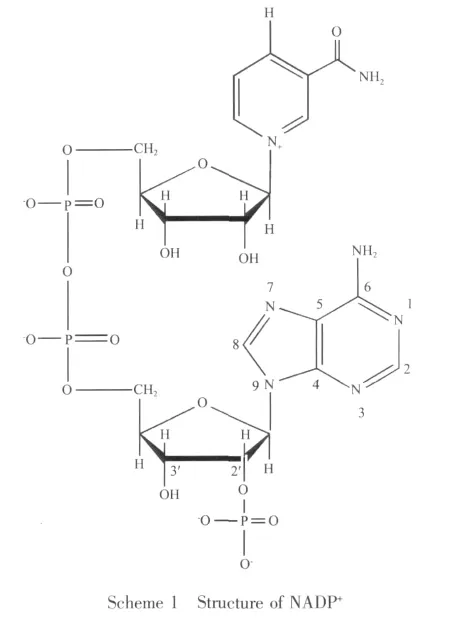
NADP+(Scheme1)isa coenzymeformany important anaerobic dehydrogenases in vivo[13].It may affect the metabolic pathway through participating in the basal metabolism with various zymoproteins.On the other hand,it is important that NADP+can be considered as the local feature of DNA structure.Interestingly, almost in all cases, unfolded conformation is required when NADP+combing with the dehydrogenase.In the decades,many researchers have investigated the interaction of low molecular weight (LMW)organic acid molecules with mineral surfaces[14-16].And they found that the adsorptions could usually be classified as inner-sphere mode or outer-sphere mode mechanisms[17-20].In addition,a number of related reports show that the nano species aluminum could affect the activity of various dehydrogenases,such asglutamate dehydrogenas,lactic dehydrogenase,lactate dehydrogenase and malate dehydrogenase.Because these dehydrogenases are NADP+-dependent enzymes,we can presume reasonably that nano species aluminum oxides may play an important role in the activity of NADP+,and it might have significance to study the interaction of nano-sized aluminum(hydr)oxides with NADP+under the simulated physiological conditions to further reveal the detailed molecular-level information about the coordination of the surface complexes.To the best of our knowledge,the adsorption of NADP+on mineral surface has not been reported.Accordingly,this work studies the adsorption of NADP+on the boehmite(γ-AlOOH)and corundum (γ-Al2O3)surfaces under a wide range of reaction conditions.
1 Experimental
1.1 Materials
Nano-corundum and nano-boehmite(purchased from Dekedaojin,China)were characterized by transmission electron microscopy(TEM)(JEM-200CX,JEOL,Japan),operating at 200 kV.Sample holders were 200 mesh copper grids.NADP+was obtained from Sigma(St.Louis.MO,USA).All other chemicals were of analytical regent grade.All solutions were made from deionized water(resistivity=18.2 MΩ·cm at 25℃),and the background electrolyte used for all experiments was NaCl solution.The pH values of solution and suspension were measured using a 215 pH meter(Denver,USA).The electrode was regularly calibrated using standard pH buffer solutions.
1.2 Batch adsorption experiments
Stock suspensions consisted of 10 g·L-1boehmite or corundum prepared using 0.01 mol·L-1NaCl were tumbled end-over-end for approximately 12 h prior to use.Allsamplesforadsorption and dissolution experiments were prepared by diluting stock suspensions with appropriate quantities and concentrations of NADP+in polypropylene centrifuge tubes.Generally,the NADP+concentration ranged from 0 to 6.25 mmol·L-1.The pH value of the suspension sample in each tube was adjusted to the desired value using 1 mol·L-1HCl or KOH.Then the tube was tumbled for 24 h at room temperature to explore the adsorption isotherms of NADP+on the particles and the effect of pH value on the adsorption.During this time,the pH value of the suspension was periodically measured and,if necessary,readjusted to the target value.Upon completion of equilibration,the suspension samples were centrifuged at 10 000 r·min-1for 20 min (H-1650,Xiangyi,China).The total concentration of NADP+in the supernatant was determined at 259 nm with a UV-Visible spectrophotometer(Cary 5000,Varian,USA)[21].
The effect of pH value on the adsorption of NADP+wasevaluated with an initialNADP+concentration of 2.5 mmol·L-1at pH value ranging from 3 to 11.
In order to investigate the ionic strength dependence on NADP+adsorption,an additional series of batch experiments were performed.A number of samples with different amounts of NaCl were added to give certain ionic strength.Then the samples were kept by stirring,and after equilibration the amount of NADP+adsorbed was finally determined as described above.In all experiments,the adsorption data were the average of three parallel experiments.
