靠近第二临界胶束浓度的C16TAB/AS/H2O系统的胶束聚集数和粘度
陈燕梅,何少青,贵元香,郝力生,南延青
(湖南师范大学化学化工学院,中国长沙 410081)
Aqueous mixtures of cationic and anionic surfactants usually exhibit complicated phase behaviors related to their strong self-assembly ability.Precipitation or turbidity generally occurs at low cT(usually slightly above),and then homogeneous transparent solution is obtained at high.The investigation of Kato et al.[7]illustrated that the apparent aggregation numbers N of SDS/OTAB/H2O homogeneous systems above the heterogeneous region decreased with the increase of cT,the viscosity η of these systems increased with the micellar growth(i.e.with the decrease of cT).The N values for aqueous mixed cationic/anionic surfactant homogenous systems become larger asapproaches 0.5 under constant.
Previous study[9]indicated that the shear viscosities η ofTAB/AS/mixed systems at 318.15 K with different x2change in different patterns as cTwas increased.η of the mixed systems with x2=0.7 increases rapidly to a viscosity peak,then decreases,and the further increase of cTleads to aqueous two-phase separation;however,η of the mixed systems with x2=0.8 and 0.9 increases slowly.While,these mixed systems have comparable CMC2values[2].In this paper,N and η of mixed C16TAB/AS/H2O systems with x2=0.7,0.8 and 0.9 near CMC2have been measured.Inorganic salt effect on the aggregation numbers and viscosities of some mixed systems has been investigated.The purpose is to try to reveal the variations of N and η with cTwhen the shape of micelles changes from spherical to rodlike,and to reveal that when cT(>CMC2)increases further,whether more rodlike micelles form or the rodlike micelles grow further.
1 Experimental Section
1.1 Materials
Sodium dodecylsulfonate(AS)purchased from Sinopharm Chemical Reagent Co.,Ltd.(purity≥97%)was recrystallized as previously[2,9].Cetyltrimethylammonium bromide(C16TAB)purchased from Sinopharm Chemical Reagent Co.,Ltd.(purity≥99%),sodium bromide(NaBr,AR)and sodium sulfate(Na2SO4,AR),were used without further purification.Pyrene(Py,Fluka,purity≥90%)was recrystallized three times from ethanol.N-cetylpyridinium chloride(CPC,Fluka,purity≥98%)was recrystallized three times from acetone-ethanol.All the above reagents were dried in a vacuum desiccator at 333.15 K for 24 h before use.Water was redistilled from potassium permanganate solution.
1.2 Determination of the aggregation number
Pyrene was used as the fluorescence probe and CPC as the quencher.The steady-state fluorescence spectra of pyrene were recorded with a Hitachi F-4500 spectrofluorometer in the range of 360~450 nm at an excitation wavelength of 335 nm.Excitation and emission band slits were 5 and 2.5 nm,respectively.
The micellar aggregation number(N)can be determined from the slope obtained from the plot of ln(I0/I)versus cQaccording to the method[10]:

where I0and I are the fluorescence intensities at 373 nm in the absence and presence of a quencher,respectively,cTis the total surfactant concentration,and cQis the quencher concentration.
The concentration of pyrene cPywas kept constant at 6.0 ×10-7mol·L-1,while cQwas varied from 0 ~87 ×10-6mol·L-1for C16TAB/H2O or AS/H2O systems;and cPy=2.0 × 10-7mol·L-1,while cQwas varied from 0 ~5 ×10-6mol·L-1for C16TAB/salt aqueous systems,C16TAB/AS/H2O systems in the absence and in the presence of salt,assuring a Poisson distribution.All measurements were performed at(318.15 ±0.01)K.
1.3 Determination of the relative viscosity
Viscosity measurements were made using an Ubbelohde suspended level capillary viscometer,suspended vertically in a thermostat with a temperature maintained at(318.15 ±0.01)K.The flow times for the studied samples are about 120 s,and the densities of these dilute surfactant solutions are very close to those of their solvents.Thus the ratio of flow time of solution to that of solvent was regarded as the relative viscosity ηr:Where η and η0are the viscosities of solution and solvent,t and t0are the flow times of solution and solvent,respectively.For each sample,ηrwas taken as the mean value of three independent experiments.Then η can be obtained by the product of η0and ηr.
