Synthesis,Crystal Structure,Luminescent and Electrochemical Properties of a New Binuclear Cd(Ⅱ)Complex with 2,4-DAA as Ligand
YANG Ying-QunLI Chang-HongLI WeiKUANG Yun-Fei(Department of Chemistry and Materials Science,Hengyang Normal University,Hengyang,Hunan 4008)(Department of Chemical Engineering,Hunan Institute of Technology,Hengyang,Hunan 400)
研究简报
Synthesis,Crystal Structure,Luminescent and Electrochemical Properties of a New Binuclear Cd(Ⅱ)Complex with 2,4-DAA as Ligand
YANG Ying-Qun*,1LI Chang-Hong2LI Wei1KUANG Yun-Fei1
(1Department of Chemistry and Materials Science,Hengyang Normal University,Hengyang,Hunan 421008)(2Department of Chemical Engineering,Hunan Institute of Technology,Hengyang,Hunan 421002)
One new complex Cd2(2,4-DAA)4(2,2′-bipy)2with 2,4-dichlorophenoxy acetic acid(2,4-DAA)and 2,2′-bipyridine(2,2′-bipy)as ligands has been synthesized and characterized.Crystal data for this complex are as follows:monoclinic,space group,a=1.049 9(2)nm,b=1.094 2(2)nm,c=1.185 7(2)nm,α=96.70(3)°,β=105.20(3)°,γ=91.75(3)°,V=1.3030(5)nm3,Dc=1.806 g·cm-3,Z=2,μ(Mo Kα)=1.295 mm-1,F(000)=704,final discrepancy factors R1=0.0421,wR2=0.1021.In the crystal,two neighboring Cd(Ⅱ)ions are bridged by four 2,4-dichlorophenoxy acetic radicals,and their end positions are coordinated with two 2,2′-bipyridine molecules,forming a binuclear cage structure.The coordination environment of each cadmium(Ⅱ) ion is CdO4N2,giving a distorted triprism coordination geometry.Luminescent property and the cyclic voltammetric behavior of the complex were investigated.CCDC:781765.
cadmium(Ⅱ)complex;2,4-dichlorophenoxy acetic acid;crystal structure;luminescent property;electrochemical property
The study of carboxyl complexes has drawn great interests,mainly because of their intriguing variety of architectures and topologies as well as potential applications in many fields,such as functional materials,biochemistry,medicine,and so on[1-2].Through research,one fact is made clear that proper selection of organic ligands is a very critical step to obtain successfully target carboxyl complexes.In the past few years,extensive studies in this field have been focused on using rigid aromatic carboxylic acids as ligands[3-6].Comparatively,except for some cases[7-8],flexible aromatic carboxylic ligands have not been studied much,which is probably because their varied geometries and conformations make it difficult to forecast and control thefinal structures of the expected complexes[9].Therefore,In order to extend our knowledge of coordination chemistry and synthesize desired flexible aromatic carboxylic complexes with predictable structures and properties,it is necessary for us to exploit flexible aromatic carboxylic acid ligands.Phenoxyacetic acid and its derivatives are a class of flexible aromatic carboxylic acid.They can be used as conventional fungicides,plant growth regulators and agricultural herbicides[10].Moreover,they can be used as ligands to build complexes[11-13].In recent years,by using 2,4-dichlorophenoxy acetic acid as a ligand,we have synthesized several complexes and studied some of their propertyies[14-17].In order to continue our research on synthesis of flexible aromatic carboxylic complexes,we report herein the synthesis method,crystal structure,luminescent and electrochemical properties of the new binuclear Cd(Ⅱ) complex Cd2(2,4-DAA)4(2,2′-bipy)2.
1 Experimental
1.1 Materials and instrumentation
All materials,including 2,4-dichlorophenoxyacetic acid,2,2′-bipyridine,cadmium acetate dehydrate,triethylamine,acetic acid and sodium acetate trihydrate,were of analytical grade and used without further purification.Crystal structure was determined with a Rigaku SATURN CCD diffractometer.Luminescent spectra were measured at room temperature with a WGY-10 fluorescence spectrophotometer.Cyclic voltammetry analysis was conducted with a LK98 electrochemical analysis system.