1.3 Adsorption isotherm fit
The adsorption isotherm of NADP+was examined by setting the concentration of boehmite and corundum at 10 g·L-1,but varying the initial concentration of NADP+from 0~6.25 mmol·L-1.The experimental data are fitted with Langmuir (1)and Freundlich(2)isotherm models[22]as follows:

where Qeis the concentration of adsorbed NADP+per unit weight of adsorbent,g·g-1;Ceis the concentration of NADP+in the aqueous phase,g·L-1;Qmis the maximum adsorption capacity per unit weight of adsorbent,g·g-1;and KL(L·g-1)and KF(g·g-1)are the corresponding constants of the system for Langmuir and Freundlich models,respectively.
The standard Gibbs energy of adsorption ΔGads⊖was calculated from theLangmuirfitusingthe equation:

where R is the gas constant(J·mol-1·K)-1;T is the temperature(K);and KLis the equilibrium constant.
1.4 ATR-FTIR spectroscopy
A Nicolet 5700 (Thermo, USA) infrared spectrometer was used to collect ATR-FTIR spectra.All samples were analyzed as liquid or wet paste.An empty cell spectrum collected prior to each sample was used as the background.Typically,512 scans with a spectral resolution of 4 cm-1were taken and averaged for each solution and wet paste sample.For each sample,the ATR-FTIR spectra of 0.01 mol·L-1NaCl solution with the same pH value were collected and subtracted from the sample spectra to remove the strong contribution from water bands.Data collection and spectral calculation were performed using OMNIC(version 7.2a,Nicolet Instrument Corp.)software.For each solution sample,this unwanted spectral response of water was removed by subtracting the ATR-FTIR spectrum ofNaClsolution.Foreach boehmite/corundum-NADP+-NaClwetpaste,the infrared absorbance of water was minimized by measuring and subtracting the ATR-FTIR spectrum of boehmite/corundum-NaCl suspension obtained at the same pH value,with the subtraction factor of 0.96~1.02[23].
1.5 Dissolution measurements
Concentrations of dissolved aluminum in the supernatants described in section 1.2 were measured by inductively coupled plasma (ICP)spectrometer(Optima DV2100,PerkinElmer)[24].Dissolution data presented are the average of results obtained at four distinct wavelengths(λ=308.2,309.2,394.4 and 396.1 nm).
1.6 TG-DTA measurements
TG-DTA measurements were performed by a thermogravimetry-differential thermal analyzer(Diamond TG/DTA, PerkinElmer, USA), with temperature ramp rate at 10 ℃·min-1in nitrogen atmosphere (100 mL·min-1).Compared with the samples after adsorption,the corresponding samples of pure NADP+,boehmite and corundum were measured at the same conditions.Before measurement,the samples after centrifugation described in batch experiment were freeze dried.
1.7 XRD measurements
The pastes after centrifugation obtained from section 1.2 and the free nanoparticles were analyzed by a X-ray diffractometer (XD-3,Beijing Purkinje General Instrument Co.,Ltd.)equipped with graphite monochromatized Cu Kα radiation (λ=0.154 18 nm),high voltage:35 kV,current:20 mA,at a scanning rate of 5°·min-1and in 2θ of 5°~80°.
1.8 Fluorescence spectra
The batch adsorption behavior was performed with nanoparticles concentration ranging from 2 to 65 g·L-1at pH value of 7 (the excitation wavelength of 275 nm and slit width of 5 nm).After centrifugation,fluorescence spectra of NADP+in the supernatant were collected by a Fluorophotometer (LS-50B,PerkinElmer,USA).
2 Results and discussion
2.1 Characterization of nanoparticles
TEM imagesforNano-corundum and nanoboehmite with the average diameters approximately from 10~20 nm are shown in Fig.1.After the samples in vacuum at 150℃for 12 h,the BET surface areas are 127 m2·g-1and 114 m2·g-1,measured by a surface area and pore size analyzer(Micrometrics ASAP 2020,USA),respectively.