2.2 急性缺血性脑卒中影响因素分析 血清脂蛋白相关磷脂酶A2水平升高、D-二聚体水平升高、高密度脂蛋白水平降低、高血压病史是急性缺血性脑卒中的独立影响因素(P<0.05)。见表2。

2 Results and Discussion
2.1 Micellar aggregation numbers of C16TAB and AS aqueous solutions
Steady-state fluorescence quenching technique has been used to determine the N values.Fig.1 shows the fluorescence emission spectra of pyrene in C16TAB/H2O systems with different quencher concentrations.Fig.2 shows the linear plot of ln(I0/I)versus cQfor C16TAB.According to equation 1[10],values of the average N for these systems have been calculated from the slope of these linear plots and the CMC1values[2].The N results for C16TAB/H2O,AS/H2O and C16TAB/0.10 mol·kg-1NaBr(or 0.050 mol·kg-1Na2SO4)/H2O systems are shown in
Tab.1.Some literature data[8,11,12]are also given.The results are well in accordance with the literature data.The results in Fig.3 indicated that the N values of aqueous solutions of C16TAB and AS increase linearly with the increase of cT(mmol·L-1)when CMC1<cT<CMC2,the linear relationships are as follows:

Based on the CMC1values of C16TAB(1.06 mmol·L-1)and AS(11.53 mmol·L-1),respectively,NC16TAB=35 and NAS=31 at CMC1were evaluated.
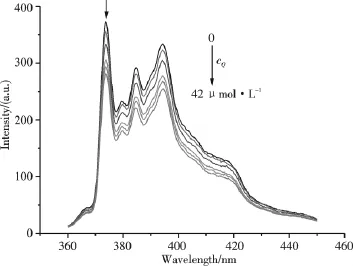
Fig.1 Variation of the emission spectra of 6 × 10-7 mol·L-1pyrene in 8.05 mmol·L-1C16TAB/H2O systems with different quencher concentrations.The vibronic peak noted by an arrow is used for quenching analysis
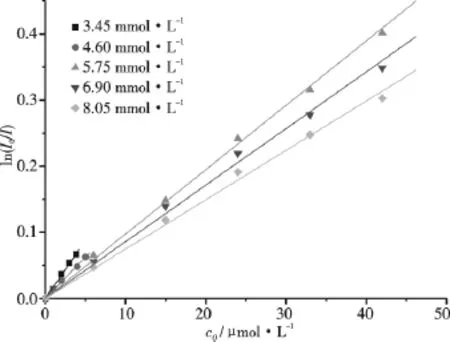
Fig.2 Linear plot of ln(I0/I)for 6 × 10-7mol·L-1pyrene in aqueous solutions of C16TAB with different concentrations as a function of the quencher CPC concentrations cQ
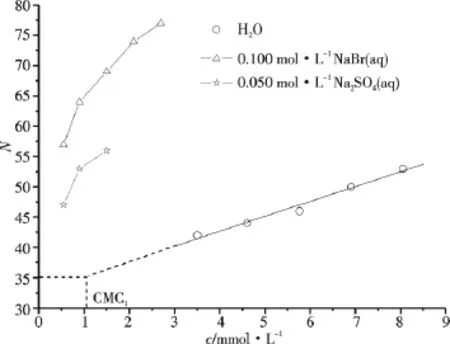
Fig.3 Micellar aggregation number of aqueous solutions of C16TAB in the absence and in the presence of sodium salt at 318.15 K
The results in Tab.1 and Fig.3 clearly indicate that the addition of NaBr or Na2SO4into C16TAB/H2O system leads to the significant increase of N,and the salt effect of NaBr on N is stronger than that of Na2SO4.Usually,the local aggregate curvature can be described by a surfactant packing parameter p=v/(lmaxa),where v and lmaxare the volume and length of the hydrophobic part,respectively and a the apparent area per molecule at the interface(hydrated headgroup)[13].If p < 1/3,the surfactant is expected to form spherical micelles;if 1/3<p<1/2,rodlike micelles tend to be formed.The addition of inorganic salt into the C16TAB/H2O system increases the number of counterions as well as the electrostatic screening effect,thus reduces the electrostatic repulsion between the headgroups and a,which is in favor of the formation and growth of micelles,and the transition from spherical micelles to rodlike micelles at lower concentration.Therefore,N is measurable at lower cTand N increases significantly with the addition of salt.
