培门冬酶联合地塞米松治疗老年进展期结外NK/T细胞淋巴瘤的临床研究
上海交通大学医学院附属第九人民医院血液科,上海 200011
培门冬酶联合地塞米松治疗老年进展期结外NK/T细胞淋巴瘤的临床研究
刘隽 唐勇 程毅敏 姚一芸 邹丽芳 汪蕾 朱琦
上海交通大学医学院附属第九人民医院血液科,上海 200011
背景与目的:进展期淋巴结外NK/T细胞淋巴瘤(extranodal NK/T-cell lymphoma,ENKTL)是一种高度侵袭性非霍奇金淋巴瘤,老年患者预后较差,目前仍无标准治疗方案。培门冬酶作为聚乙二醇包裹的天冬酰胺酶可诱导肿瘤性NK细胞凋亡,并且具有良好耐受性。本研究探讨培门冬酶联合地塞米松(pegaspargase combined with dexamethasone,PegAD)方案治疗老年进展期ENKTL (鼻型)的临床疗效和不良反应。方法:15例老年患者均经病理确诊为ENKTL (鼻型),临床分期Ⅲ~Ⅳ期,采用PegAD方案治疗:培门冬酶2 500 U/(m2·d),肌内注射,第1天;地塞米松20 mg/d,静脉滴注,第1~4天,21 d为1个疗程。对该方案的临床疗效和安全性进行分析和评估。结果:①15例患者共完成45个疗程PegAD方案化疗,中位化疗3个疗程,2例获得完全缓解,7例达到部分缓解,总有效率为60%,总体平均生存时间10个月。②初治患者有效率75%明显高于诱导化疗失败的难治性患者(75% vs 43%,P<0.05),而且初治患者平均生存时间长于难治性患者(14个月 vs 6个月)。③PegAD方案化疗的主要不良反应为肝功能损害和凝血功能异常,其中3~4级肝功能损害发生率为20%,纤维蛋白原含量下降、凝血酶原时间或活化部分凝血酶时间延长的发生率达60%,血液学毒性轻微。结论:PegAD方案是治疗老年进展期ENKTL患者有效,且耐受性较好的化疗方案。
结外NK/T细胞淋巴瘤;老年患者;培门冬酶
在淋巴结外NK/T细胞淋巴瘤(extranodal NK/T-cell lymphoma,ENKTL)中,鼻型(nasal type)是2001年WHO淋巴造血组织肿瘤新分类中的独立类型。ENKTL属于非霍奇金淋巴瘤中一种少见的类型,但包括中国在内的亚洲地区并不少见。相对于早期局限性ENKTL,进展期患者对常规淋巴瘤化疗方案反应率较低且预后较差[1-3],而老年进展期ENKTL患者因其组织器官功能衰退以及并发症较多等因素而成为临床治疗难题。因此,有必要寻找老年进展期ENKTL患者可耐受的新型化疗方案。近来研究报道显示,进展期ENKTL患者经包含左旋门冬酰胺酶的化疗方案治疗后取得良好疗效[4],但左旋门冬酰胺酶多次静脉内用药所导致的不良反应(例如过敏反应、胰腺炎和血栓等)限制了其在老年患者中的应用。培门冬酶作为聚乙二醇包裹的天冬酰胺酶具有与左旋门冬酰胺酶相同的抗肿瘤机制,而且与左旋门冬酰胺酶相比,具有过敏反应发生率低、半衰期长和给药方便等优点[5]。2011年1月—2012年12月上海交通大学医学院附属第九人民医院血液科采用培门冬酶联合地塞米松(pegaspargase combined with dexamethasone,PegAD)方案治疗15例老年进展期鼻型ENKTL患者,现将结果报告如下。