1.2 Synthesis
A mixture of 2,4-dichlorophenoxyacetic acid(0.67 mmol),2,2′-bipyridine (0.13 mmol)and cadmium acetate dihydrate(0.47 mmol)was dissolved in 20 mL ethanol.The pH value of the resultant mixture was adjusted to about pH=5.0 by adding triethylamine solution.Under water-bath condition,the reaction was kept at 70 ℃ for nearly 16 h.Afterwards,the resultant solution was filtrated,and the filtrate waskept untouched and evaporated slowly at room temperature.Colorless block-shaped single crystals suitable for X-ray diffraction determination were obtained about three weeks later.Yield:31%.
1.3 Crystal structure determination
A crystal with dimensions of 0.18 mm×0.18 mm×0.16 mm was put on Rigaku SATURN CCD diffractometer equipped with a graphite-monochromatic Mo Kα radiation (λ=0.071 070 nm).The crystal was determined by using ω~φ scan mode at 113(2)K.Of the total 8 930 reflections collected in the range of 1.79°≤θ≤25.02°,4 459 were independent with Rint=0.027 3 and 4 236 were considered to be observed(I>2σ(I)).The observed reflections were used in the succeeding refinement.The crystal structure was solved directly with SHELXS-97 program,and it was refined with SHELXL-97 program[18].Corrections for Lp factors and empirical adsorption adjustment were applied and all non-hydrogen atoms were refined with anisotropic thermal parameters.The final refinement including hydrogen atoms converged to R1=0.0421,wR2=0.1021;w=1/[σ2(Fo2)+(0.0790P)2+0.1290P],where P=(Fo2+2Fc2)/3,(Δ/σ)max=0.003,S=1.123.Crystallographic data of the complex are shown in Table 1.
CCDC:781765.

Table 1 Crystallographic data of the title complex

Continued Table 1
2 Results and discussion
2.1 Crystal structure analysis
Selected bond lengths and bond angles are shown in Table 2.The crystal structure is revealed in Fig.1 and its coordination polyhedron is shown in Fig.2.As shown in Fig.1,the crystal structure contains two Cd(Ⅱ)ions,four 2,4-dichlorophenoxy acetic radicals,and two 2,2′-bipyridine molecules.Two Cd(Ⅱ) ions are bridged by four 2,4-dichlorophenoxy acetic radicals,and their end positions are coordinated with two 2,2′-bipyridine molecules,forming a binuclear cage-like structure.The distance between Cd(1)and Cd(1)iis 0.359 3 nm,shorter than those of analogues[Cd(cinnamato)2(Phen)]2(Cd-Cd 0.3780 nm)[19]and[Cd2(phen)2(C15H11O3)4]·(H2O)(Cd-Cd 0.419 8 nm)[20].In the title complex,only the bidentate bridging coordination mode of 2,4-dichlorophenoxy acetic radicals could be observed,and the folding angle O(2)-C(8)-O(3)is 125.9(3)°,which is characteristic of bridging carboxylic groups.Each Cd(Ⅱ)ion is coordinated with two nitrogen atoms of 2,2′-bipyridine and four oxygen atoms of four bridging 2,4-dichlorophenoxy acetic radicals to give a distorted triprism coordination geometry(Fig.2),where O(2),N(1)and O(6)locate at the top plane while O(5)i,N(2)and O(3)ioccupy the bottom plane.Their least-square plane equations are 6.792x-0.543y+6.679z=11.4903 and 6.608x-2.296y+6.873z=12.9578,respectively.The angle between the top plane and the bottom plane is 9.3°,suggesting that the two planes are nearly parallel to each other.The distances of Cd(1)-N(1)and Cd(1)-N(2)are 0.2365(3)nm and 0.2370(2)nm,respectively,and the average of Cd-N is 0.236 8 nm,which is longer than those of Cd-N in some other Cd(Ⅱ)complexes[21-23].The bond lengths of Cd(1)-O(2),Cd(1)-O(6),Cd(1)-O(3)iand Cd(1)-O(5)iare 0.233 7(2),0.231 6(2),0.230 6(2)and 0.2262(2)nm,respectively,and the average of Cd-O is 0.2305(2)nm.All in all,a conclusion could be drawn that the central Cd(Ⅱ)ions is in a distorted triprism coordination environment.