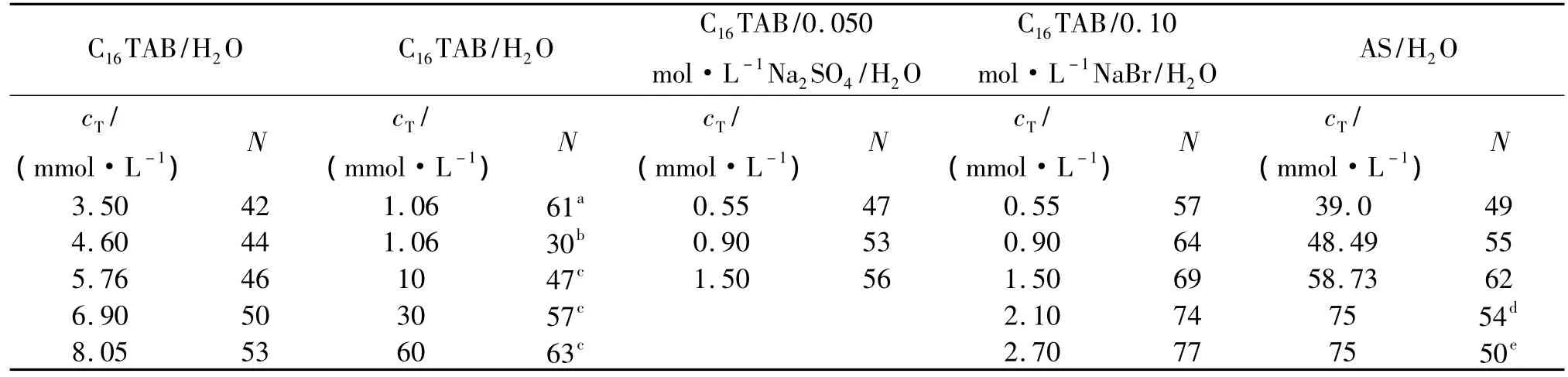
Tab.1 The aggregation number for the studied C16TAB or AS systems at 318.15 K
a and b[12]:steady-state fluorescence quenching technique(SSQT,(303 ± 0.1)K)and simulation method,respectively;c[11]:SSQT,(298.15 ± 0.1)K;d and e[12]:SSQT and time-resolved fluorescence quenching technique,respectively,313.15 K.
The specific ion effects on CMC1of ionic surfactant generally depend on counterions and usually follow direct or reversed Hofmeister series.According to Collins'concept of matching water affinities[14],the chaotropic headgroups of C16TAB should come in close contact with chaotropic counterions like Br-,making it more possible to form direct ion pairs,whereas kosmotropic SO2-4remain further away.Such ion pairs will be much less hydrated than separate ions and headgroups.This smaller hydration is reflected in smaller effective headgroup areas a while does not significantly influence v and lmax[15],thus leading to larger packing parameter and larger aggregates.That is why the salt effect of NaBr on N of C16TAB is stronger than that of Na2SO4.
2.2 Micellar aggregation numbers of C16TAB/AS mixed systems
The C16TAB/AS/H2O mixed systems with x2=0.7,0.8 and 0.9 were chosen as samples of N measurements,in order to try to interpret the variation tendency of η at higher cTin our previous work[9].Fig.4 presents the N values of C16TAB/AS/H2O mixed homogeneous transparent systems with different composition near CMC2.Our results are totally different from the experimental results reported by Kato et al.[7]For the mixed systems with x2=0.9,N increases significantly first when cT< CMC2,then increases slightly when cTis very near CMC2,and finally becomes near constant when cT>CMC2.It indicates that N increases significantly as the micelles change from spherical to rodlike.Whereas for the rodlike micelles,N is almost unchanged with the increase of cTwhen CMC2<cT<2CMC2.The micellar aggregation number increases with the decrease of x2from 0.9 to 0.7 under the same cT.For the mixed systems with x2=0.8,N increases slightly as cTis very near CMC2(i.e.cTincreases from 1.5 mmol·L-1to 1.69 mmol·L-1),and N is almost constant as cTis somewhat larger than CMC2.However,different from the case for the mixed systems with x2=0.8 and 0.9,N increases successively for the mixed systems with x2=0.7 as cTincreases from 1.50 mmol·L-1(<CMC2)to 2.70 mmol·L-1(>CMC2).It suggests that for the mixed systems with x2=0.7,N increases as the micelles change from spherical to rodlike,meanwhile for the formed rodlike micelles,N increases further with the increase of cT.