2.2 Macroscopic adsorption studies
2.2.1 Influence of pH value and ionic strength on adsorption
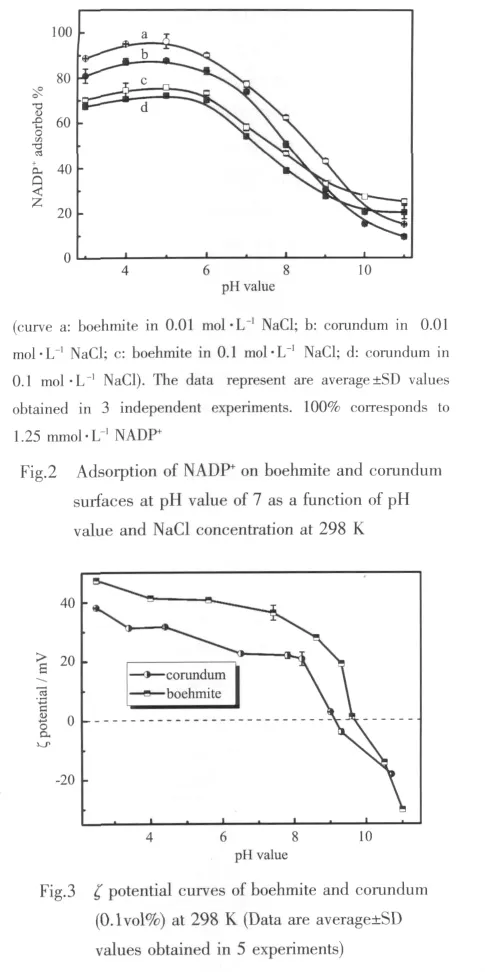
The macroscopic adsorption properties of NADP+on boehmite and corundum surfaces are shown as a function of both pH values and NaCl concentrations in Fig.2.The adsorption process of NADP+is suppressed by alkaline pH value and increasing ionic strength.Furthermore,the extent of adsorption reaches the maximum at pH value of 5 and then systematically decreases under higher basic conditions,due to the strong dependence of boehmite and corundum surface charge on the pH value (Fig.3).Collected by an Electroacoustic spectrometer (Nano-ZS90,Malvern Instruments Ltd.England),the isoelectric points(pI)of the corundum and boehmite are 9.10±0.05 and 9.65±0.05 (n=5),respectively.For example,when pH value is lower than the pI,the surface is positively charged.In contrast,when the pH value is higher than the pI,the surface is negatively charged.Consequently,the amountofNADP+adsorbed decreases significantly at higher pH value condition because of a net repulsive electrostatic interaction between the negatively charged surface and NADP+anions.When pH value<5,however,the amount of adsorbed NADP+also falls below that predicted,due to the competition of solutionbased Alバ.It can also be interpreted as the reduction of the particle concentration and active surface sites caused by the dissolution of boehmite and corundum.
In addition,the effect of salt added is obviously different at the range of pH values.This might be interpreted as the shielding effect of the salt.For instance,at pH value<pI,the salt added could hinder the electrostatic attraction between the surface and the NADP+,in contrast,at pH value>pI,the salt particles might effectively shield the electrostatic repulsion between them.Therefore,at low pH values,the amount of NADP+adsorbed decreases with increasing NaCl concentration,and on the contrary,it increases at higher pH values.
According to the analogous results previously reported fortheadsorption oforganic acidson hydroxide minerals at low ligand concentrations[25-26],themain contribution ofelectrostaticbindingis indeed strongly supported by the observation that the application of salts leads to a concentration-dependent desorption of NADP+from the surface.
The charging behavior of the surfaces using the following acid-base reactions is shown in equations(4)and(5):

The surface species≡AlOH (including≡AlO-,≡AlOH0and≡AlOH2+,the surface site density of 1.7 sites per nm2for boehmite and 1.03 sites per nm2for corundum[27],respectively)represents the total surface sites capable of adsorbing/desorbing protons over the pH value range investigated.Eqs.4 and 5 are on the basis of that,they do not consider the behavior of multinuclear hydroxylspecies atthe corundum surface.For example,Al2OH species are prevalent on the hydrated 001 surface of corundum[28].However,Al2OH species are not proton active in the pH value range from 2 to10[29].Furthermore,Al2OH possessing a net neutral charge is not expected to contribute significantly to the interaction with NADP+in this study,in contrast,NADP+will be electrostatically attracted to AlOH2+.The high negative charge of the species may result in surface charge “saturation”ratherthan saturation ofsurface binding sites.Compared to the surface density ofcorundum,boehmite displays a larger proton exchange capacity,which implies that the average distance between the surface hydroxyls on the boehmite surface is shorter.And this is connected to an increase in the contribution from electrostatic forces.[27]
2.2.2 Adsorption isotherm fit
To assess more information of the mechanism of the adsorption on the surface,the experimental data are simulated with Langmuir and Freundlich models in Fig.4.Obviously,the single-site Langmuir isotherm can provide a good fitting to the experimental data,with a constant of the system KL=45.45 and 37.04 L·g-1,respectively.In all cases,the adsorption capacity eventually reaches a platform finally and the adsorption capacity of boehmite is greater than that of corundum.Moreover,the corresponding standard Gibbs energy of adsorption ΔGads⊖=-25.98 and-25.47 kJ·mol-1for boehmite and corundum,respectively.Both of ΔGads⊖are negative,suggesting that the reactions are spontaneous,and these values agree with the typicalstrength ofhydrogen bond surfacemolecule interaction[30].