1 资料和方法
1.1 病例选择
15例老年鼻型ENKTL患者均经病理组织学确诊,其中男性10例,女性5例;年龄61~75岁,平均65岁;临床分期(Ann Arbor-Cotswolds分期):ⅢB期6例,ⅣB期9例;初治患者8例,诱导化疗失败的难治性患者共7例,曾用过的化疗方案包括CMOP、CEOP、COP等,疗程数2~4(中位数为3);患者全身功能状态ECOG评分0~2分(表1);经签署化疗知情同意书后,给予PegAD方案治疗。
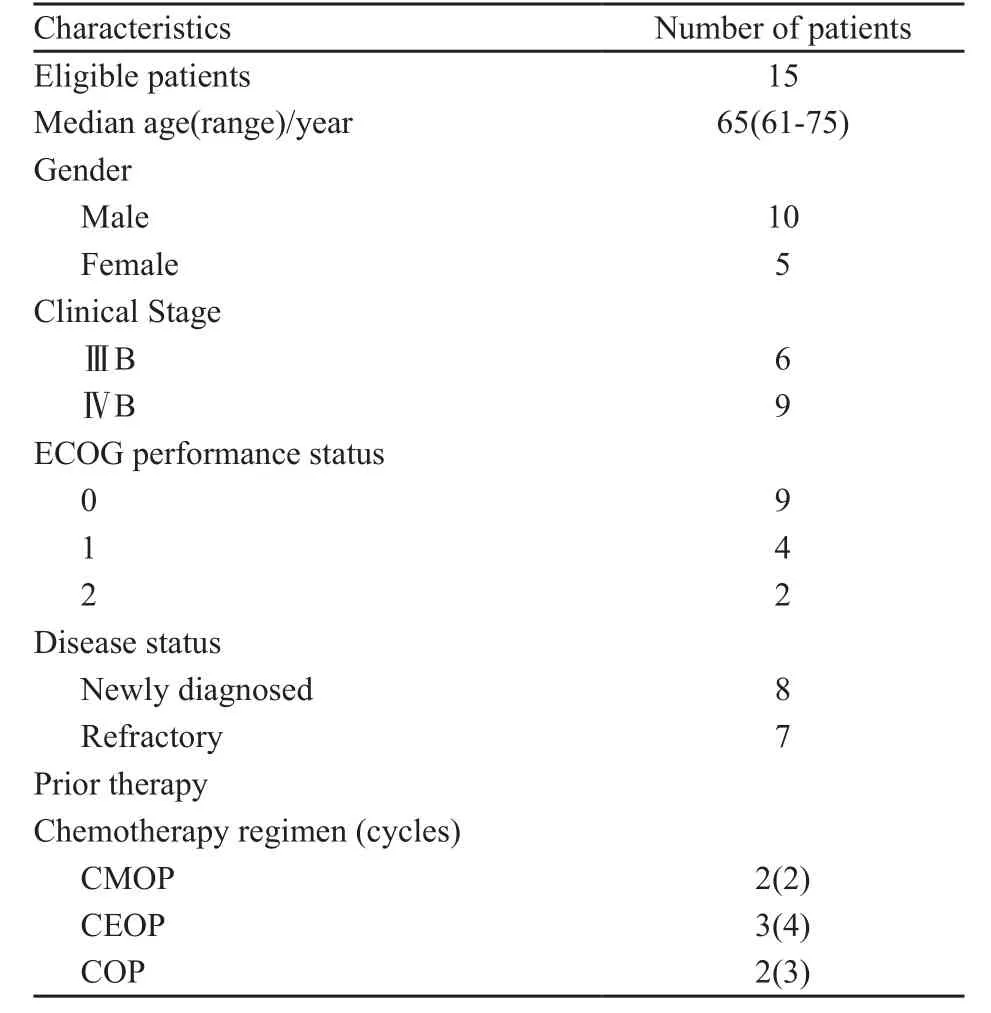
表 1 临床资料Tab. 1 Patient baseline characteristics
1.2 治疗方案
PegAD方案:培门冬酶2 500 U/(m2·d),肌内注射,第1天;地塞米松20 mg/d,静脉滴注,第1~4天,21d为1个疗程。化疗前预防恶心呕吐,常规给予5-HT3受体拮抗剂(恩丹西酮或格拉司琼)。治疗前后及过程中密切观察患者的临床表现并作血常规、肝肾功能、心电图、B超、影像学等检查,以此作为评价依据。
1.3 评价标准
化疗2个疗程后评价疗效,按照WHO恶性淋巴瘤的疗效评价标准进行临床评估[6],疗效包括完全缓解(CR):症状缓解、阳性体征消失,持续4周以上;部分缓解(PR):肿块减小50%以上并持续4周以上,无新的病变出现;稳定(SD):症状减轻或缓解,肿块缩小不足50%,或肿块增大25%以下,无新的病变出现;疾病进展(PD):肿块增大25%以下,或有新的病变出现。总体有效率(OR)为CR加PR。生存时间计算:患者接受PegAD方案治疗的第1天至患者失访日或死亡日;平均生存时间(月)=病例总的生存时间/病例数;不良反应则按照WHO评价标准[6]分为0~4级。
1.4 统计学处理
应用SPSS 13.0统计软件,将全部数据输入数据库进行统计分析,组间比较采用χ2检验。P<0.05为差异有统计学意义。
2 结 果
2.1 临床疗效和平均生存时间
15例老年进展期ENKTL患者共接受45个疗程的PegAD方案治疗,中位疗程数3个(1~6个),2例获得CR(2/15),7例达到PR(7/15),有效率为60%,总体平均生存时间为10个月;8例初治患者接受化疗的中位疗程数3个(2~6个),其中2例获得CR(2/8),4例达到PR(4/8),有效率为75%,平均生存时间为14个月;7例难治性患者接受化疗的中位疗程数2个(1~4个),无患者获得CR,3例达到PR(3/7),有效率为43%,平均生存时间为6个月;显示初治患者的疗效优于诱导化疗失败的难治性患者(P<0.05,表2)。
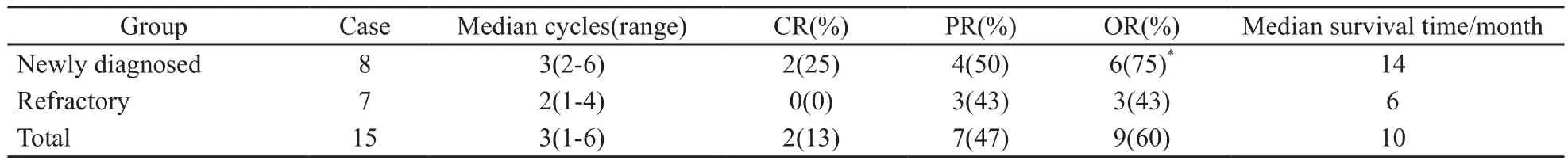
表 2 PegAD治疗方案的临床疗效和平均生存时间Tab. 2 Response and survival time after PegAD therapy in elderly patients with ENKTL