The micellar compositions of the mixed cationic/anionic surfactants at CMC1are nearly equimolar for a wide range of mixing ratios.Whereas at cTmuch higher than CMC1,the micelle composition approaches the bulk solution mixing ratio[16,17].Thus the micellar compositions at CMC2are more close to the bulk compositions.For the mixed systems with x2=0.7,0.8 and 0.9,the compositions of rodlike micelles are more far from equimolar with the increase of x2.Micelle composition more close to equimolar corresponds to stronger electrostatic attraction between the oppositely charged headgroups,and weaker electrostatic repulsion between the like-charged headgroups in excess,thus means smaller effective headgroup areas a.According to the simple geometrical model,the smaller the a value,the larger the N value,that is why N increases with the decrease of x2under the same cT.For the mixed systems near the equimolar composition,floc or precipitate is easy to form(unsuitable for N measurement),indicating the significant increasing tendency of N as the equimolar composition is approached.
Israelachvili et al.[13]have argued that rodlike micelles form spherical end-caps in order to reduce the free energy cost of hydrocarbon ends in contact with water.For rodlike micelles[5],since a is at its optimum value everywhere except an end-cap part,the surfactant molecules residing in the end-cap parts have higher chemical potential than those in the cylindrical part.This unfavorable end-cap energy determines the growth tendency of rodlike micelles.For the mixed systems with x2=0.8 or 0.9,the end-cap energy of the rodlike micelles is low due to larger a value in favor of forming higher curvature structures such as end-caps,and the increase of cTleading to the formation of large amount of small rodlike micelles other than the formation of larger rodlike micelles.That is why their N values above CMC2are almost constants.On the other hand,for the mixed systems with x2=0.7,the end-cap energy of the rodlike micelles is high due to smaller a,thus leading to the enhancement of micellar growth in the axial dimension(i.e.the increase of N)with the increase of cT.
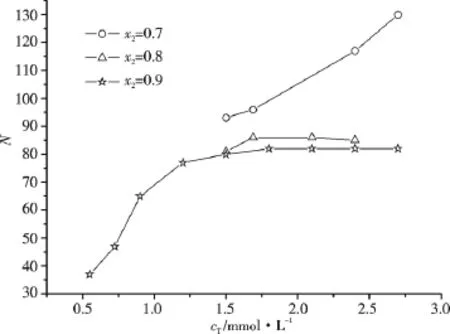
Fig.4 The aggregation numbers of C16TAB/AS/H2O mixed systems
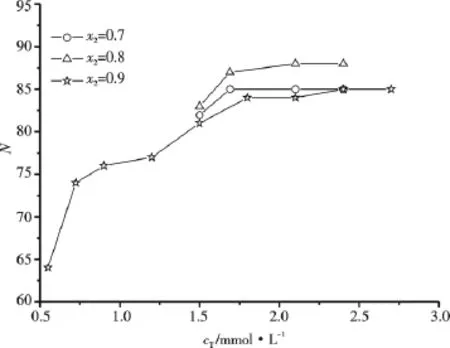
Fig.5 The aggregation numbers of C16TAB/AS/(0.1 mol·L-1NaBr aq)mixed systems
Fig.5 shows the N values of C16TAB/AS/0.1 mol·L-1NaBr/H2O mixed systems.Fig.6 shows the influences of NaBr and Na2SO4on N of C16TAB/AS/H2O mixed systems with x2=0.9.In comparison with the results shown by Fig.4,for the mixed systems with x2=0.8 and 0.9,the addition of NaBr or Na2SO4does not change the variation tendency of N with cT,but leads to the significant increase of N for the C16TAB/AS/H2O mixed systems with cT<CMC2,and leads to the slight increase or near invariableness of N for the C16TAB/AS/H2O mixed systems with cTnear or larger than CMC2.This is mainly due to a decrease in the a value with the addition of salt,which does not significantly influence v and lmax[14],thus leads to an increase of p and is beneficial for the formation of rodlike micelles at lower cTand the increase of N.Similar to the case of C16TAB,the salt effect of NaBr on N of C16TAB/AS/H2O mixed systems with x2=0.9 is stronger than that of Na2SO4.In these mixed systems C16TAB is in large excess,the mixed micelles are positively charged,the counterions Br-screens more efficiently the positive charge on the mixed micelles than counterions.However for the C16TAB/AS/H2O mixed systems with x2=0.7,the addition of NaBr leads to the decrease of N.The larger N values and the increase of N with the increase of cTfor the C16TAB/AS/H2O mixed systems with x2=0.7 shown by Fig.4 suggest that p is suitable for the formation of larger rodlike micelles and the elongation of rodlike micelles with the increase of cT.The addition of NaBr leads to the increase of p,thus is less suitable for the elongation of rodlike micelles.