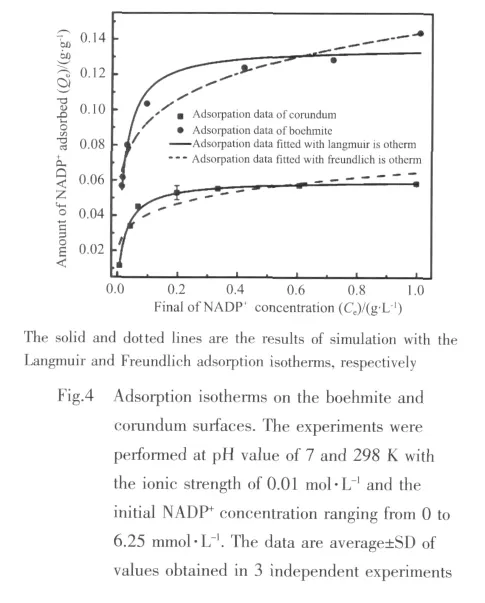
In a word,the results of macroscopic adsorption experiments show that outer-sphere complexes are dominantly formed on the boehmite and corundum surfaces over the range of pH values.
2.3 Microscopic adsorption studies
2.3.1 XRD measurement
According to the XRD characteristic peaks of free nanoparticles,the boehmite and corundum(Fig.5)show good crystallization and single crystalline phase.If NADP+diffuses into the particles,the inner layer structure of crystal would be destroyed.Compared with the XRD feature of pure nanoparticles,there is no obvious change for the crystal structure after reaction,which proves indirectly that the adsorption of NADP+by boehmite and corundum might occur on the surface of the particles rather than into the inner layer.

2.3.2 ATR-FTIR spectra
The ATR-FTIR spectra of NADP+in aqueous solution tabulated in this study(Table 1)are consistent with that previously reported by Iwaki et al[33].Ribose section does not absorb significantly in the 1 700~1 200 cm-1window of the ATR-FTIR spectra.Thus,all the major bands in the adenosine spectrum above 1 200 cm-1must arise from the nicotinamide and adenine ring system.For instance,the bands at 1 650,1 608~1 610,1 579~1 581,1 475~1 481,1 423~1 428,1 380,1 326~1 332 and 1 301~1 309 cm-1dominate in the spectra of aqueous NADP+,which could be assigned to the adenine moiety of the dinucleotides.The band at 1 650 cm-1is mainly due to NH2group(adenine)with a small contribution from dihydronicotinamide and the band at 1 608~1 610 cm-1arises from the stretch of C5-C6(adenine).And the band located at 1 579~1 581 cm-1is due to the stretching of the C-N bond within the carboxamide group.Moreover,both the nicotinamide and adenine moieties (C4-N9-C8-H)may have contributions to the bands centered at 1 421~1 423 cm-1and important bands of 1 330 cm-1is assigned to the stretching of C5-N7+C8=N7.The important peaks at 1 076~1 078 and 1 236~1 238 cm-1suggest that these bands correspond to the characteristic of the asymmetric and symmetric stretching vibrations ofpyrophosphate bridge linking the nicotinamide and adenosine moieties (O=P-O-)adsorption region.Other bands around 1 100 cm-1are dominated by P-O groups,supposed to be particularly strong in the NADP+spectra because of the additional 2-phosphate on the adenine ribose.However,they are observed weakly compared with the bands at 1 076 cm-1in solution in this study.The ribose OH bonds are masked by the phosphate at the same region[36-37,39].On the basis of the above peak assignments,ourfurtherATR-FTIR investigations are just limited to a study of the role of the most prevalent NADP+functional groups in the adsorption of NADP+on boehmite and corundum surfaces.