Fig.1 Molecular structure of the title complex with the ellipsoids presented at 30%probability level

Table 2 Selected bond lengths(nm)and bond angles(°)of the complex

Fig.2 Coordination polyhedron of Cd(Ⅱ)
2.2 Luminescence property
Fig.3 shows the emission spectrum of the title complex in the solid state measured at ambient temperature in the range of 550~730 nm.When the excitation wavelength is at 694 nm,the title complex exhibits an intense photoluminescence,and the maximum emission wavelength is at 702 nm.Comparably,under the same conditions,2,4-dichlorophenoxy acetic acid also displays emission at 702 nm (λex=694 nm).Moreover,luminescence peak intensity and shape of the free ligand 2,4-dichlorophenoxy acetic acid and those of the title complex are nearly identical.Therefore,the luminescence of the title complex may be mainly ascribed to electronic transition of the intraligand 2,4-dichlorophenoxy acetic acid.

Fig.3 Emission spectrum of the title complex in solid state at ambient temperature
2.3 Electrochemical property
The CV curve of the title complex is given in Fig.4.In the CV measurement,a conventional threeelectrode system was employed.Glass/C was the working electrode,a saturated calomel electrode(SCE)was the reference electrode and a platinum electrode was the counter electrode.HAc-NaAc buffer solution(pH=4.50)was used as the supporting electrolyte.The title complex was dissolved in the solvent of methanol with a complex condensation of 1 ×10-5mol·L-1.The scanning range is-1.2~0.2 V and scanning rate is 0.05 V·s-1.The results show that there exists only one couple of oxidation/reduction peaks with peak potential ofEpa=-0.686 V,Epc=-0.794 V,and ΔE=0.108 V,indicating that the electron transfer in the electrode reaction is quasi-reversible.
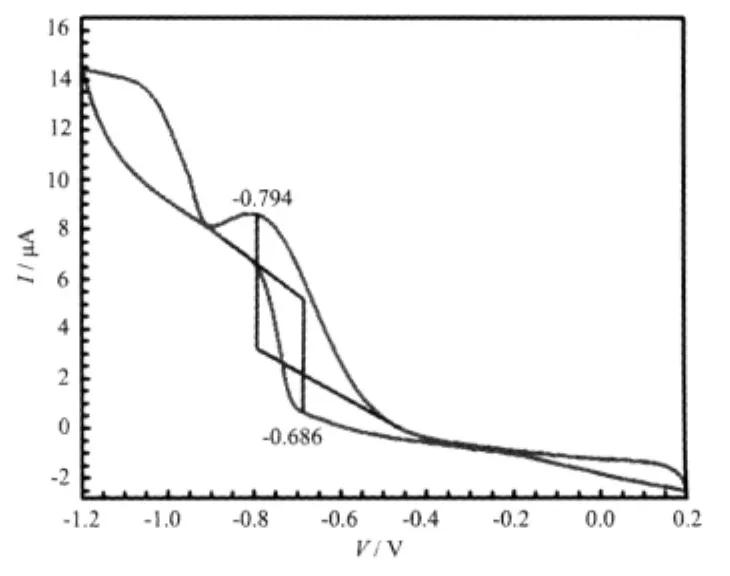
Fig.4 Cyclic voltammogram of the title complex
[1]Simon-Manso E,Valderrama M,Arancibia V,et al.Inorg.Chem.,2000,39:1650-1654
[2]Cao R,Sun D,Hong M,et al.Inorg.Chem.,2002,41:6161-6168
[3]Zhang S S,Niu S Y,Wang L,et al.Asian J.Chem.,2006,18:1885-1887
[4]Sharma R,Sharma R P,Bala R,et al.J.Coord.Chem.,2007,60:495-504
[5]Zhu X F,Zhou Y H,Guan L,et al.Acta Crystallogr.,Sect.E,2009,65:994-995
[6]MiyazawaM,IrieY,KashimotoK,etal.Inorg.Chem.Commun.,2009,12:336-339
[7]Jitsukawa K,Morioka T,Masuda H,et al.Inorg.Chim.Acta,1994,216:249-252
[8]Morin G,Shang M,Smith B D.Acta Crystallogr.,Sect.C:Cryst.Struct.Commun.,2000,56:544-547
[9]Bi W H,Cao R,Sun D F,et al.Inorg.Chem.Commun.,2003,6:1426-1428
[10]Lin Yu(林 郁).Handbook of Besticide Application(农药应用手册).Beijing:Agriculture Press,1982.