2.3 Viscosities of C16TAB/AS mixed systems
The viscosities of surfactant systems are generally related to their microstructures[18].In order to verify the above experimental N results,as well as to replenish the η data of the C16TAB/AS mixed systems at low cT,the viscosities of some C16TAB/AS mixed systems near CMC2have been measured.Fig.7 gives the viscosities of some C16TAB/AS/H2O mixed systems at 318.15 K.The η variation of the mixed systems with x2=0.8 and 0.9 is very small as cTincreases due to that surfactant solutions containing spherical micelles or short rodlike micelles tend to have low η near to or slightly higher than η of solvent.The experimental η results also illustrate that the C16TAB/AS/H2O mixed systems with x2=0.8 and 0.9 are less viscous than the mixed systems with x2=0.7,which is in line with the experimental N results.For the mixed systems with x2=0.7,η increases more apparently when cT>CMC2due to the growth of rodlike micelles.These experimental η results for the dilute C16TAB/AS/H2O mixed systems with x2=0.7,0.8 and 0.9 are in good agreement with our previous shear viscosity results of these mixed systems at higher cTrange[9],the variation tendencies of η with cTare consistent.
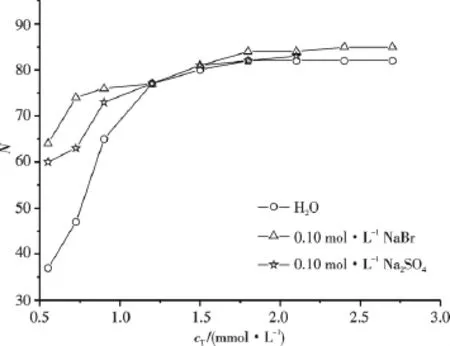
Fig.6 Aggregation numbers of C16TAB/AS/H2O with x2=0.9 in the absence or in the presence of salt at 318.15 K
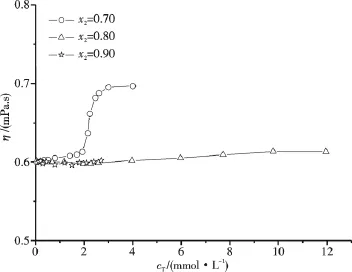
Fig.7 Viscosity of C16TAB/AS/H2O with different compositions at 318.15 K
Fig.8 gives the viscosities of some C16TAB/AS/(0.1 mol·L-1NaBr aq)mixed systems at 318.15 K.Different from the case with pure water as solvent,the mixed systems with x2=0.8 are more viscous than those systems with x2=0.7 and 0.9 at the same cT,the η sequence shown in Fig.8 is in accordance with the N sequence shown in Fig.5,i.e.,C16TAB/AS/(0.1 mol·L-1NaBr aq)mixed systems containing micelles with larger N are more viscous.In comparison with the result shown in Fig.7,the addition of NaBr into the C16TAB/AS/H2O mixed systems with x2=0.8 and 0.9 leads to η increase due to the increase of N.However,for the mixed systems with x2=0.7,the situation is different.The experimental η results in Fig.9 clearly illustrate that for the aqueous mixed systems with cT<2 mmol·L-1,addition of NaBr leads to η increase due to the decrease ofand the η increase of solvent when 0.1 mol·L-1NaBr aqueous solution substitutes water as solvent[19].Whereas when cT≥2 mmol·L-1,addition of NaBr results in the decrease of η due to the decrease of N.