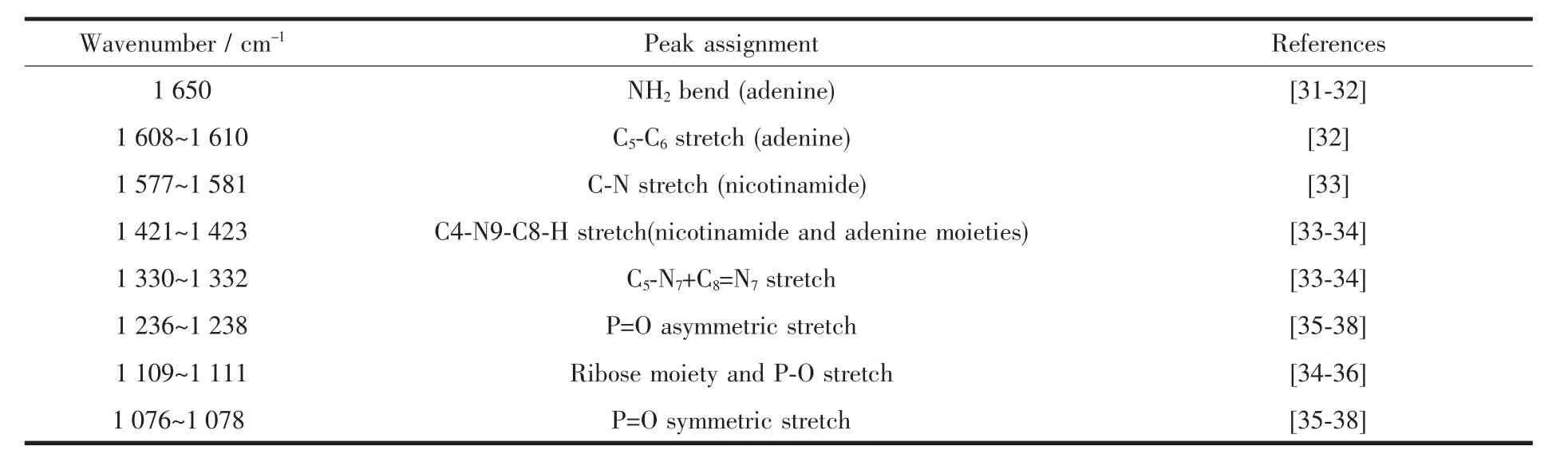
Table 1 ATR-FTIR peak assignment for aqueous NADP+
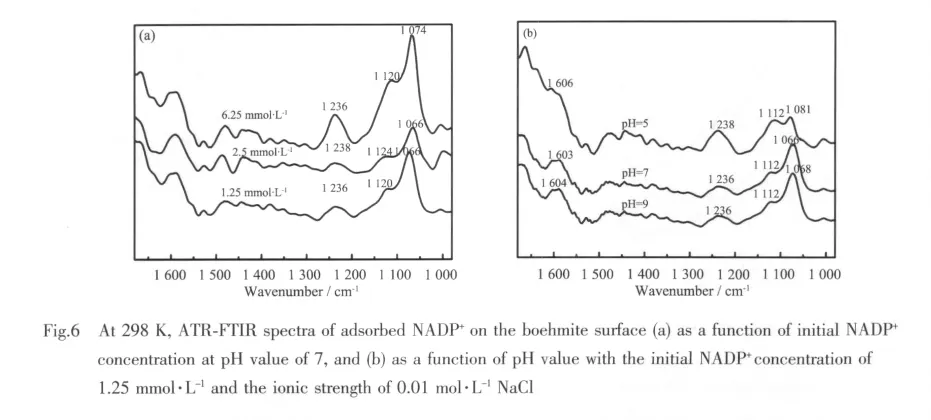
To assess the effects of initial concentration of NADP+and the suspension pH value on the adsorption at the boehmite and water interfaces,ATRFTIR spectra are illustrated in Fig.6. Because phosphate moieties are expected to be the functional groupsresponsible forthe complexation between NADP+and nanoparticle,the analysis of ATR-FTIR spectra of adsorbed NADP+focus on the stretching vibration of the P-O bond.In general,if the phosphate groups coordinate with the metal ion(s)on the surface by forming inner-sphere complexes, the bands corresponding to the vibration of phosphate groups will split into two or three bands or experience shifts.If outer-sphere complexes are formed,their vibrations should shift only slightly due to slight distortion in adsorbed phosphate groups via Van der Waals forces and the number of bands will not change[40-41].It is noted that the spectra(Fig.6)bear striking similarities to those feature of aqueous NADP+listed in Table 1.For example,the spectra show a strong peak around 1 066~1 081 cm-1,and another prominent peak at 1 236 ~1 238 cm-1.However,two important subtle differences are evident;namely,(1)the positions of the major peaks shift slightly;and(2)the peaks in the adsorbed spectrum are significantly broader than those in theaqueousspectra.Forexample,the peak centered at 1 076 cm-1in solution moves by 2~10 cm-1wavenumbers approximately,and the peak of 1 109~1 111 cm-1shifts to higher wavenumbers at around 1 112 ~1 124 cm-1.Similar results have been previously reported about the adsorption modes of LMW organic anions on mineral surface and generally indicate an outer-sphere mode of adsorption for the organic species[42-45].In addition,the phosphate groups in the NADP+molecule are negatively charged.So,at high pH values,if they are in a direct contact with the negatively charged surface,the phosphate groups would be repelled away from the surface due to the electrostatic repulsion.However,in the spectra demonstrated in Fig.6b,the intensity of the bands located at 1 236~1 238 cm-1does not change significantly with pH value,which indicates that the phosphate groups do not change their orientation when the solution pH valueschange.Meanwhile,the intensity of bands at 1 112~1 124 cm-1in the spectra after adsorption are enhanced compared to aqueous NADP+,due to the formation of outer-sphere complexes by the electrostatic attraction between the 2-phosphate and boehmite surfaces.