2.2 不良反应
PegAD化疗方案的主要不良反应为肝功能损害和凝血功能异常,3~4级肝功能损害发生率为20%,纤维蛋白原含量下降、凝血酶原时间或活化部分凝血酶时间延长的发生率达60%,各有1例患者发生3~4级白细胞和血小板减少,另有4例患者出现1~2级恶心、呕吐等消化道症状,此外,各有1例患者出现1~2级心脏、肾脏和周围神经不良反应(表3)。值得一提的是,无一例患者出现过敏反应、淀粉酶升高或治疗相关性胰腺炎。
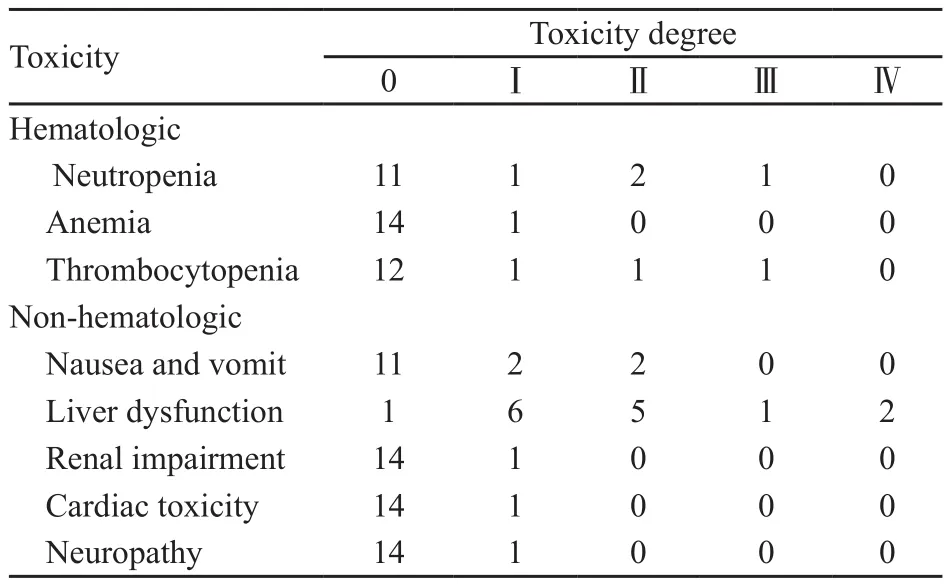
表 3 PegAD治疗方案的不良反应Tab. 3 Toxicity of PegAD in the treatment of elderly patients with relapsed NHL
3 讨 论
进展期ENKTL治疗以化疗为主,但各种传统化疗方案(如CHOP及CHOP样含蒽环类药物的化疗方案)在进展期患者所取得的整体疗效并不理想,而老年ENKTL患者的预后更差。由于淋巴细胞包括NK/T本身缺少门冬酰胺合成酶,以左旋门冬酰胺酶为基础的解救化疗方案取得较好疗效[7-9],但老年患者由于其化疗耐受性较差,通常不适用含左旋门冬酰胺酶强烈化疗方案(如SMILE方案等),必须另辟蹊径积极寻找适合老年ENKTL的治疗方案。培门冬酶是门冬酰胺酶的聚乙二醇偶联化合物,不仅保持了左旋门冬酰胺酶的活性,同时降低了蛋白质的免疫原性, 其半衰期长达6 d,避免了患者反复注射的痛苦,也减少了过敏反应的发生概率。与左旋门冬酰胺酶相比,培门冬酶毒性和不良反应发生率较低[10]。国内外已多个治疗小组应用含培门冬酶的化疗方案治疗中高度恶性淋巴瘤,取得了较为满意的临床疗效[11-13]。本研究采用PegAD方案治疗15例老年进展期ENKTL患者,结果显示有效率为60%,平均生存时间为10个月,其中初治患者的疗效较为理想,有效率达75%,平均生存时间为14个月,部分患者至今仍无病生存,提示PegAD方案可治愈部分初治老年ENKTL患者。虽然诱导化疗失败的难治性患者的缓解率较低,但仍有部分难治性患者取得PR,显示PegAD方案具有一定逆转耐药的能力。老年患者由于其器官功能的退化,化疗的不良反应与整体疗效密切相关,而既往研究发现培门冬酶的主要不良反应也都可以通过积极治疗得到有效控制[14-15]。本研究结果显示,PegAD方案的主要不良反应为肝功能损害和凝血功能异常,但通过积极支持治疗,未导致化疗相关死亡。重要的是,血液学毒性以及心脏、肾脏和周围神经不良反应多为1~2级,而且发生率较低。这些结果均提示,老年ENKTL对PegAD方案耐受性良好。
虽然本研究的样本量较少,远期疗效的数据也不充分,但这个方案对治疗老年进展期ENKTL仍具有积极意义,若将该方案与新型靶向药物结合,有望进一步提高老年ENKTL患者临床疗效和长期生存率。
[1] OSHIMI K. Progress in understanding and managing natural killer-cell malignancies [J]. Br J Haematol, 2007, 139(4): 532-544.