[11]GAO Shan(高 山),YUE Yu-Mei(岳玉梅),MA Dong-Sheng(马 东 升 ),et al.Chinese J.Struct.Chem.(Jiegou Huaxue),2004,23(7):825-828
[12]Zhao J G,Gu C S,Huo L H,et al.Acta Crystallogr.,Sect.E,2005,61:76-77
[13]Gulino F G,Lauceri R,Frish L,et al.Chem.-Eur.,2006,12:2722-2725
[14]YANG Ying-Qun(杨颖群),LI Chang-Hong(李昶红),LI Wei(李 薇),et al.Chemical Journal of Chinese Universities(Gaodeng Xuexiao Huaxue Xuebao),2008,29(3):449-452
[15]YANG Ying-Qun(杨颖群),LI Chang-Hong(李昶红),LI Wei(李 薇),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(9):1531-1534
[16]LI Wei(李 薇),LI Chang-Hong(李昶红),YANG Ying-Qun(杨颖群),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(2):269-372
[17]YANG Ying-Qun(杨颖群),LI Chang-Hong(李昶红),LI Wei(李 薇),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(6):1120-1123
[18]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of Gottingen,Germany,1997.
[19]TANG Si-Ping(唐斯萍).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(6):977-980
[20]LI Wei(李 薇),LI Chang-Hong(李昶红),YANG Ying-Qun(杨颖群),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(1):166-170
[21]Wang H S,Shi W,Zhai B,et al.J.Mol.Struct.,2007,833:102-105
[22]Wang J J,Yang M L,Hu H M,et al.Z.Anorg.Allg.Chem.,2007,633:341-343
[23]Song Y S,Yan B,Chen Z X.J.Solid.State Chem.,2006,179:4037-4040
2,4-二氯苯氧乙酸构筑的双核镉(Ⅱ)配合物的合成、晶体结构、荧光和电化学性质
杨颖群*,1李昶红2李 薇1匡云飞1
(1衡阳师范学院化学与材料科学系,衡阳 421008)(2湖南工学院化学化工系,衡阳 421002)
镉(Ⅱ)配合物;2,4-二氯苯氧乙酸;晶体结构;荧光性质;电化学性质
O614.24+2
:A
:1001-4861(2010)10-1890-05
2010-04-06。收修改稿日期:2010-06-22。
杨颖群,女,39岁,副教授;研究方向:功能配合物。
衡阳市科技计划项目(No.2009KJ26),湖南省重点建设学科项目资助。
*通讯联系人。 E-mail:yyingqun@126.com
- 无机化学学报的其它文章
- Solvothermal Synthesis,Crystal Structure and Photoluminescence Property of a Coordination Polymer Based on 1,1′-Ethynebenzene-3,3′,5,5′-tetracarboxylate
- Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
- Synthesis and Characterization of Tungsten Oxide Nanostructures
- Syntheses,Crystal Structure and Optical Property of Two Bis-ligand Silver(Ⅰ)Complexes Containing Diphenic Acid and Bidentate N-donor Ligands
- Syntheses and Structures of Two Copper(Ⅱ)Complexes with Salicyl Mono-oxime Ligands
- Graphene-RuO2Nanocomposites:Hydrothermal Synthesis and Electrochemical Capacitance Properties