ATR-FTIR spectra of adsorbed NADP+as a function of initial NADP+concentration and pH value on corundum surface are shown in Fig.7.An important subtle change is that the intensity of peaks around 1 112 cm-1are close to that of 1 079 cm-1after adsorption compared with that in aqueous solution.And this may be taken as additional evidences that NADP+is predominantly combined with corundum via the phosphate and the surface hydroxyl groups as outer-sphere adsorption complex(≡AlOH2+…NADP+)at all experimental conditions.The spectra are most notable for the overlapped band at 1 603~1 606 cm-1,which corresponds to a combination of NH2bending.And C5-C6stretchingobserved in theadsorption NADP+spectra is much weaker than that in aqueous NADP+spectra,which may be due to another outersphere adsorption mode by hydrogen bond formed via amino group and surface hydroxyl groups[34].Moreover,with low surface coverage,the centrifuged paste obtained contained a relatively low particle concentration,and therefore a reduced ATR-FTIR signal is observed,compared with the other NADP+concentrations investigated (Fig.7a).It is important that with the concentration increased,a new peak(1 143 cm-1~1 151 cm-1)gradually appears[24],probably owing to small quantities of inner-spherically complexes,in which a direct bond is formed between Al and NADP+phosphate[46-47].

Considering the less effect of the inner-sphere complexes on the extent of adsorbed NADP+on the corundum displayed in the batch experiments,we conclude that the inner-sphere complexes are minor.So,it can be confirmed that NADP+is predominantly adsorbed at the boehmite/water interface as outersphere fashions by electrostatic interaction over the investigated pH conditions.In contrast,on the corundum surface,two outer-sphere fashions by electrostatic interaction and hydrogen bond interactions in all cases as well as another minor inner-sphere adsorption complex at low pH values are formed.Though the minor complexes are unlikely to contribute appreciably to the overall macroscopic adsorption characteristics of NADP+,it might have a significant effect on the corundum dissolution behavior[23].This is further discussed in this study.
2.3.3 TG-DTA measurement[48-49]
TG curves (Fig.8a)exhibit the first weight loss step for free NADP+at 100~200 ℃ (peak temperature of169.2 ℃ ismarked).Itisfollowed bythe exothermic effect of the decomposition reaction at 194.6 ℃.For boehmite,the TG-DTA curve for the onsettemperature and endothermic temperatures occurs at 374.4 ℃ and 433.7 ℃,and shifts to 434.7℃ and 471.7 ℃ for the paste after adsorption,respectively.Although there is no more explicit peak in DTA curve for the paste on the boehmite surface,it confirms that the mass loss and endothermic temperature are marked at higher temperature than that of pure boehmite obviously.The results suggest that the paste after adsorption was the complex through weak interaction between NADP+and boehmite instead of the simple mixture of them.
However,in the case of corundum powder,there is one mass loss step in TG curve at 201.4 ℃ and the DTA curve involves a single endothermic peak at 221.6 ℃ (Fig.8b).Both temperatures after adsorption are at higher temperature of 219 ℃ and 238.4 ℃,respectively,which shows that the stability of NADP+is improved after adsorption.It is interesting that the decomposition is changed from exothermic to endothermic peak forthe paste afteradsorption compared with free NADP+in DTA curve.As a consequence,the changes in TG-DTA curves reveal the possibility of stronger interaction between NADP+and corundum than thatbetween NADP+and boehmite.
2.3.4 Impact of NADP+adsorption on the dissolution behavior

The dissolution behavior of boehmite and corundum as a function of NADP+concentration and pH value is shown in Fig.9. Itdisplays low concentration for dissolved Alバin the intermediate pH value from 7 to 9 in the absence of NADP+,which agrees with the slow rate of dissolution typical of aluminum (hydro)oxides reported in this pH value range by Walther[50].The concentration of dissolved Alバ increases markedly under acidic (pH<7)conditions.Such increases in solubility as the pH value moves away from near-neutral conditions,which is consistentwith an increased attack on the nanoparticle surface by dissolution-enhancing proton and hydroxyl species,and this is typical of aluminum oxides minerals.In this study,the presence of NADP+inhibits the dissolution of boehmite and corundum,this may be due to NADP+anions adsorbed as outersphere complexes.Because outer-sphere complexes do nottransfersignificantelectron density into the coordination sphere of the surface cations,consequently,they have slightinfluence on the bridging metal-oxygen bonds in the mineral surface.Therefore,they have little directeffecton the dissolution kinetics.In this study,though adsorbed in outer-sphere modes,NADP+anions tightly bond to specific surface sites via electrostatic and hydrogen bonding interactions. Importantly, under this mechanism,NADP+does not have a significant polarizing effect on bridging Al-O bonds in the corundum and boehmite surface.As a result,the dissolved Alバ concentrationsofboehmite and corundum decrease in the presence of NADP+.It also shows that,as the concentration of NADP+increased,the concentrations of dissolved Alバprogressively decrease at pH<5.By contrast,at pH>9 (where the extent of NADP+adsorption is very low),the presence of NADP+has little effect on the extent of adsorbent dissolution.The results are similar to those previously obtained for the interaction of maleate with corundum by Johnson et al[51].