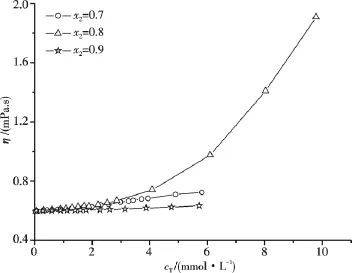
Fig.8 Viscosity of C16TAB/AS/(0.1 mol·L-1NaBr aq)with different compositions at 318.15 K
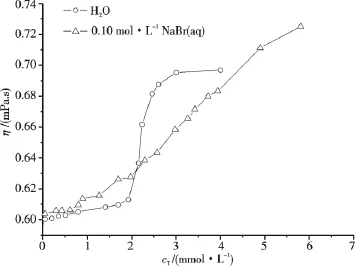
Fig.9 Viscosity of C16TAB/AS/H2O or C16TAB/AS/(0.1 mol·L-1NaBr aq)mixed systems with x2=0.7 at 318.15 K
The above results illustrate that the η variation of mixed cationic/anionic surfactant systems with cTis the reflection of the N variation with cT.For the rodlike micelles,larger N values mean more viscous.Similar to the investigation of Kato et al.[7],our investigation also suggests that η increases with the micellar growth of the mixed systems.In spite of that for the C16TAB/AS/H2O mixed systems with x2=0.7 and 0.8 in the absence or in the presence of NaBr,there is a heterogeneous region between CMC1and CMC2,which is similar to case of SDS/OTAB/H2O system.However,totally different N variation tendency with cTwas obtained for the clear solutions formed by these two cationic/anionic surfactant systems at concentrations above the heterogeneous region.This difference may be originated from the difference of average chain length of these two systems.Shorter chain length corresponds to stronger hydrophilicity and weaker hydrophobicity,and vice versa.It is deserved to mention that for the studied C16TAB/AS mixed systems in the heterogeneous region,the amount of floc or the insoluble solid is very small,and the formation of floc or insoluble solid shows almost no influence on the electrical conductivity vs cTcurve and the viscosity vs cTcurve.For these C16TAB/AS mixed systems,the micellar compositions at or slightly larger than CMC1are nearly equimolar for a wide range of mixing ratios,strong electrostatic attraction exists between the oppositely charged headgroups in the micelles.As cTincreases first,it is beneficial for the formation of catanionic complex(CTA+AS-)which is slightly soluble in water[20].Then,as cTincreases further,the micellar compositions are gradually far from equimolar ratios and approach to their bulk compositions,the formation of catanionic complex becomes unbeneficial and the originally formed complex is solubilized into mixed micelles.That is why these heterogeneous systems are formed at cTslightly above CMC1.Finally,as cTincreases above certain concentrations,the solutions become clear again,and sphere-to-rod micelle transition[2]first and then the growth of rodlike micelles or the increase of the number of small rodlike micelles were observed with increasing cT,that is why N increases with increasing cTfor these C16TAB/AS mixed systems.
3 Conclusions
The N values of the C16TAB/AS/H2O mixed systems with excess C16TAB at dilute concentrations above the heterogeneous region,increase with increasing cTat the same x2,or increase with decreasing x2at the same cT.Experimental η results increase with the micellar growth of the mixed systems,verifies the experimental N results.For the aqueous mixed systems with x2=0.8 and 0.9,low end-cap energy of the rodlike micelles is beneficial for the formation of large amount of small rodlike micelles other than larger rodlike micelles with increasing cT,thus N at concentrations slightly above CMC2are almost constants.However,for the aqueous mixed systems with x2=0.7,high end-cap energy of rodlike micelles is beneficial for the growth of rodlike micelles,thus increasing cTleads to the increase of N.
The addition of inorganic salt into the C16TAB/AS/H2O mixed systems with different x2gives different influ-ences on N.The addition of salt into the C16TAB/AS/H2O mixed systems with x2=0.8 and 0.9 leads to the significant increase of N at cT<CMC2and the slight increase or near invariableness of N at cTnear or larger than CMC2,respectively.The salt effect of NaBr on N is stronger than that of Na2SO4.The chaotropic counterions Br-screens more efficiently the positive charge of the excess chaotropic headgroups of C16TAB on the micelles than the kosmotropic,leading to lower CMC2and larger aggregates.However,the addition of NaBr into the C16TAB/AS/H2O mixed systems with x2=0.7 leads to decreasing N.Since the addition of NaBr leads to the increase of p,is less suitable for the elongation of rodlike micelles.
[1]ZANA R,LÉVY H,DANINO D,et al.Mixed micellization of cetyltrimethylammonium bromide and an anionic dimeric(gemini)surfactant in aqueous solution [J].Langmuir,1997,13(3):402-408.
[2]HAO L S,DENG Y T,ZHOU L S,et al.Mixed micellization and the dissociated margules model for cationic/anionic surfactant systems[J].J Phys Chem B,2012,116(17):5213-5225.