[2] CHIM C S, MA S Y, AU W Y, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index [J]. Blood,2004, 103(1): 216-221.
[3] KIM B S, KIM D W, IM S A, et al. Effective second-line chemotherapy for extranodal NK/T-cell lymphoma consisting of etoposide, ifosfamide, methotrexate, and prednisolone [J]. Ann Oncol, 2009, 20(1): 121-128.
[4] JACCARD A, PETIT B, GIRAULT S, et al. L-Asparaginasebased treatment of 15 Western patients with extranodal NK/ T-cell lymphoma and leukemia and a review of the literature[J]. Ann Oncol, 2009, 20(1): 110-116.
[5] ASSELIN B L, WHITIN J C, COPPOLA D J, et al. Comparative pharmacokinetic studies of three asparaginase preparations[J]. J Clin Oncol, 1993, 11(9): 1780-1786.
[6] CHESON B D, HORNING S J, COIFFIER B, et al. Report of an international workshop to standardize response criteria for non- Hodgkin's lymphomas. NCI Sponsored International Working Group [J]. J Clin Oncol, 1999, 17(4): 1244-1253.
[7] TROTTI A, COLEVAS A D, SETSER A, et al . CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment [J]. Semin Radiat Oncol, 2003, 13(3): 176-181.
[8] YONG W, ZHENG W, ZHU J , et al. L-asparaginase in the treatment of refractory and relapsed extranodal NK/Tcell lymphoma, nasal type[J]. Ann Hematol, 2009, 88 (7): 647-652.