In addition,the presence of inner-sphere complex mightbe expected to significantly enhance the dissolution of corundum.However,the effects are very small,compared with the surface site-blocking role displayed by outer-spherical mode,which may support indirectly that the inner-sphere complexes are minor.Therefore,the overalleffectofNADP+on the dissolution rate of corundum is inhibition.Moreover,it can be seen with the increase of initial NADP+concentration,the effect of suppress for boehmite is greater than that for corundum.These results further confirm that NADP+is predominantly adsorbed by outer-sphere mode on the boehmite and corundum,as well as some minor inner-sphere fashion at low pH values on the corundum.
2.4 Biological effects
As mentioned in the introduction,we also used an approach involving Fluorescence measurement to observe NADP+conformation affected by the adsorption.Fluorescence spectra of supernatants with increasing corundum concentration at pH value of 7 are shown in Fig.10.The NADP+concentration is 2.5 mmol·L-1in all cases.The efficient fluorescence spectra are only available for the folded conformation of NADP+,as demonstrated in previous works[12,52-54].

The results of ICP measurement suggest that the amount of dissolved Alバis nearly zero at pH value of 7,so it indicates that the change of NADP+conformation should be due to the addition of nano-corundum.It is assumed that the fluorescence intensity of NADP+would increase or decrease correspondingly if the conversion between folded and unfolded conformation is present in the adsorption.Thus,the fluorescence spectra may indirectly monitor the conformational changes after adsorption.As demonstrated,the fluorescence intensity of NADP+gradually falls with increasing corundum concentration,which can be illustrated as the increase of folded conformation.However,at the boehmite/water interface,it presents an irregularchange with increasing amountof boehmite.It may be ascribed to the complexity of the heterogeneity of surface,and this can also prove partially that the transformation of conformation occurs on the boehmite surface.
The unfolded conformation of NADP+in all cases is required to take part in all kinds of physiological reactions.Therefore,we presume reasonably that the exchange between folded and unfolded conformation by adsorption may influence the activity of NADP+-dependant dehydrogenase in the biological system.
3 Conclusions
In this paper,the adsorption behavior of NADP+on the nano-boehmite(γ-AlOOH)and nano-corundum(γ-Al2O3)surfaces was investigated over the range of pH values,ionic strengths and initial NADP+concentrations.The appropriate combination of techniques can give very accurate pictures of the interactions of small biomolecules with nanoparticles.The results suggest that the adsorption displayed strong ionicstrength dependence and it is consistent with NADP+adsorbed predominantly in the form of outer-sphere complexes via electrostatic interactions on boehmite and corundum surfaces.Meanwhile,there are two additional species on corundum:an outer-sphere complex by hydrogen bond and small inner-sphere species(≡AlNADP+),and this is confirmed partially by the dissolution rate of the adsorbents.In addition,the adsorption behavior can be described by the Langmuir adsorption isotherm and are both highly spontaneous.According to the changes of NADP+conformation,we assume that the adsorption may influence the activity of dehydrogenase depending on NADP+in the biological system.
Acknowledgements:The project is supported by the National Natural Science Foundations of China (Grant Nos.20875047),Ministry of Water Resources(201201018)and Priority Academic Program Development of Jiangsu Higher Education Institutions.