[3]DANINO D,TALMON Y,ZANA R.Alkanediyl-α,ω-bis(dimethylalky1ammonium bromide)surfactants(dimeric surfactants).5.Aggregation and microstructure in aqueous solutions[J].Langmuir,1995,11(5):1448-1456.
[4]ZANA R,LEVY H,PAPOUTSI D,et al.Micellization of two triquaternary ammonium surfactants in aqueous solution[J].Langmuir,1995,11(10):3694-3698.
[5]KWON S Y.Length control in rigid cylindrical nanoassembly by tuning molecular interactions in aqueous solutions[J].Langmuir,2008,24(19):10674-10679.
[6]HAYASHI S,IKEDA S.Micelle size and shape of sodium dodecyl sulfate in concentrated sodium chloride solutions[J].J Phys Chem,1980,84(7):744-751.
[7]KATO T,TAKEUCHI H,SEIMIYA T.Change in size and composition of mixed micelles with concentration in anionic/cationic surfactant solutions[J].J Colloid Interface Sci,1990,140(1):253-257.
[8]LI G Z,LI F,ZHENG L Q,et al.Fluorescence-probe study of anionic/cationic surfactant systems 2.Alkylsulfonate/alkyltrimethylammonium bromide[J].Colloids Surfaces A:Physicochem Eng Aspects,1993,76:257-265.
[9]YOU Y L,HAO L S,NAN Y Q.Phase behavior and viscous properties of cetyltrimethylammonium bromide and sodium dodecyl sulfonate aqueous mixtures[J].Colloids Surfaces A:Physicochem Eng Aspects,2009,335(1-3):154-167.
[10]TURRO N,YEKTA A.Luminescent probes for detergent solutions.A simple procedure for determination of the mean aggregation number of micelles[J].J Am Chem Soc,1978,100(18):5951-5952.
[11]GUO R,ZHU X J,GUO X.The effect of β-cyclodextrin on the properties of cetyltrimethylammonium bromide micelles[J].Colloid Polym Sci,2003,281(9):876-881.
[12]RAY G B,CHAKRABORTY I,GHOSH S,et al.Self-aggregation of alkyltrimethylammonium bromides(C10-,C12-,C14-,and C16TAB)and their binary mixtures in aqueous medium:a critical and comprehensive assessment of interfacial behavior and bulk properties with reference to two types of micelle formation [J].Langmuir,2005,21(24):10958-10967.
[13]ISRAELACHVILI J N,MITCHELL D J,NINHAM B W.Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers[J].J Chem Soc,Faraday Trans 2,1976,72:1525-1568.
[14]COLLINS K D.Ion hydration:implications for cellular function,polyelectrolytes,and protein crystallization[J].Biophys Chem,2006,119(3):271-281.
[15]VLACHY N,JAGODA-CWIKLIK B,KUNZ W,et al.Hofmeister series and specific interactions of charged headgroups with aqueous ions[J].Adv Colloid Interface Sci,2009,146(1-2):42-47.
[16]HERRINGTON K L,KALER E W,MILLER D D,et al.Phase behavior of aqueous mixtures of dodecyltrimethylammonium bromide(DTAB)and sodium dodecyl sulfate(SDS)[J].J Phys Chem,1993,97(51):13792-13802.
[17]KATO T,TAKEUCHI H,SEIMIYA T.Concentration dependence of micellar size and composition in mixed anionic/cationic surfactant solutions studied by light scattering and pulsed-gradient FT-NMR spectroscopy[J].J Phys Chem,1992,96(16):6839-6843.
[18]RAGHAVAN S R,FRITZ G,KALER E W.Wormlike micelles formed by synergistic self-assembly in mixtures of anionic and cationic surfactants[J].Langmuir,2002,18(10):3797-3803.
[19]ISONO T.Density,viscosity,and electrolytic conductivity of concentrated aqueous electrolyte solutions at several temperatures.Alkaline-earth chlorides,lanthanum chloride,sodium chloride,sodium nitrate,sodium bromide,potassium nitrate,potassium bromide,and cadmium nitrate[J].J Chem Eng Data,1984,29(1):45-52.
[20]MARQUES E F,BRITO R O,WANG Y,et al.Thermotropic phase behavior of triple-chained catanionic surfactants with varying headgroup chemistry[J].J Colloid Interface Sci,2006,294(1):240-247.