[9] MATSUMOTO Y, NOMURA K, KANDA-AKANO Y, et al. Successful treatment with Erwinia L-asparaginase for recurrent natural killer/T cell lymphoma [J]. Leuk Lymphoma, 2003, 44(5): 879-882.
[10] SILVEMAN L B, SUPKO J G, STEVENSON K E, et al. Intravenous PEG-asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia [J]. Blood, 2010, 115 (7): 1351-1353.
[11] REYES V E Jr, AL-SALEEM T, ROBU V G, et al. Extranodal NK/T-cell lymphoma nasal type: efficacy of pegaspargase. Report of two patients from the United Sates and review of literature [J]. Leuk Res, 2010, 34(1): 50-54.
[12] WANG L, WANG Z H, CHEN X Q, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extronodal natural killer/T-cell lymphoma[J]. Cancer, 2013, 119(2): 348-355.
[13] 季建美, 徐小红. 培门冬酶治疗两例鼻型NK/T细胞淋巴瘤病例报道并文献回顾[J]. 齐齐哈尔医学院学报, 32(7): 1060-1061.
[14] MALBORA B, AVCI Z, OZBED N. Treatment of severe hypertriglyceridemia associated with accidental pegylated asparaginase push in a child with relapsed acute lymphoblastic leukemia[J]. Drug Chem Toxicol, 2012, 35(4): 463-466.
[15] STOCK W, DOUER D, DEANGELO D J, et al. Prevention and management of asparaginase/pegasparaginase-associated tocixities in adults and older adolescents: recommendations of an expert panel[J]. Leuk Lymphoma, 2011, 52(12): 2237-2253.
Clinical study on pegaspargase combined with dexamethasone in the treatment of elderly patients with extranodal NK/T-cell lymphoma
LIU Juan, TANG Yong, CHEN Yi-min, YAO Yi-yun, ZOU Lifang, WANG Lei, ZHU Qi (Department of Hematology, Shanghai 9thPeople’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China)
ZHU Qi E-mail: zhuqi70@hotmail.com
Background and purpose: Advanced extranodal NK/T-cell lymphoma is a highly aggressive disease with a grim prognosis in elderly patients. No therapeutic strategy is currently identified in these setting of patients. Pegaspargase formed by covalently attaching polyethylene glycol to asparaginase has been shown to induce apoptosis of tumoral NK cells in vitro and well tolerated. This clinical study was to investigate the clinical efficacy and toxicity of pegaspargase combined with dexamethasone (PegAD) regimen in the treatment of elderly patients with extranodal NK/T-cell lymphoma, nasal type (ENKTL). Methods: A total of 15 elderly patients with pathologically diagnosed ENKTL and presented with Ann Arbor stage Ⅲ-Ⅳ were treated with PegAD regimen, which consisted of pegaspargase 2 500 U/(m2·d) given as intramuscular injection on day 1 and dexamethasone 20 mg/d administered intravenously on day1 through 4. The PegAD regimen was repeated every 21 days. Clinical efficacy and safty profiles of PegAD regimen was systemically reviewed and analysed. Results: ① All the 15 patients
a total of 45 cycles of PegAD regimen. The median cycles was 3. Two patients achieved complete response, while seven cases obtained partial response. The overall response rate was 60%. The median survival time was 10 months. ②In newly diagnosed patients, overall response rate (OR) reached 75%, which was significantly higher than that in refractory cases, whose OR were 43% (P<0.05). In addition, the median survival duration of newly diagnosed patients was longer than that of refractory cases, whose median survival time was 14 and 6months, respectively. ③The major adverse events was liver dysfunction and disturbances of blood coagulation with grade 3-4 hepatitis in 20% cases and low levels of fibrinogen aswell as prolonged PT and APTT in 60% patients. However, hematologic toxicities were moderate. Conclusion: PegAD regimen was an effective and well tolerated therapeutic schedule for elderly patients with ENKTL.
Extranodal NK/T-cell lymphoma; Elderly patients; Pegaspargase
10.3969/j.issn.1007-3969.2013.04.010
R733.1
:A
:1007-3639(2013)04-0298-04
2013-02-01
2013-03-15)
朱琦 E-mail:zhuqi70@hotmail.com