[1]Service R F.Science,2008,321(5892):1036-1037
[2]Win-Shwe T T,Fujimaki H.Int.J.Mol.Sci.,2011,12(9):6267-6280
[3]Ganguly P,Poole W J.Mater.Sci.Eng.,A,2003,352(1):46-54
[4]Gilbert N.Nature,2009,460(7258):937-937
[5]Sharma H S,Sharma A.Prog.Brain Res.,2007,162:245-273
[6]Orringer D A,Koo Y E,Chen T,et al.Clin.Pharmacol.Ther.,2009,85(5):531-534
[7]Ralph L,Twiss M R.Bull.Environ.Contam.Toxicol.,2002,68(2):261-268
[8]Yang L,Watts D J.Toxicol.Lett.,2005,158(2):122-132
[9]Cai L,Xie Y F,Li L,et al.Colloids Surf.,B,2010,81(1):123-129
[10]Pakrashi S,Dalai S,Sabat D,et al.Chem.Res.Toxicol.,2011,24(11):1899-1904
[11]Coleman J G,Johnson D R,Stanley J K,et al.Environ.Toxicol.Chem.,2010,29(7):1575-1580
[12]Pauluhn J.Toxicology,2009,259(3):140-148
[13]Aliverti A,Pandini V,Pennati A,et al.Arch.Biochem.Biophys.,2008,474(2):283-291
[14]YUAN Xiao-Wei(袁晓卫),YANG Qian(杨骞),LIU Qi(刘琦),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(2):285-292
[15]Johnson S B,Brown G E,Healy T W.Langmuir,2005,21(14):6356-6365
[16]Silverio F,dos Reis M J,Tronto J,et al.J.Mater.Sci.,2008,43(2):434-439
[17]Ha J Y,Yoon T H,Wang Y G,et al.Langmuir,2008,24(13):6683-6692
[18]Purgel M,Takacs Z,Josson C M,et al.J.Inorg.Biochem.,2009,103(11):1426-1438
[19]Sheals J,Sjöberg S,Persson P.Environ.Sci.Technol.,2002,36(14):3090-3095
[20]Jonsson C M,Persson P,Sjöberg S,et al.Environ.Sci.Technol.,2008,42(7):2464-2469
[21]Nordin J,Persson P,Laiti E,et al.Langmuir,1997,13(15):4085-4093
[22]Dubey A,Shiwani S.Int.J.Environ.Sci.Technol.,2012,9(1):15-20
[23]Johnson S B,Yoon T H,Brown G E.Langmuir,2005,21(7):2811-2821
[24]Johnson S B,Yoon T H,Slowey A J,et al.Langmuir,2004,20(26):11480-11492
[25]Filius J D,Hiemstra T,Van Riemsdijk W H.J.Colloid Interface Sci.,1997,195(2):368-380
[26]Yoon T H,Johnson S B,Brown G E,et al.Langmuir,2004,20(14):5655-5658
[27]Laiti E,Ohman L O.J.Colloid Interface Sci.,1996,183(2):441-452
[28]Eng P J,Trainor T P,Brown G E,et al.Science,2000,288(5468):1029-1033
[29]Hiemstra T,Yong H,Van Riemsdijk W H.Langmuir,1999,15(18):5942-5955
[30]Von oepen B,Kordel W,Klein W.Chemosphere,1991,22(3/4):285-304
[31]Yue K T,Martin C L,Chen D,et al.Biochemistry,1986,25(17):4941-4947
[32]Savoie R,Jutier J J,Prizant L,et al.Spectrochim.Acta:Part A,1982,38(5):561-568
[33]Iwaki M,Cotton N P J,Quirk P G,et al.J.Am.Chem.Soc.,2006,128(8):2621-2629
[34]Damian A,Omanovic S.Langmuir.2007,23(6):3162-3171
[35]Bin X M;Zawisza L;Goddard J D,et al.Langmuir.2005,21(1):330-347
[36]Brewer S H,Anthireya S J,Lappi S E,et al.Langmuir,2002,18(11):4460-4464
[37]Takeuchi H,Murata H,Harada I.J.Am.Chem.Soc.1988,110(2):392-397
[38]Benedetti E,Bramanti E,Papineschi F,et al.Appl.Spectrosc.,1997,51(6):792-797
[39]Barth A,Mantele W.Biophys.J.,1998,75(1):538-544
[40]Arai Y,Sparks D L.J.Colloid Interface Sci.,2001,241(2):317-326
[41]ConnorPA,McQuillanAJ.Langmuir,1999,15(8):2916-2921
[42]Johnson B B,Sjöberg S,Persson P.Langmuir,2004,20(3):823-828
[43]Persson P,Nordin J,Rosenqvist J,et al.J.Colloid Interface Sci.,1998,206(1):252-266
[44]Boily J F,Persson P,Sjöberg S.Geochim.Cosmochim.Acta,2000,64(20):3453-3470
[45]Rosenqvist J,Axe K,Sjöberg S,et al.Colloids Surf.,A,2003,220(1/2/3):91-104
[46]Guan X H,Shang C,Zhu J,et al.J.Colloid Interface Sci.,2006,293(2):296-302
[47]Guan X H,Liu Q,Chen G H,et al.J.Colloid Interface Sci.,2005,289(2):319-327
[48]Kandori K,Oda S,Fukusumi M,et al.Colloids Surf.,B,2009,73(1):140-145
[49]Larsericsdotter H,Oscarsson S,Buijs J.J.Colloid Interface Sci.,2004,276(2):261-268
[50]Carroll-Webb S A,Walther J V.Geochim.Cosmochim.Acta,1988,52(11):2609-2623
[51]Johnson S B,Yoon T H,Kocar B D,et al.Langmuir,2004,20(12):4996-5006
[52]Yang X D,Bi S P,Yang L,et al.Spectrochim.Acta:Part A,2003,59(11):2561-2569
[53]Yang X D,Cai L,Peng Y,et al.Sensors,2011,11(6):5740-5753
[54]Hull R V,Conger P S,Hoobler R J.Biophys.Chem.,2001,90(1):9-16